Abstract
Aim:
Described in this manuscript is the first ever reported, new, simple, high-performance thin-layer chromatographic method for the determination of mycophenolate mofetil in bulk and tablet dosage form.
Materials and Methods:
The drug was separated on aluminum plates precoated with silica gel 60 F254 with toluene, acetone, and methanol in the ratio of 6:2:2 (v/v/v) as the mobile phase. Quantitative analysis was performed by densitometric scanning at 254 nm.
Results:
The method was validated for linearity, accuracy, precision, and robustness. The calibration plot was linear in the range of 100–500 ng band-1 for mycophenolate mofetil. The method was successfully applied to the analysis of the drug in a pharmaceutical dosage form.
Keywords: Accuracy, mycophenolate mofetil, tablet dosage form
INTRODUCTION
Mycophenolate mofetil is chemically 2-(morpholin-4-yl) ethyl (4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydroisobenzofuran-5-yl)-4-methylhex-4-enoate.[1] Mycophenolate mofetil [Figure 1] is an immunosuppressant and prodrug of mycophenolic acid, extensively used to prevent rejection in organ transplantation. It acts as a noncompetitive, selective, and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH) in purine biosynthesis, guanine synthesis to be specific, which is necessary for the growth of T cells and B cells. A few high-performance liquid chromatography (HPLC)[2–7] and liquid chromatography–mass spectrometry (LC-MS)[8,9] methods for its determination have been reported. A simultaneous determination of mycophenolic acid and valproic acid in human plasma by HPLC[10] has also been reported.
Figure 1.
Structure of mycophenolate mofetil
No high-performance thin layer chromatography (HPTLC) method for the quantitative analysis of mycophenolate mofetil as bulk and as formulation or for stability studies was found by a computer-assisted survey of the literature either from chemical abstracts or by using the CAMAG Bibliography Service. Hence, the main objective of the work discussed in this paper was to develop a rapid and reliable HPTLC method for the analysis of mycophenolate mofetil in bulk and its dosage form. The method was validated in accordance with the guidelines of the International Conference on Harmonization (ICH) International Conference on Harmonization and remove “o”.[11] Here we describe the investigation in detail.
The usage of HPTLC is well appreciated and accepted all over the world. Many methods are being established to standardize the assay methods. HPTLC remains one step ahead when compared with other tools of chromatography. HPTLC is used for the identification of constituents, determination of impurities, and quantitative determination of active substances. The use of modern apparatus such as video scanners, densitometers, and new chromatographic chambers, more effective elution techniques and high-resolution sorbents with selected particle size or chemically modified surface, the possibility of combining with other instrumental methods, and the development of computer programs for method optimization make HPTLC an important alternative method to HPLC or gas chromatography. Specifically, HPTLC is one of the ideal TLC techniques for analytical purposes because of its increased accuracy, reproducibility, and ability to document the results, compared with standard TLC. Because of this, HPTLC technologies are also the most appropriate TLC techniques for conformity with Good Manufacturing Practices (GMPs). Today, the comprehensive use of TLC in pharmaceutical analysis is demonstrated by the great number of articles published in this field. So the ultimate aim of the present study is to develop and validate the HPTLC method for the determination of mycophenolate mofetil in bulk drug and dosage form. The optimization of the method separation, validation parameters, and quantification of mycophenolate mofetil as bulk and as formulation are reported in the following sections.
MATERIALS AND METHODS
Chemicals and reagents
The authentic sample of mycophenolate mofetil was procured from Intas Pharmaceuticals Ltd., Ahmedabad. The pure drug obtained had 99.9% w/w assay value, and was used without further purification. All chemicals and reagents used were of analytical grade. Mycophenolate mofetil is available as commercial tablets under the brand name Mycofit 250 mg from Intas Pharmaceuticals Ltd., and Mycofit 250 mg was procured from the local pharmacy.
Preparation of the standard stock solution
Analyte (10, 20, 30, 40, and 50 mg) was accurately weighed and separately dissolved in methanol in 100 mL volumetric flasks to furnish solutions in the concentration range of 100–500 ng μL-1. These solutions were used for the working range.
