Abstract
Aim
Survival after cardiac arrest (CA) is limited by the profound neurologic insult from ischemia–reperfusion injury. Therapeutic options are limited. Previous data suggest a benefit of coenzyme Q10 (CoQ10) in post-arrest patients. We hypothesized that plasma CoQ10 levels would be low after CA and associated with poorer outcomes.
Methods
Prospective observational study of post-arrest patients presenting to a tertiary care center. CoQ10 levels were drawn 24 h after return of spontaneous circulation (ROSC) and compared to healthy controls. Levels of inflammatory cytokines and biomarkers were analyzed. Primary endpoints were survival to discharge and neurologic status at time of discharge.
Results
23 CA subjects and 16 healthy controls were enrolled. CoQ10 levels in CA patients (0.28 µmol L−1, inter-quartile range (IQR): 0.22–0.39) were significantly lower than in controls (0.75 µmol L−1, IQR: 0.61–1.08, p < 0.0001). The mean CoQ10 level in CA patients who died was significantly lower than in those who survived (0.27 vs 0.47 µmol L−1, p = 0.007). There was a significant difference in median CoQ10 level between patients with a good vs poor neurological outcome (0.49 µmol L−1, IQR: 0.30–0.67 vs 0.27 µmol L−1, IQR: 0.21–0.30, p = 0.02). CoQ10 was a statistically significant predictor of poor neurologic outcome (adjusted p = 0.02) and in-hospital mortality (adjusted p = 0.026).
Conclusion
CoQ10 levels are low in human subjects with ROSC after cardiac arrest as compared to healthy controls. CoQ10 levels were lower in those who died, as well as in those with a poor neurologic outcome.
Keywords: Cardiac arrest, Coenzyme Q10, Cytokines, Biomarkers, Inflammatory cascade
1. Introduction
Cardiac arrest (CA) occurs in nearly 350,000 patients in the United States each year with an estimated mortality of 60% in those surviving the initial arrest.1 Moreover, the overall prognosis for survivors is often limited by neurologic injury.2 Two randomized control trials have demonstrated that therapeutic hypothermia (TH) after CA improves survival and reduces neurologic morbidity.3,4 As a result of these studies, TH has become the standard of care in post-CA patients. While the mechanism of action for TH is unknown, one potential target following ischemia–reperfusion injury is mitochondrial function in the injured cell and attenuation of potentially damaging oxygen-free radicals. Specifically, optimizing mitochondrial function and reducing oxygen free radicals may enhance cellular function and mitigate cellular injury thereby leading to improved outcome.5
Coenzyme Q10 (CoQ10) is an essential mitochondrial co-factor and free radical scavenger that has been identified as possibly beneficial in various neurodegenerative disorders such as Parkinson’s and Huntington’s diseases.6–10 Whether CoQ10 can provide neuroprotection in acute ischemia–reperfusion remains less clear, but it has been recognized by the American Heart Association as a potentially promising neuroprotective agent.11 CoQ10, or ubiquinone, is located within the inner mitochondrial membrane and acts as an electron transport mediator from complex I or complex II to complex III.12 While a small portion of CoQ10 may be obtained from dietary sources (including food and oral supplementation), CoQ10 is primarily synthesized endogenously in the endoplasmic reticulum from tyrosine and mevalonate and vitamins B2, B9, B12 and C and is transported in the plasma on low-density lipoprotein (LDL).13 Lower CoQ10 levels may result from impairment in CoQ10 synthesis or increased requirement (such as during conditions that increase oxidative stress), or any combination of these factors.14
We recently reported CoQ10 levels to be low in patients suffering from septic shock and inversely associated with inflammatory biomarkers such that patients with lower CoQ10 levels had higher levels of inflammatory biomarkers.15 The mechanism for lower plasma CoQ10 levels in septic shock patients remains unknown but we proposed that this occurred from a reduction of the carrier, LDL cholesterol. Whether CoQ10 levels are low in post-cardiac arrest patients remains unknown. One previous investigation suggested that provision of CoQ10 might be neuroprotective in this population when combined with mild therapeutic hypothermia but the mechanism remains unclear.16 A potential link is that post-arrest patients may have depleted CoQ10 levels with concomitant increase of oxygen free radicals, which are known to be injurious to neurons in many disease states. We hypothesized that, as in sepsis, patient post-cardiac arrest would have decreased CoQ10 levels. We further hypothesized that lower levels would correlate with clinical manifestations, particularly death or worse neurological outcome, as well as increased inflammatory cytokines and vascular endothelial biomarkers. In order to test these hypotheses, we performed a prospective observational study of post-cardiac arrest patients.
