Abstract
Aim:
Three simple, accurate, and reproducible spectrophotometric methods have been developed and validated for simultaneous estimation of telmisartan (TELM) atorvastatin (ATV) in combined tablet dosage form.
Materials and Methods:
The first method is based on first-order derivative spectroscopy. The sampling wavelengths were 223 nm (zero crossing of TELM) where ATV showed considerable absorbance and 272 nm (zero crossing of ATV) where TELM showed considerable absorbance. The second method Q-analysis (absorbance ratio), involves formation of Q-absorbance equation using respective absorptivity values at 280.9 nm (isobestic point) and 296.0 nm (λmax of TELM). The third method involves determination using multicomponent mode method; sampling wavelengths selected were 296.0 and 246.9 nm.
Results:
TELM and ATV followed linearity in the concentration range of 5–40 and 4–32 μg/ml for method I, 5–30 μg/ml and 2–24 μg/ml for method II and III, respectively. Mean recoveries for all three methods were found satisfactory. All methods were validated according to International Conference on Harmonization Q2B guidelines.
Conclusion:
The developed methods are simple, precise, rugged, and economical. The utility of methods has been demonstrated by analysis of commercially available tablet dosage form.
Keywords: Absorbance ratio, atorvastatin, derivative spectroscopy, multicomponent analysis, telmisartan
INTRODUCTION
Telmisartan (TELM) chemically 4’-[(1, 4’-Dimethyl-2’-propyl-[2, 6’-bi-1H-benzimidazol]-1’-yl) methyl]-[1, 1’-biphenyl]-2-carboxylic acid, is a nonpeptide angiotensin-II receptor antagonist, which selectively and insurmountably inhibits angiotensin-II AT1 receptor subtype without affecting other systems involved in cardiovascular regulation [Figure 1]. Atorvastatin (ATV) calcium chemically [R-(R*, R*)]-2-(4-fluorophenyl)-β, δ, dihydroxy-5-(1-methyl ethyl)-3-phenyl-4 [(phenyl-amino)-carboxyl]-1 H-pyrrole-1-heptanoic acid calcium salt is a second generation synthetic 3-hydroxy-3-methyl glutaryl-coenzyme A (HMG-CoA) reductase inhibitor, which decreases de novo cholesterol synthesis [Figure 2]. ATR decreases the amount of low-density lipoprotein (LDL)-cholesterol in blood, reduces blood levels of triglycerides and slightly increases levels of high-density lipoprotein (HDL)-cholesterol.[1–3] Literature survey reveals several methods for determination of TELM and ATV individually in biological fluids and formulation like HPLC, TLC-densitometric, and derivative spectrophotometry.[4–14] HPLC and HPTLC methods were reported for determination of TELM and ATV in combination.[15,16].
Figure 1.
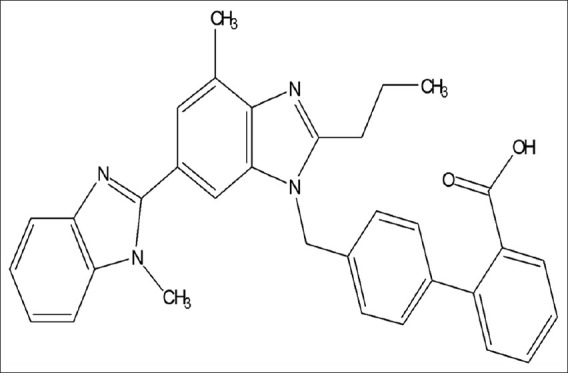
Chemical structure of telmisartan
Figure 2.