Chromatographic conditions
Chromatography was performed on 10 × 10 cm aluminum plates precoated with 250 μm layers of silica gel 60 F254 (E. Merck, Darmstadt, Germany). Before use, the plates were prewashed with methanol and activated at 110° for five minutes. The samples were applied to the plates as bands that were 6 mm wide and 10 mm apart by means of a Camag Linomat V sample applicator (Camag, Muttenz, Switzerland) equipped with a 100 μl syringe (Hamilton, Bonaduz, Switzerland). Linear ascending development was performed in a 10 × 10 cm twin trough glass chamber (Camag), with toluene, acetone, and methanol in the ratio 6:2:2 (v/v/v) as the mobile phase and the chamber was presaturated with mobile phase vapor for 10 minutes. The development distance was 8.5 cm with a development time of approximately 60 minutes. After chromatography, the plates were dried in a current of air by using air-blowing drier. Densitometric scanning was performed with a Camag TLC Scanner 3 at 254 nm for all measurements. The scanner was operated by Wincats software version 1.2.3. The source of radiation was a deuterium lamp emitting a continuous ultraviolet (UV) spectrum between 200 and 400 nm. The slit dimensions were 5 × 0.45 mm and the scanning speed was 20 mm s-1. After chromatographic development, the bands were scanned in the range of 200–400 nm (spectrum scan speed: 20 nm s-1) so that the drug could be estimated at 254 nm, which is ascertained by taking the spectrum at different concentrations between 100 and 500 ng with 100 ng increment. Further, it is also observed that the spectra are similar in their behavior.
Procedure for the standard
The standard stock solution of mycophenolate mofetil was applied on a TLC plate, (1 μL) by using the Linomat V sample applicator and the 100 μl syringe. The plate was developed and scanned under the conditions described above. Each amount was analyzed five times and peak areas were recorded. A calibration plot of peak area against the respective amount was established for mycophenolate mofetil.
Procedure for the sample
Twenty tablets were weighed accurately and finely powdered. A quantity of powder equivalent to 10 mg mycophenolate mofetil was weighed and transferred to a 100 mL volumetric flask containing approximately 50 mL methanol. The mixture was ultrasonicated for five minutes; then, the final dilution was made with methanol. The solution was filtered using Whatmann 41 paper, and 3 μl of the filtrate was applied on a TLC plate. After the development of the chromatogram, the peak area of the bands was measured at 254 nm and the amount of drug in each tablet was determined from the calibration plot. The analytical procedure was repeated six times for the homogenous powder sample.
Method validation
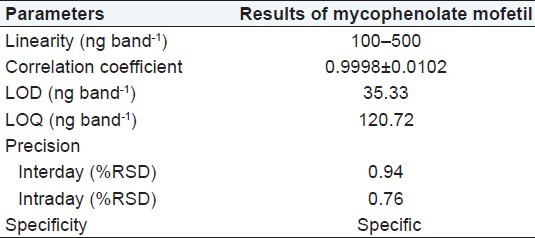
The limit of detection (LOD) and limit of quantitation (LOQ) for mycophenolate mofetil was calculated from the linearity data using relative standard deviation of the response and slope of the calibration curve for mycophenolate mofetil. The LOD of a compound is defined as the lowest concentration that can be detected. LOD value was found to be 35.33 ng/band for mycophenolate mofetil. LOQ is the lowest concentration of a compound that can be quantified with acceptable precision and accuracy. The LOQ value was found to be 120.72 ng/band for mycophenolate mofetil. To study intraday and interday precision, three different concentrations of sample solutions were prepared (100, 300, and 500 ng/ band) and applied to the TLC plates. All the solutions were analyzed in triplicate on the same day and on three different days to record intraday and interday variations in the results, respectively. To check the accuracy of the method, recovery measurements were performed by the addition of the standard drug solution at three different levels (50, 100, and 150%) to the preanalyzed sample solution (200 ng/band for mycophenolate mofetil so that after the addition of standards, the samples would be in the linear range). Three replicate estimations were carried out for each concentration level. The specificity of the method was determined by analyzing the drug standard and test samples. The peak for mycophenolate mofetil in the test sample was confirmed by comparing its RF and spectrum with those of the standard. The peak purity of mycophenolate was determined by comparing the spectra acquired at three different positions on the spot, that is, peak start (S), peak apex (M), and peak end (E). By introducing small changes in the mobile phase composition, the effects on the results were examined for studying robustness. The mobile phase having different compositions like toluene-acetone-methanol was tried and chromatograms were run. The amount of mobile phase, temperature, and relative humidity varied in the range of ±5%. Robustness of the method was done at three different concentration levels: 100, 300, and 500 ng/band, respectively.