2. Methods
2.1. Study design
This is a prospective observational study of post-cardiac arrest patients presenting to an urban tertiary care center. All patients presenting to the hospital’s emergency department or intensive care units following an out of hospital cardiac arrest (OHCA) with subsequent return of spontaneous circulation were approached for enrollment in the study. The study was approved by the Institutional Review Board (IRB) at Beth Israel Deaconess Medical Center.
2.2. Study setting and population
All patients who presented with OHCA were screened for enrollment. Patients were screened by trained research assistants via the hospital’s electronic medical record system. Inclusion criteria for the initial trial consisted of adult patients (age ≥ 18 years) who had experienced an OHCA with subsequent return of spontaneous circulation (ROSC) and were comatose at the time of enrollment. Exclusion criteria consisted of pregnancy, traumatic cardiac arrest, and patients who were already comatose pre-arrest. Upon screening for enrollment, an eligible patient’s surrogate was approached for informed consent.
2.3. Data collection
Pertinent patient demographics including age, sex, race, past medical history, and events leading up to the arrest were recorded at the time of enrollment. Specific data collected regarding the arrest event included the location of the arrest, total low-flow and no-flow times, whether or not the arrest was witnessed, and intra-arrest interventions performed. Vital signs and laboratory data, including complete blood count, electrolytes, arterial blood gas measurements and lactate were recorded at the time of enrollment and at serial time intervals. Outcome measures included in-hospital mortality, as well as neurologic function at the time of hospital discharge as described by the dichotomized Cerebral Performance Category (CPC) score, where CPC score of 1–2 indicates a good neurologic outcome and CPC score of 3–5 indicates a poor neurologic outcome.3,4
2.4. Primary data analysis
2.4.1. Plasma coenzyme Q10 measurement
Blood was collected at the 24 h post-ROSC time point into evacuated tubes containing ethylenediaminetetraacetic acid from an existing venous or arterial catheter. The blood was immediately centrifuged at 2800 × g at 4 °C for 10 min; 0.5 mL of plasma was then transferred into cryogenic vials and stored at −80 °C immediately after centrifugation.
Concentration of CoQ10 was measured using high performance liquid chromatography (HPLC) with electrochemical detection system. Briefly, 50 µL of thawed plasma was mixed in an Eppendorf tube with 50 µL of ethanol solution containing 25 ng CoQ9 (used as internal standard). After the addition of 900 µL of 1-propanol, the tube was vortex mixed for 2 min and then centrifuged at high speed in the cold room for 10 min. An aliquot (~300 µL) of the supernatant was filtered through a 0.2 µm filter column and transferred to an HPLC sample injection vial and an aliquot of 50 µL of the extract was injected into HPLC system equipped with a C18 reversed-phase column and an ESA Coulochem II electrochemical detector (ESA, Inc., Chelmsford, MA). We also measured plasma CoQ10 in a sample of 16 healthy control patients at our center, and found a median value of 0.75 µmol L−1 (IQR 0.61–1.08), which is similar to the established healthy control range established in the literature (1.04 ± 0.33 µmol L−1).
2.4.2. Inflammatory markers measurement
Plasma was collected and stored at −80 °C until thawed for assaying cytokines and soluble cardiovascular biomarkers. Analytes were measured by Luminex Mutiplex Analysis (BioRad, Hercules, CA) on 96-well multiplex plates (Millipore, Billerica, MA). Analytes measured were: IL-1ra, IL-2, IL-6, IL-8, IL-10, TNF-α, VEGF, soluble E-selectin, soluble VCAM, and soluble ICAM. For vascular biomarkers (E-selectin, VCAM, and ICAM) plasma samples were measured at either dilution factor 1:50 or 1:100. For cytokines and VEGF plasma samples were measured undiluted.