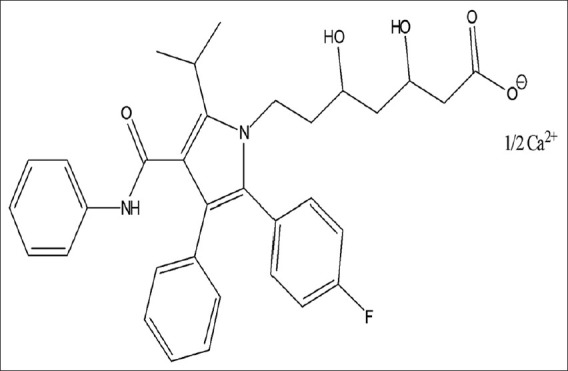
Chemical structure of atorvastatin calcium
However, due to lack of such equipments in many resources-limited countries and high costs of HPLC grade solvents and columns, alternative methods are needed to facilitate and increase the speed of analysis, with relatively few costs. Spectrophotometry continues to be very popular, because of its simplicity, versatility and low cost. In this paper, a successful attempt has been made to estimate two drugs simultaneously by UV spectrophotometric analysis. This paper describes three simple, rapid, accurate, reproducible, and economical methods for simultaneous determination of TELM and ATV in tablet formulation using first order derivative, Q-analysis, and multicomponent mode method.
MATERIALS AND MEHODS
Chemicals and reagents
Pharmaceutical grade TELM and ATV were supplied by Atoz laboratories, Chennai, India. Tablets labeled to contain 40 mg TELM and 10 mg ATV were manufactured and supplied by Dr. Reddy's Laboratories Ltd., Hyderabad, India. Methanol (analytical grade) was obtained from Merck Chemicals, Mumbai, India.
Equipment
A double beam UV/Visible spectrophotometer (Schimadzu, Japan) model UV-1700 with quartz cell 1 cm path length, connected to HP computer version 2.21 was used. Shimadzu balance (AUW-120D) was used for all weighing.
Standard stock solution
Standard stock solution (1.0 mg/ml) each of TELM and ATV was separately prepared by dissolving in methanol. These stock solutions were further diluted to get working standard stock solutions (each 100 μg/ ml).
Sample preparation
Twenty tablets were accurately weighed and tablet powder equivalent to 100 mg of TELM was transferred into a 100 ml volumetric flask; 50 ml methanol was added, dissolved and completed to 100 ml with same solvent. The resulting solution is filtered through Whatmann filter paper, discarding first few millilitres. From the above solution suitable aliquots were completed to volume with methanol to get concentration in the ratio of 4:1, taking into consideration its amount present in combined tablet formulation.
Method I
First order derivative spectroscopy
The first derivative (D1) spectra of TELM and ATV was found to show zero crossing point and assisted in their simultaneous estimation [Figure 3]. First derivative values of TELM and ATV were measured at 272 and 223 nm. Calibration curves were constructed by analysis of working standard solutions of TELM and ATV with six different concentrations in the range between 5–40 and 4–32 μg/ml for TELM and ATV, respectively. Each concentration was analyzed thrice. In assay of tablet formulation, the sample solution of final concentration 20 μg/ml of TELM and 5 μg/ ml of ATV was analyzed by first-order derivative spectroscopic method. The absorbance was measured at 272 and 223 nm. The procedure was repeated five times for sample analysis. The concentration of TELM and ATV were calculated from calibration graph.
Figure 3.
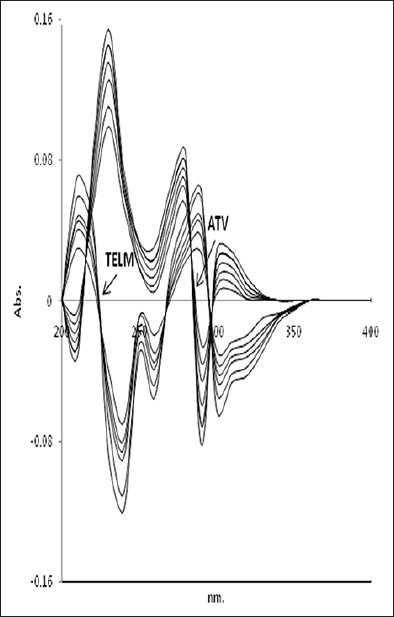
First order derivative spectra of TELM and ATV for different linear concentrations
Method II
Q-analysis method (Absorbance ratio)
Zero order absorption spectra of TELM shows λmax at 296.0 nm [Figure 4]. Similarly, ATV shows λmax at 246.9 nm [Figure 5]. For Q-analysis, the absorption spectra of prepared solutions were recorded in the range of 200–400 nm and absorbance values at 296.0 nm (λmax of TELM) and 280.9 nm (isobestic point) were measured [Figure 6]. The absorptivity values for both drugs at selected wavelengths were calculated and the average values were taken. The method employs Q values and the concentrations of both drugs were determined using following equation.