RESULTS AND DISCUSSION
Different mobile phases containing toluene, methanol, acetic acid, propanol, acetone, ethyl acetate, and dichloromethane in different proportions were examined; of these, the mixture of toluene, acetone, and methanol in a ratio of 6:2:2 (v/v/v) Was found to be most suitable for the studies. The Rf value of standard mycophenolate mofetil was 0.55 ± 0.02. [Figure 2]. The densitogram obtained from a sample solution of mycophenolate mofetil is depicted in Figure 3. The calibration plot was found to be linear in the range of 100–500 ng/band for tapentadol hydrochloride, with a correlation coefficient of 0.9998 ± 0.0102. The LOD and LOQ were 20.33 and 60.72 ng/band, respectively. The proposed HPTLC methods were validated for intra and interday variations. The values of percent relative standard deviations (RSDs) were found to be 0.76 and 0.94, respectively which indicate that the method was precise. The method was also evaluated by the assay of commercially available tablets containing mycophenolate mofetil. Six replicate analyses were performed on accurately weighed amount of tablets. The percent assay was found to be 99.29 ± 0.77 for mycophenolate mofetil. To study the accuracy of the method, recovery studies were performed. For mycophenolate mofetil, the recovery ranged from 99.26 to 100.5%, with values of percent RSD ranging from 0.29 to 0.72, indicating that the proposed HPTLC method was highly accurate [Table 1]. When the specificity of the method was checked, it was found that the Rf and UV spectrum of the drug standard were the same as those from the sample. The study of robustness of the method revealed that the peak areas were unaffected (RSD < 2%) by small changes in the operating conditions, and can be inferred to be more robust. The method validation parameters are presented in Table 2.
Figure 2.
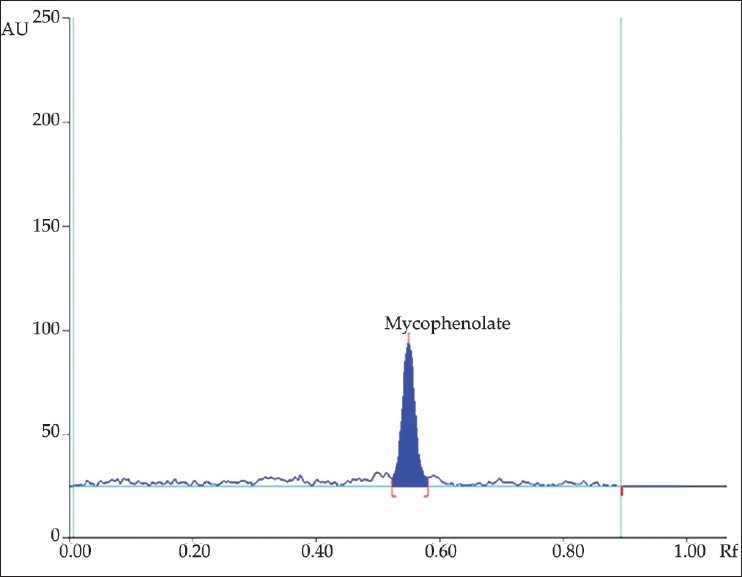
Typical HPTLC densitogram of mycophenolate mofetil standard (Rf: 0.55 ± 0.02)
Figure 3.
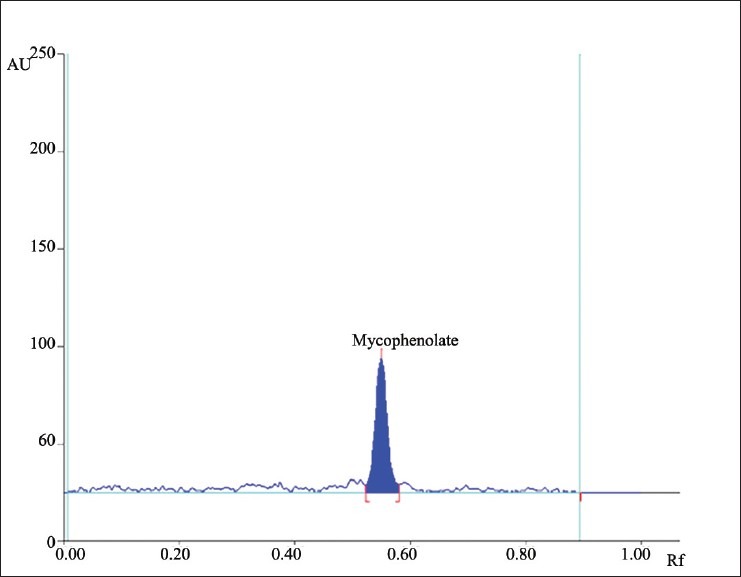
Typical HPTLC densitogram of mycophenolate mofetil extracted from tablet formulation (Rf: 0.55 ± 0.02)
Table 1.
