Abstract
The control points of the Embden-Meyerhof-Parnas pathway in germinating castor bean (Ricinus communis) endosperms are sought in two ways: (a) by measuring the amounts of various glycolytic intermediates at intervals during the germination; (b) by determining the crossover points appearing during anoxia.
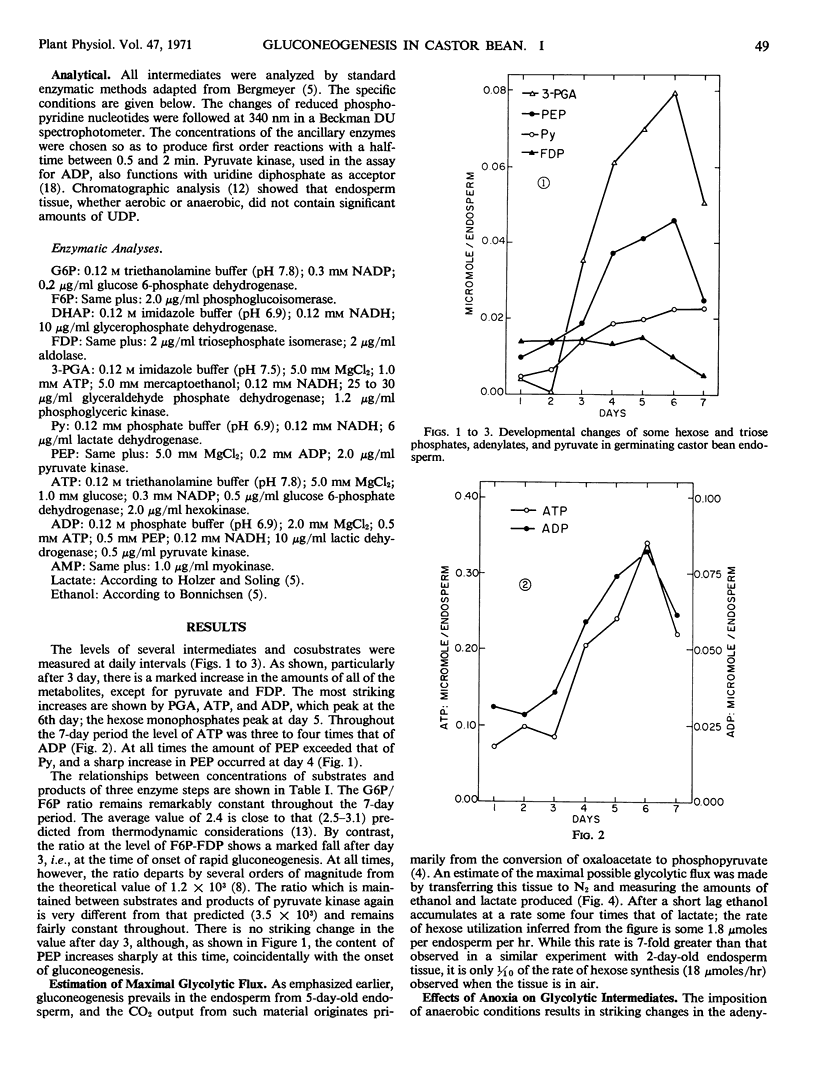
A significant departure from thermodynamic equilibrium between substrates and products is found at the level of fructose 1,6-diphosphatase and phosphofructokinase. A definite shift of this ratio is observed at the onset of active gluconeogenesis. The concentrations of phosphoenolpyruvate and 3-phosphoglyceric acid increase at the same time. Another departure from the expected equilibrium is also observed at the level of the pyruvate kinase.