Figure 4.
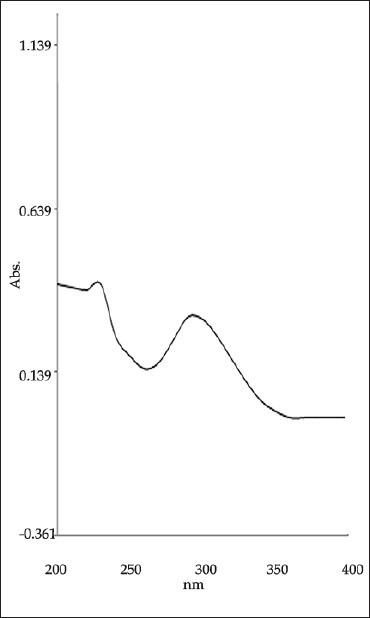
Zero order absorption spectra of TELM
Figure 5.
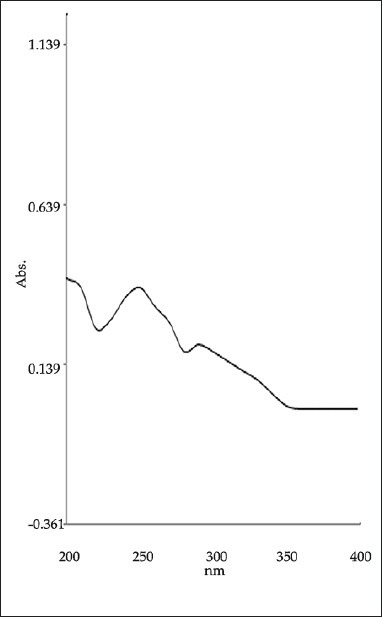
Zero order absorption spectra of ATV
Figure 6.
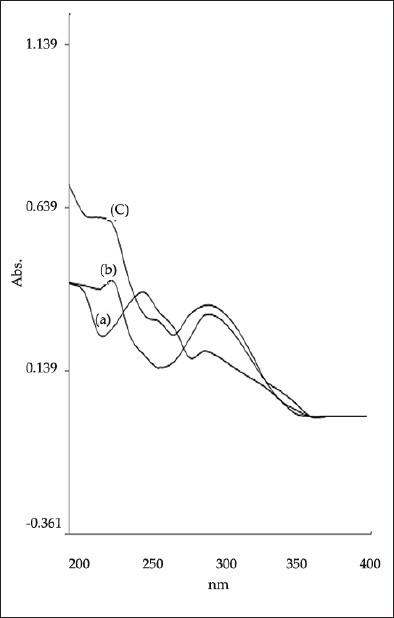
Zero order overlain spectra of (a) TELM, (b) ATV and (c) binary mixture of TELM and ATV
Cx = (Qm – Qx/Qx – Qy) × A1/ax1 (1)
Cy = (Qm – Qy/Qy – Qx) × A1/ay1 (2)
where Cx and Cy are concentrations of TELM and ATV in μg/ml, respectively, Qm is absorbance of sample at 296.0 nm/absorbance of sample at 280.9 nm; Qx is absorptivity of TELM at 296.0 nm/absorptivity of TELM at 280.9 nm; Qy is absorptivity of ATV at 296.0 nm/absorptivity of ATV at 280.9 nm; ax1 is absorptivity of TELM at 280.9 nm; ay1 is absorptivity of ATV at 280.9 nm; and A1 is absorbance of the sample at 280.9 nm. The absorbance of laboratory prepared mixtures at 296.0 and 280.9 nm were recorded; absorptivity were calculated and substituted in the equations mentioned above, in order to obtain the concentration of both drugs.