Results from recovery studies of mycophenolate mofetil
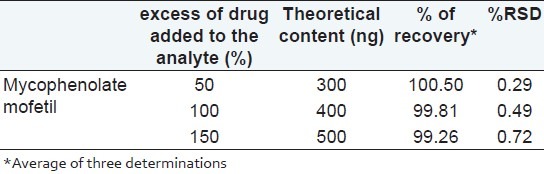
Table 2.
Method validation parameters of mycophenolate mofetil
CONCLUSION
The developed HPTLC technique is precise, specific, and accurate. Statistical analysis proves that the method is suitable for the analysis of mycophenolate mofetil as bulk drug and as formulation without interference from its excipients. It may be extended for the quantitative estimation of the said drug in plasma and other biological fluids.
ACKNOWLEDGMENT
The authors wish to thank Intas Pharmaceuticals Ltd., Ahmedabad, for providing the gift sample of mycophenolate mofetil. They are also grateful to the management of the Hindu College of Pharmacy for providing the necessary facilities to carry out this project.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.The Merck Index. 13th ed. White House Station, NJ: Merck Research Laboratories; 2001. [Google Scholar]
- 2.Na-Bangchang K, Supasyndh O, Supaporn T, Banmairuroi V, Karbwang J. Simple and sensitive high-performance liquid chromatographic. J Chromatogr B Biomed Sci Appl. 2000;738:169–73. doi: 10.1016/s0378-4347(99)00487-9. [DOI] [PubMed] [Google Scholar]
- 3.Hosotsubo H, Takahara S, Kokado Y, Permpongkosol S, Wang JD, Tanaka T, et al. Rapid and simple determination of mycophenolic acid in human plasma by RP-HPLC with fluorescence detection. J Pharm Biomed Anal. 2001;24:555–60. doi: 10.1016/s0731-7085(00)00442-8. [DOI] [PubMed] [Google Scholar]
- 4.Teshima D, Kitagawa N, Otsubo K, Makino K, Itoh Y, Oishi R. Simple determination of mycophenolic acid in human serum by column-switching high-performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;780:21–6. doi: 10.1016/s1570-0232(02)00410-5. [DOI] [PubMed] [Google Scholar]
- 5.Srivatsan V, Dasgupta AK, Kale P, Verma R, Joshi P, Soni D, et al. Determination of mycophenolic acid human plasma by high-performance liquid chromatography. J Chromatogr A. 2004;1031:259–64. doi: 10.1016/j.chroma.2003.08.073. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn J, Prante C, Kleesiek K, Götting C. Measurement of mycophenolic acid and its glucuronide using a novel rapid liquid chromatography-electrospray ionization tandem mass spectrometry assay. Clin Biochem. 2009;42:83–90. doi: 10.1016/j.clinbiochem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Plätzer M, Jahn K, Wohlrab J, Neubert RH. Quantification of mycophenolate mofetil in human skin extracts using high performance liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;755:355–9. doi: 10.1016/s0378-4347(01)00064-0. [DOI] [PubMed] [Google Scholar]
- 8.Shen B, Li S, Zhang Y, Yuan X, Fan Y, Liu Z, et al. Determination of total, free and saliva mycophenolic acid with LC-MS/MS method: Application to pharmacokinetic study in healthy volunteers and renal transplant patients. J Pharm Biomed Anal. 2009;50:515–21. doi: 10.1016/j.jpba.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Benech H, Hascoet S, Furlan V, Pruvost A, Durrbach A. Devolopment and validation of an LC-MS/MS assay for mycophenolic acid in human peripheral blood mononuclear cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;853:168–74. doi: 10.1016/j.jchromb.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Zhong Y, Jiao Z, Yu Y. Simultaneous determination of mycophenolic acid and valproic acid based on derivatization by high-performance liquid chromatography with fluorescence detection. Biomed Chromatogr. 2006;20:319–26. doi: 10.1002/bmc.566. [DOI] [PubMed] [Google Scholar]
- 11.International federation of Pharmaceutical Manufacturers and Associations (IFPMA), “Good manufacturing practice guide for active pharmaceutical ingredients,” in proceedings of the International conference on Harmonization, (ICH’05), Methodology Q7A. Geneva, Switzerland: 2005. [Google Scholar]