2.4.3. Statistical methods
Basic demographics and patient characteristics are reported using descriptive statistics. Continuous data are reported as medians with inter-quartile ranges (IQRs) and categorical data are reported as frequency with percentages. CoQ10 levels in cardiac arrest patients and healthy controls were compared using a Mann–Whitney U-test. Non-normal data were transformed with logarithmic function, and the relationship between CoQ10 and inflammatory and vascular endothelial biomarkers was assessed using either a Pearson or Spearman correlation based on the normality of the data, with corrections for multiple testing.17 Statistical analyses were performed using the software package Graphpad Prism v5.04 (Graphpad Software Inc., LaJolla, CA, USA). A p < 0.05 was considered significant for statistical tests of the data; when correcting for multiple testing for the secondary analysis of inflammatory cytokines and vascular endothelial biomarkers, a p < 0.008 (0.05/6) was considered significant.
3. Results
We evaluated a total of 23 post-cardiac arrest subjects and 16 healthy controls. The baseline characteristics of our cohort are shown in Table 1. Overall, the mean age of the population was 71 ± 19 years with 7/23 (30%) female. The overall in-hospital mortality was 61% and survival with good neurological function (CPC score 1–2) was 30%.
Table 1.
Baseline characteristics of the post-arrest cohort.
| Characteristic | Value |
|---|---|
| Number | 23 |
| Age – median (IQR) | 71 (19) |
| Female – no. (%) | 7 (30) |
| Past medical history – no. (%) | |
| Hypertension | 15 (65) |
| Coronary artery disease | 11 (48) |