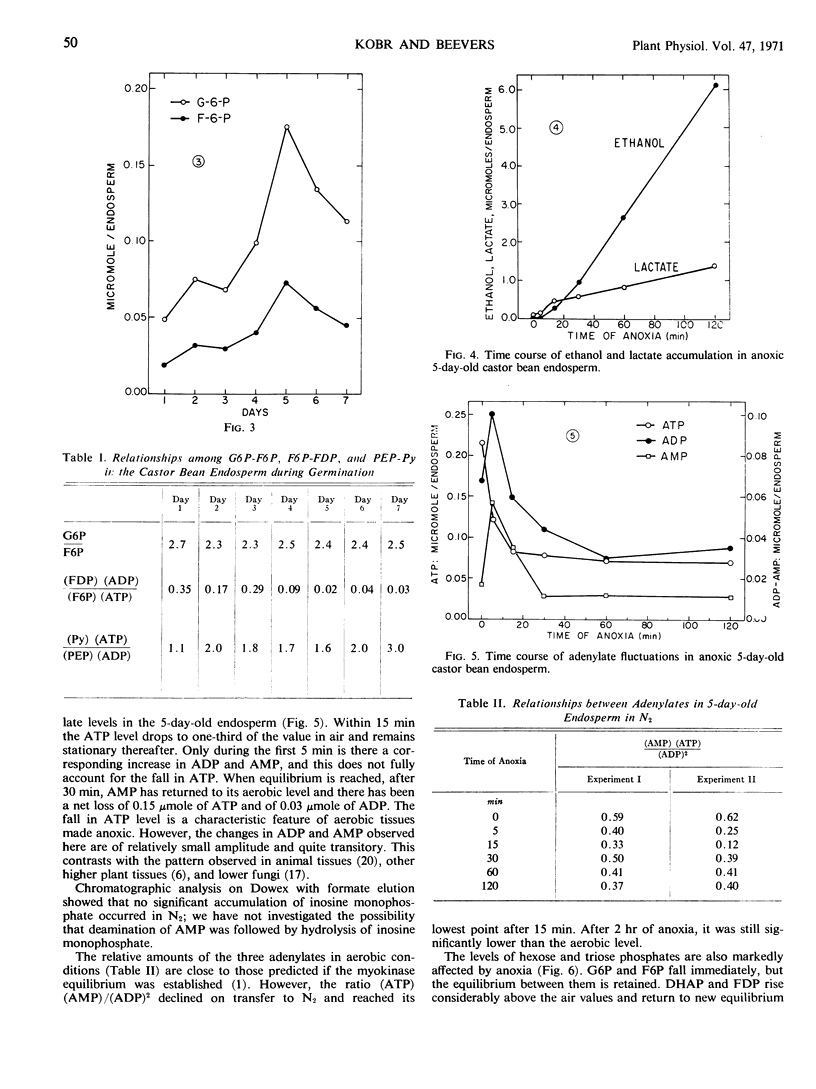
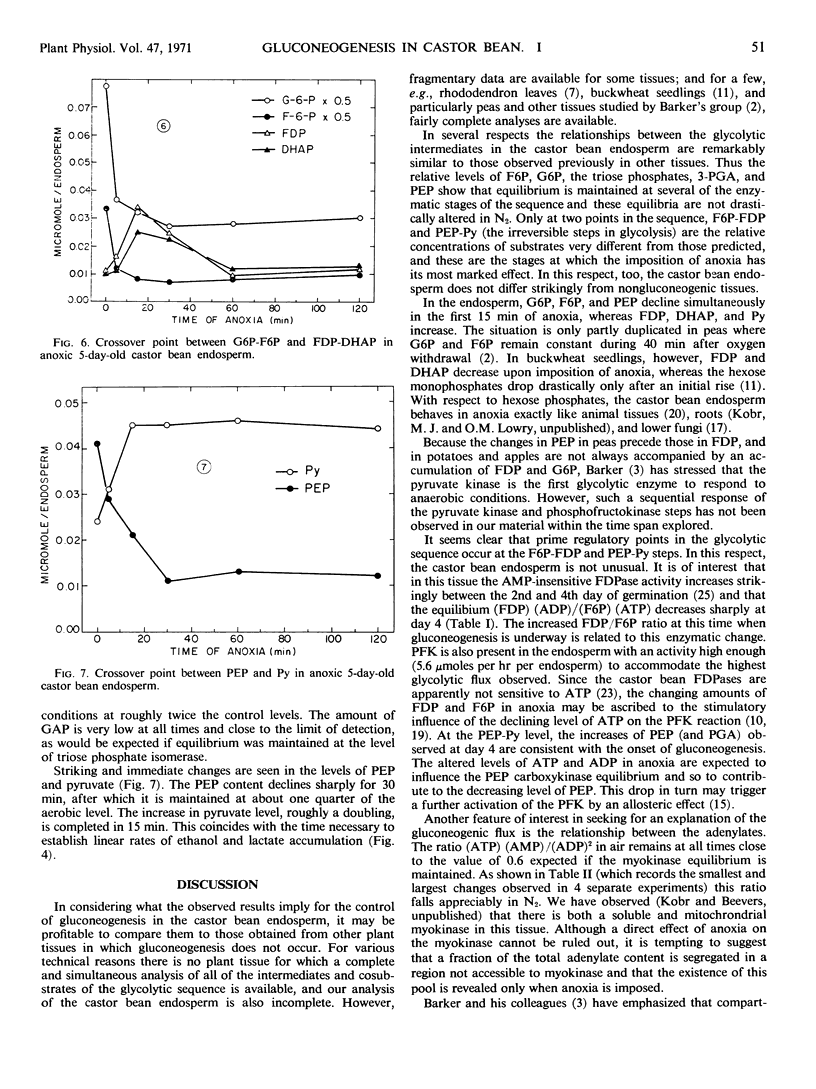
The imposition of anoxia on 5-day-old endosperms reveals two crossover points, at the level of the same enzymes. It is therefore concluded that they regulate the glycolytic flow.