Method III
Multicomponent mode method
For this method 296.0 nm (λmax of TELM) and 246.9 nm (λmax of ATV) were selected as two sampling wavelengths for TELM and ATV and multicomponent mode of spectrophotometer was used. Similarly, sample solutions were scanned in the multicomponent mode of instrument at selected sampling wavelengths [Figure 7]. The overlain spectra of five standard binary mixtures were employed to determine the concentration of drugs in sample solutions by analysis of spectral data of sample solutions with reference to mixture standards.
Figure 7.
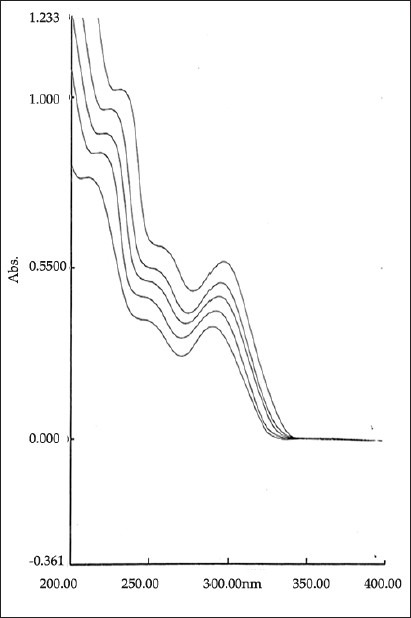
Overlain spectra of binary mixtures of TELM and ATV in multicomponent mode
RESULTS AND DISCUSSION
The aim of this work is to establish and validate simple, sensitive, and accurate spectrophotometric method according to ICH guidelines[17] with satisfactory precision and accuracy.
Linearity and sensitivity
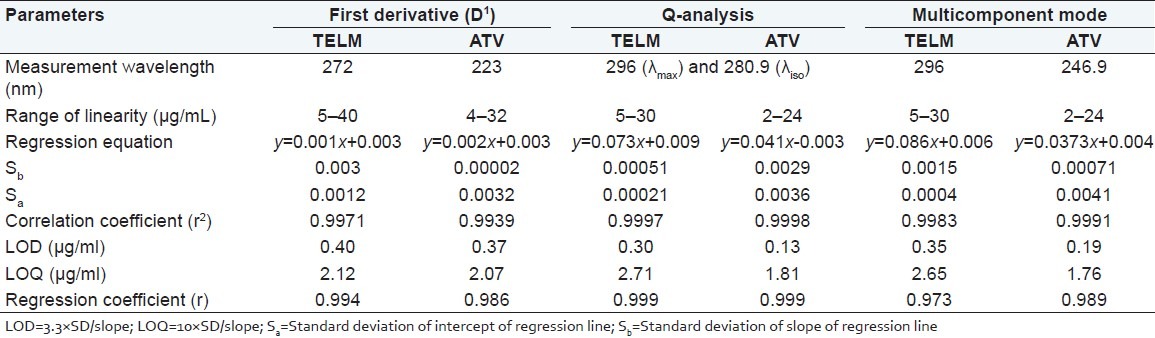
The linearity of methods was evaluated by analyzing six concentrations of each drug and each concentration was repeated three times. Linear regression equation was obtained over the concentration ranges. Table 1 reveals the correlation coefficients along with standard deviation of slope (Sb) and that of intercept (Sa). The detection and quantitation limits were calculated based on standard deviation of response and slope. The detection and quantification limits obtained for TELM and ATV for derivative, absorbance ratio and multicomponent mode methods were tabulated.
Table 1.