| Myocardial infarction | 8 (35) |
| Congestive heart failure | 3 (13) |
| Chronic obstructive pulmonary disease | 5 (22) |
| Diabetes | 3 (13) |
| Rhythm – no. (%) | |
| Asystole | 1 (4) |
| PEA | 9 (39) |
| VF | 11 (47) |
| VT | 1 (4) |
| Unknown | 1 (4) |
| Witnessed – no. (%) | 16 (70) |
| Bystander CPR – no. (%) | 15 (65) |
| Median downtime – min (IQR) | 32 (21) |
| Median no flow interval –min (IQR) | 1 (5) |
| Median low flow interval – min (IQR) | 18 (17) |
| Vasopressor use – no. (%) | 15 (65) |
| Temperature – F (±SD) | 97.9 ± 1.99 |
| HR – bpm (±SD) | 91 ± 27 |
| Systolic BP – mmHg (±SD) | 124 ± 33 |
| Diastolic BP – mmHg (±SD) | 65 ± 20 |
| GCS – mean (±SD) | 4.3 ± 2.7 |
| Lactate – mean (±SD) | 6.1 ± 2.9 |
| Induced hypothermia – no. (%) | 14 (61) |
| APACHE II – mean (±SD) | 24 ± 8 |
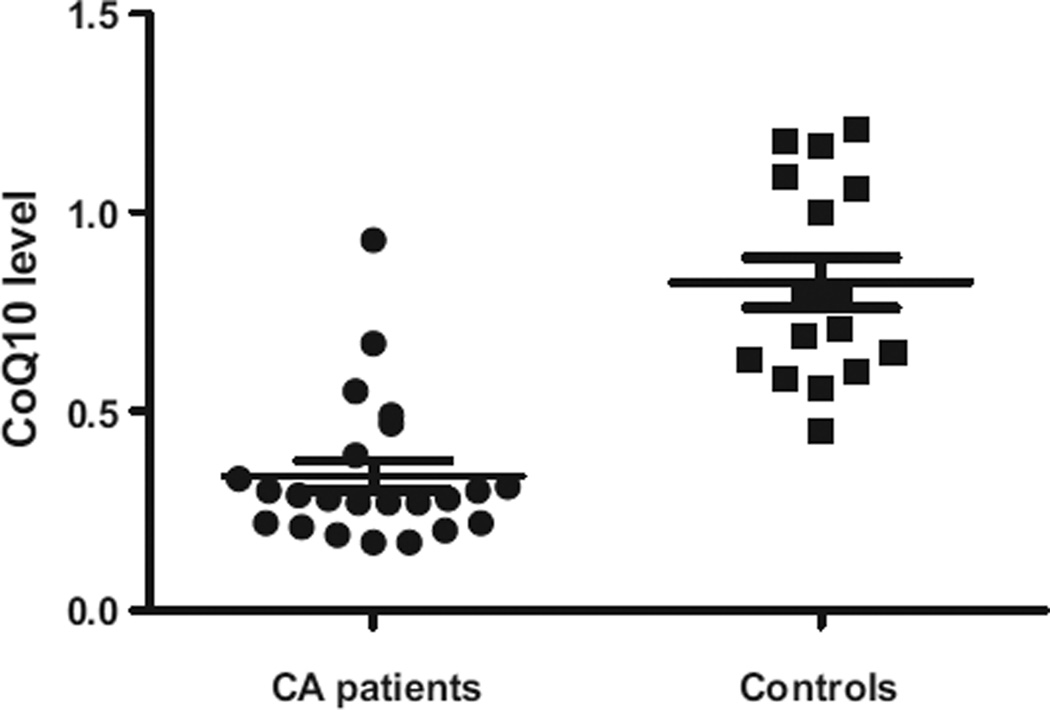
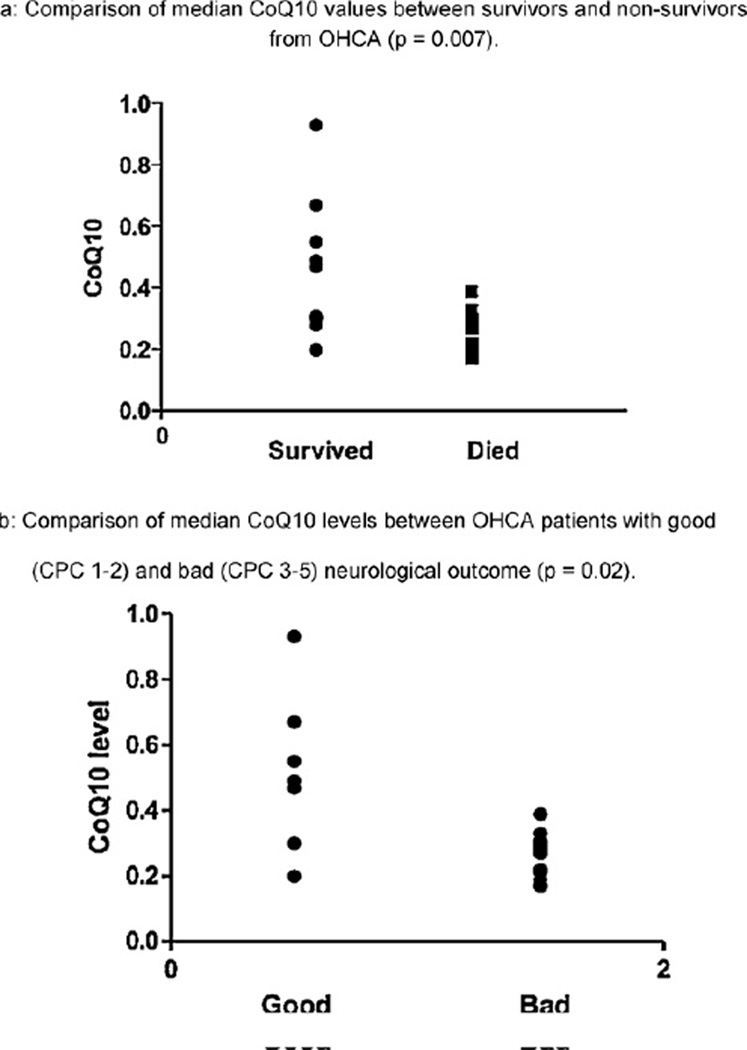
CoQ10 levels were statistically significantly lower in post-arrest patients than in healthy controls (median 0.28 µmol L−1 [IQR 0.22–0.39] vs 0.75 µmol L−1 [IQR 0.61–1.08], p < 0.0001; Fig. 1). Of note, our control values of CoQ10 were similar to previously established (literature-based) healthy control ranges, thus validating our methods.18 CoQ10 levels in cardiac arrest patients who died were statistically significantly lower than in those who survived to hospital discharge (0.27 µmol L−1 [IQR 0.21–0.29] vs 0.47 µmol L−1 [IQR 0.29–0.61], p = 0.007; Fig. 2a). There was a statistically significant difference in median CoQ10 level between patients with a good vs poor neurological outcome (0.49 µmol L−1 [IQR 0.30–0.67] vs 0.27 µmol L−1 [IQR 0.21–0.30], p = 0.02; Fig. 2b).