The maximal glycolytic flow, however, is only 1/10 of the gluconeogenic one. To account for this high gluconeogenic efficiency, it is postulated that gluconeogenesis and glycolysis occur in separate intracellular regions. The consistent departure from equilibrium between adenylates observed during the early stages of anoxia supports the concepts that the pools of glycolytic and gluconeogenic intermediates are indeed compartmented and that the two processes are independently regulated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON M. R., BURTON R. M., MORTON R. K. Equilibrium constant of phosphoryl transfer from adenosine triphosphate to galactose in the presence of galactokinase. Biochem J. 1961 Apr;78:813–820. doi: 10.1042/bj0780813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER J., KHAN M. A., SOLOMOS T. MECHANISM OF THE PASTEUR EFFECT. Nature. 1964 Mar 14;201:1126–1127. doi: 10.1038/2011126a0. [DOI] [PubMed] [Google Scholar]
- Barker J., Khan M. A., Solomos T. Mechanism of the Pasteur effect. Nature. 1966 Jul 30;211(5048):547–548. doi: 10.1038/211547a0. [DOI] [PubMed] [Google Scholar]
- Benedict C. R., Beevers H. Formation of sucrose from malate in germinating castor beans. I. Conversion of malate to phosphoenol-pyruvate. Plant Physiol. 1961 Sep;36(5):540–544. doi: 10.1104/pp.36.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel J. L., Pradet A. Study of adenosine 5'-mono-,di- and triphosphates in plant tissues. IV. Regulation of the level of nucleotides, in vivo, by adenylate kinase: theoretical and experimental study. Biochim Biophys Acta. 1968 Aug 20;162(2):230–242. doi: 10.1016/0005-2728(68)90105-9. [DOI] [PubMed] [Google Scholar]
- Bourne D. T., Ranson S. L. Respiratory Metabolism in Detached Rhododendron Leaves. Plant Physiol. 1965 Nov;40(6):1178–1190. doi: 10.1104/pp.40.6.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Dennis D. T., Coultate T. P. Phosphofructokinase, a regulatory enzyme in plants. Biochem Biophys Res Commun. 1966 Oct 20;25(2):187–191. doi: 10.1016/0006-291x(66)90578-x. [DOI] [PubMed] [Google Scholar]
- Effer W. R., Ranson S. L. Some effects of oxygen concentration on levels of respiratory intermediates in buckwheat seedlings. Plant Physiol. 1967 Aug;42(8):1053–1058. doi: 10.1104/pp.42.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURLBERT R. B., SCHMITZ H., BRUMM A. F., POTTER V. R. Nucleotide metabolism. II. Chromatographic separation of acid-soluble nucleotides. J Biol Chem. 1954 Jul;209(1):23–39. [PubMed] [Google Scholar]
- KAHANA S. E., LOWRY O. H., SCHULZ D. W., PASSONNEAU J. V., CRAWFORD E. J. The kinetics of phosphoglucoisomerase. J Biol Chem. 1960 Aug;235:2178–2184. [PubMed] [Google Scholar]
- KREBS H. A., KORNBERG H. L., BURTON K. A survey of the energy transformations in living matter. Ergeb Physiol. 1957;49:212–298. [PubMed] [Google Scholar]
- Kelly G. J., Turner J. F. Inhibition of pea-seed phosphofructokinase by phosphoenolpyruvate. Biochem Biophys Res Commun. 1968 Jan 25;30(2):195–199. doi: 10.1016/0006-291x(68)90470-1. [DOI] [PubMed] [Google Scholar]
- Kelly G. J., Turner J. F. The regulation of pea-seed phosphofructokinase by phosphoenolpyruvate. Biochem J. 1969 Nov;115(3):481–487. doi: 10.1042/bj1150481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobr M. J., Turian G. Metabolic changes during sexual differentiation in Allomyces. Arch Mikrobiol. 1967 Jun 21;57(3):271–279. doi: 10.1007/BF00405952. [DOI] [PubMed] [Google Scholar]
- LELOIR L. F., GOLDEMBERG S. H. Synthesis of glycogen from uridine diphosphate glucose in liver. J Biol Chem. 1960 Apr;235:919–923. [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V. A COMPARISON OF THE KINETIC PROPERTIES OF PHOSPHOFRUCTOKINASE FROM BACTERIAL, PLANT AND ANIMAL SOURCES. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964 May 11;248:185–194. doi: 10.1007/BF00246673. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- NEAL G. E., BEEVERS H. Pyruvate utilization in castor-bean endosperm and other tissues. Biochem J. 1960 Feb;74:409–416. doi: 10.1042/bj0740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala J., Patrick C., Macbeth G. FDPases of the castor bean endosperm and leaf: properties and partial purification. Arch Biochem Biophys. 1968 Sep 20;127(1):576–584. doi: 10.1016/0003-9861(68)90265-8. [DOI] [PubMed] [Google Scholar]