Optical characteristics obtained for TELM and ATV by first derivative, Q-analysis and multicomponent method
Accuracy
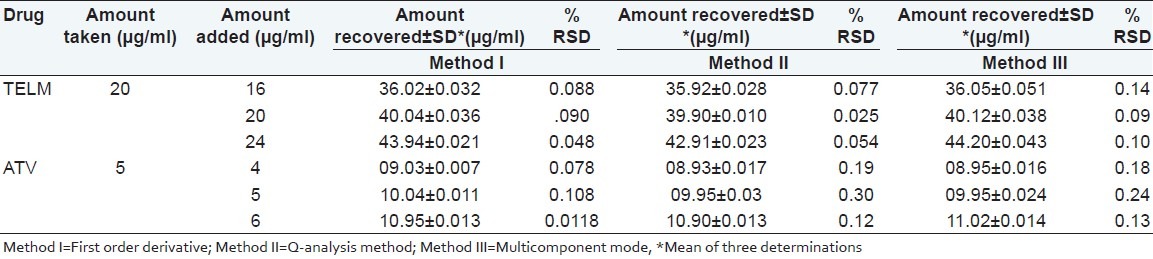
Accuracy was assured by standard addition technique, performed by addition of known amounts of pure TELM and ATV to known concentrations of sample solution. The resulting mixtures were assayed in triplicate and results obtained were compared with expected results. The good recoveries as revealed in Table 2 indicate accuracy of the proposed methods.
Table 2.
Results of recovery study
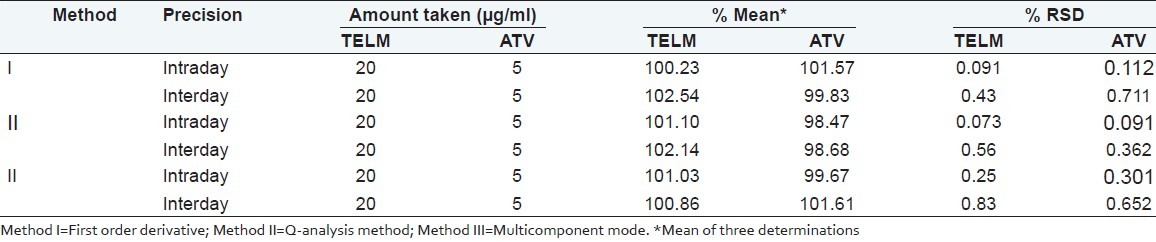
Precision
Precision was ascertained by triplicate estimation of standard drugs on same day (intraday) and on three consecutive days (interday). The percentage relative standard deviation reveals good precision [Table 3].
Table 3.
Results of intraday and interday precision
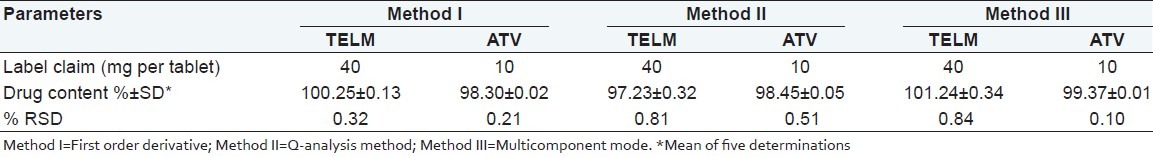
Assay of tablet formulation
The results of analysis of tablet formulation (labeled to contain TELM 40 mg and ATV 10 mg) for three methods are shown in Table 4. The standard deviation of five replicate analysis for all three methods were found to be <1. The assay values indicate that interference of excipients matrix is insignificant in the estimation of TELM and ATV by proposed methods.
Table 4.