Fig. 1.
Comparison of mean CoQ10 levels between OHCA patients and healthy controls (p < 0.0001).
Fig. 2.
(a) Comparison of median CoQ10 values between survivors and non-survivors from OHCA (p = 0.007). (b) Comparison of median CoQ10 levels between OHCA patients with good (CPC 1–2) and bad (CPC 3–5) neurological outcome (p = 0.02).
The relationships between CoQ10 and inflammatory and vascular endothelial markers were as follows: IL-6 (r = −0.45; p = 0.03), IL-8 (r = −0.49; p = 0.02), VCAM (r = −0.24; p = 0.3), TNF-α (r = −0.14; p = 0.5), E-selectin (r = −0.3; p = 0.24), IL-1ra (r = −0.08; p = 0.7), or VEGF (r = 0.25; p = 0.25). With correction for multiple testing for the biomarkers analysis, none of these values remained statistically significant.
In univariate logistic regression models, CoQ10 was a significant predictor of poor neurologic outcome (p = 0.019) and in-hospital mortality (p = 0.025). Given that CoQ10 levels are thought to decrease with age,18,19 a multivariate logistic regression model controlling for age demonstrated that CoQ10 remained a statistically significant predictor of poor neurologic outcome (adjusted p = 0.02) and in-hospital mortality (adjusted p = 0.026).
Given the proposed mechanism of CoQ10 depletion relating to the carrier LDL, these levels were tested as well from the same samples in the post-arrest cohort. As often seen in critically ill patients, LDL levels were lower in our cohort than what is seen in the general population (mean 48 ± 22 mg/dL). The ratio of CoQ10 to LDL was calculated for each patient to determine the influence of LDL level on the CoQ10 level, and potentially on the outcomes. CoQ10:LDL ratio was not associated with mortality (p = 0.3). Serum LDL level was also not associated with mortality (p = 0.14), suggesting that CoQ10 levels correlate with mortality independent of the level of the carrier LDL. However, there does appear to be a moderate correlation between LDL levels and CoQ10 (r = 0.53; p = 0.017).
4. Discussion
Patients who suffer a cardiopulmonary arrest and achieve return of spontaneous circulation often suffer devastating neurologic injury which limits survival and meaningful recovery. Currently, the single most effective therapeutic intervention for patients who are comatose after cardiac arrest is mild induced hypothermia. Despite this intervention, many patients still succumb in the post-arrest period; often care is withdrawn due to profound neurologic injury, and novel therapies for the mitigation of neurologic injury after cardiac arrest are needed.
Coenzyme Q10, as an essential mitochondrial co-factor and free radical scavenger, may offer some insight into the pathophysiology of the post-arrest state, and may represent an area for possible intervention. To the best of our knowledge, this is the first report on the plasma levels of CoQ10 in human subjects after cardiac arrest. Previous human data have suggested a benefit of CoQ10 administration in a post-arrest cohort.16 Damian and colleagues performed a randomized placebo-controlled trial in which 49 comatose post-arrest patients were randomized to either therapeutic hypothermia (TH) and placebo or to TH and CoQ10. CoQ10 or placebo was administered to the study group via nasogastric tube. CoQ10 was given in an initial dose of 250 mg, then 150 mg three times daily for five days. The investigators found a significantly higher three month survival in the CoQ10 group compared with placebo (68% vs 29.2%). The mechanism of this apparent improved survival is unclear, and these findings have never been reproduced. In our study, we demonstrated that among OHCA patients admitted to the hospital in a comatose state, CoQ10 levels are extremely low early in the post-arrest period when compared to healthy adult controls. Furthermore, we found that CoQ10 levels are statistically significantly lower in patients who died or had a poor neurologic outcome, when compared to those who survived and had a more favorable neurologic outcome. In a multivariate logistic model, we found that CoQ10 was a significant predictor of poor neurologic outcome as well as in-hospital mortality, further evidence that low CoQ10 levels are associated with worse outcome.
Coenzyme Q10 is an essential component of the electron transport chain, acting as an electron transporter between mitochondrial complexes I, II and complex III. Disruption of this mechanism can compromise the oxidative capacity of the mitochondria and lead to a decreased level of energy production. Mitochondria are the primary organelle for cellular energy production and mitochondrial dysfunction can be detrimental in critical illness.20–22 The presence of mitochondrial dysfunction in the post-arrest period has been described previously, but the exact components and reasons for dysfunction are not completely known.23–25 The possibility that CoQ10 deficiency contributes to mitochondrial dysfunction remains a plausible hypothesis. Our findings that plasma CoQ10 levels are abnormally low in a post-cardiac arrest cohort compared to healthy adults supports a potential link that warrants further exploration.
The post-cardiac arrest syndrome, which includes neurologic injury as well as hemodynamic derangements, has been described as a “sepsis-like syndrome”.26 Previous studies have demonstrated that inflammatory cytokines and vascular endothelial markers are abnormally elevated in septic shock, indicating both inflammation and activation of the vascular endothelium. Within septic populations, elevation of these biomarkers has been associated with worse outcome.27–30 In our cohort of post-arrest patients, we found a suggestion of an inverse relationship between IL-6 and IL-8 and CoQ10 levels, although when accounting for multiple comparisons these associations did not remain significant; future investigations should assess these potential associations with a larger cohort of patients. In a previously published study on septic patients, we demonstrated a correlation between lower CoQ10 levels and higher levels of multiple inflammatory markers, including IL-6 and IL-8.15 This suggests that the post-cardiac arrest state may share similar features with the sepsis syndrome, and therapeutic interventions, which may be effective for one disease could ultimately prove to be effective for the other. In experimental models, pretreatment with CoQ10 before induction of sepsis restored hepatic ATP levels, suppressed markers of lipid peroxidation, and increased survivability.12 In a canine model of septic shock, Lelli et al. found that pretreatment with CoQ10 lead to improvements in cardiac output and mean arterial pressure though they did not find statistically significant differences in several biomarkers including TNF-α and IL-6. However, they had a very small sample size (ten dogs, five in each group) and they indicated that IL-6, for instance, had an approximate 3000% increase over baseline level (prior to injection with Escherichia coli) compared to an approximate 500% increase in the group that was administered CoQ10 in addition to E. coli. Although this did not reach statistical significance in such a small sample size, the large difference between the two groups suggests that a statistically significant relationship might have been noted with a larger sample.31 Thus, the improvements in cardiovascular function that these investigators found may be linked to modifications of vascular or inflammatory biomarkers. It is reasonable to hypothesize that similar findings could be seen in the post-cardiac arrest state, and further investigation is warranted.
The finding of abnormally low CoQ10 levels is consistent with the expected pathophysiology in the post-cardiac arrest state, given the similarities between post-cardiac arrest pathophysiology and that of sepsis.32 Since LDL is the primary transport molecule for CoQ10 in the plasma, we measured LDL levels and found that these levels were low, which is in agreement with reports in the literature on lipoprotein variations in sepsis and septic shock.33 The decrease in the carrier may lead to depletion of CoQ10, although whether this reflects decreased bioavailability of CoQ10 remains unknown, and further investigation is needed.
Regardless of the exact pathophysiologic mechanisms, identifying low CoQ10 levels in the post-cardiac arrest state is significant, as the compound is essential to mitochondrial function and may play an important role in the mitochondrial dysfunction seen during the ischemia–reperfusion injury that follows cardiac arrest. In contrast to previous investigations of mitochondrial dysfunction, our finding that CoQ10 is low in the early post-arrest period raises the possibility for a potential therapeutic intervention as CoQ10 can be administered exogenously and carries a very favorable risk profile.
The current study has limitations which must be considered when interpreting the data. First, the sample size for this study is small. Second, while these data are intriguing, the mechanism and significance of depletion of CoQ10 in this population remains unclear. Larger prospective observational trials measuring CoQ10 in a post-cardiac arrest population are needed.
5. Conclusion
Coenzyme Q10 levels are abnormally low in human subjects with return of spontaneous circulation after cardiac arrest as compared to healthy controls. The clinical significance of this abnormality remains unknown but should be explored given the essential role that CoQ10 plays in the electron transport chain and its potential role in mitochondrial dysfunction. Finding a means by which to mitigate neurologic injury after cardiac arrest is of critical importance, and the current study may provide important information about future directions for investigation.