Results of tablet analysis
CONCLUSION
The developed methods were found to be precise and accurate. The methods can be used for routine simultaneous spectrophotometric analysis of TELM and ATV in pharmaceutical preparations. Moreover, the developed methods have the advantages of simplicity, convenience and quantification of TELM and ATV for assay of their dosage form.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lea AP, McTavish D. Atorvastatin. A review of its pharmacology and therapeutic potential in the management of hyperlipidaemias. Drugs. 1997;53:828–47. doi: 10.2165/00003495-199753050-00011. [DOI] [PubMed] [Google Scholar]
- 2.Ramsay LE, Dennis Johnston G, Graham AM, Lucilla P, Poston L, Potter JF. British hypertension society guidelines for hypertension management. BMJ. 1999;319:630–35. doi: 10.1136/bmj.319.7210.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mc Clellan KJ, Markham A. Telmisartan. Drugs. 1998;56:1039–44. doi: 10.2165/00003495-199856060-00007. [DOI] [PubMed] [Google Scholar]
- 4.Altuntasa TG, Erkb N. Liquid chromatographic determination of atorvastatin in bulk drug, tablets, and human plasma. J Liq Chromatogr Related Technol. 2004;27:83–93. [Google Scholar]
- 5.Ghosh C, Jain I, Gaur S, Patel N, Upadhyay A, Chakraborty BS. Simultaneous estimation of atorvastatin and its two metabolites from human plasma by ESI-LC-MS/MS. Drug Test Anal. 2011;3:352–62. doi: 10.1002/dta.228. [DOI] [PubMed] [Google Scholar]
- 6.Zarghi A, Shafaati A, Foroutan SM, Khoddam A. A simple and rapid HPLC method for the determination of atorvastatin in human plasma with UV detection and its application to pharmacokinetic studies. Arzneimittelforschung. 2005;55:451–4. doi: 10.1055/s-0031-1296887. [DOI] [PubMed] [Google Scholar]
- 7.Pallad MS, Chatter M, Rajesh PM, Bhat AR. Difference spectrophotometric determination of telmisartan in tablet. Indian J Pharm Sci. 2006;68:685–6. [Google Scholar]
- 8.Bahrami G, Mohammadi B, Mirzaeei S, Kiani A. Determination of atorvastatin in human serum by reversed-phase high-performance liquid chromatography with UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;826:41–5. doi: 10.1016/j.jchromb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Stanisz B, Kania L. Validation of HPLC method for determination of atorvastatin in tablets and for monitoring stability in solid phase. Acta Pol Pharm. 2006;63:471–6. [PubMed] [Google Scholar]
- 10.Rao RN, Guru Prasad K, Naidu Gangu CH, Maurya PK. Development of a validated liquid chromatographic method for determination of related substances of telmisartan in bulk drugs and formulations. J Pharm Biomed Anal. 2011;56:471–8. doi: 10.1016/j.jpba.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 11.Farahani H, Norouzi P, Beheshti A, Sobhi HR, Dinarvand R, Ganjali MR. Quantitation of atorvastatin in human plasma using directly suspended acceptor droplet in liquid-liquid-liquid microextraction and high-performance liquid chromatography-ultraviolet detection. Talanta. 2009;80:1001–6. doi: 10.1016/j.talanta.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Rao RN, Sen S, Nagaraju P, Reddy VS, Krishnamurthy Radha P, Udaybhaskar S. HPLC determination of telmisartan in bulk and pharmaceutical formulations. Asian J Chem. 2006;18:775–82. [Google Scholar]
- 13.Li Y, Bing GP, Xiao YD, Wang X. Determination of In PHM telmisartan in human plasma by HPLC mass spectrometry and studies on its pharmacokinetics and relative bioavailability study. Chin J Clin Pharm. 2006;15:200–3. [Google Scholar]
- 14.Christel H, Liane G, Ulrich K, Uwe K. Determination of telmisartan in human blood plasma: Part II: Liquid chromatography-tandem mass spectrometry method development, comparison to immunoassay and pharmacokinetic study. Anal Chim Acta. 2006;560:35–40. [Google Scholar]
- 15.Patil UP, Gandhi SV, Sengar MR, Rajmane VS. High-performance thin-layer chromatographic determination of etoricoxib and thiocolchicoside in combined tablet dosage form. J AOAC Int. 2010;93:783–6. [PubMed] [Google Scholar]
- 16.Manoj SC, Abhiner G, Chakole RD. Simultaneous determination of atorvastatin calcium and telmisartan in pharmaceutical formulations by reverse phase-high performance liquid chromatography. Int J Pharm Chem. 2012;2:1–6. [Google Scholar]
- 17.ICH Q2 (R1), Validation of Analytical Procedures: Text and Methodology, CPMP/ICH/381/95. Geneva: 1995. pp. 1–15. [Google Scholar]