Acknowledgments
The authors would like to thank Dr. Peter Weller for his generous assistance with multiplex analysis. The authors would also like to thank Francesca Montillo for her assistance in preparing this manuscript.
This project was funded (in part) by the Eleanor Shores Grant/Harvard Medical School, and supported by Grant Number UL1 RR025758 – Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Dr. Praveen Akuthota is supported by NIH F32AI081513. Dr. Catherine Buettner is supported by NIH K23AR055664. Dr. Michael W. Donnino is supported by the American Heart Association (0735533T). Dr. Michael N. Cocchi is supported by the American Heart Association (10CRP2640126).
Footnotes
A Spanish translated version of the abstract of this article appears as Appendix in the final online version at doi:10.1016/j.resuscitation.2012.03.023.
Conflict of interest statement
None of the authors declare any conflict of interests.
Authors’ contributions
MWD and MNC designed the study and were responsible for all aspects of this investigation. BG, JS and KB were responsible for primary data collection and substantial portions of the data interpretation and analysis. SG provided oversight of the statistical analysis. PA was responsible for vascular endothelial biomarker and inflammatory cytokine assays. AN was responsible for coenzyme Q10 assays and CB provided oversight on interpretation of coenzyme Q10 results. All authors were involved in writing of the manuscript and have approved the final version.
References
- 1.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.Young GB. Clinical practice. Neurologic prognosis after cardiac arrest. N Engl J Med. 2009;361:605–611. doi: 10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 4.Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 5.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann NY Acad Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 6.Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr. 2004;36:381–386. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- 7.Beal MF. Therapeutic effects of coenzyme Q10 in neurodegenerative diseases. Methods Enzymol. 2004;382:473–487. doi: 10.1016/S0076-6879(04)82026-3. [DOI] [PubMed] [Google Scholar]
- 8.A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology. 2001;57:397–404. doi: 10.1212/wnl.57.3.397. [DOI] [PubMed] [Google Scholar]
- 9.Muller T, Buttner T, Gholipour AF, Kuhn W. Coenzyme Q10 supplementation provides mild symptomatic benefit in patients with Parkinson’s disease. Neurosci Lett. 2003;341:201–204. doi: 10.1016/s0304-3940(03)00185-x. [DOI] [PubMed] [Google Scholar]
- 10.Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 11.Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 12.Dare AJ, Phillips AR, Hickey AJ, et al. A systematic review of experimental treatments for mitochondrial dysfunction in sepsis and multiple organ dysfunction syndrome. Free Radic Biol Med. 2009;47:1517–1525. doi: 10.1016/j.freeradbiomed.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Tomasetti M, Alleva R, Solenghi MD, Littarru GP. Distribution of antioxidants among blood components and lipoproteins: significance of lipids/CoQ10 ratio as a possible marker of increased risk for atherosclerosis. Biofactors. 1999;9:231–240. doi: 10.1002/biof.5520090218. [DOI] [PubMed] [Google Scholar]
- 14.Marcoff L, Thompson PD. The role of coenzyme Q10 in statin-associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49:2231–2237. doi: 10.1016/j.jacc.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 15.Donnino MW, Cocchi MN, Salciccioli JD, et al. Coenzyme Q10 levels are low and are associated with the inflammatory cascade in septic shock. Crit Care. 2011;15:R189. doi: 10.1186/cc10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damian MS, Ellenberg D, Gildemeister R, et al. CoEnzyme Q10 combined with mild hypothermia after cardiac arrest. Circulation. 2004;110:3011–3016. doi: 10.1161/01.CIR.0000146894.45533.C2. [DOI] [PubMed] [Google Scholar]
- 17.Curtin F, Schulz P. Multiple correlations and Bonferroni’s correction. Biol Psychiatry. 1998;44:775–777. doi: 10.1016/s0006-3223(98)00043-2. [DOI] [PubMed] [Google Scholar]
- 18.Miles MV, Horn PS, Tang PH, et al. Age-related changes in plasma coenzyme Q10 concentrations and redox state in apparently healthy children and adults. Clin Chim Acta. 2004;347:139–144. doi: 10.1016/j.cccn.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Ravaglia G, Forti P, Maioli F, et al. Coenzyme Q10 plasma levels and body composition in elderly males. Arch Gerontol Geriatr. 1996;22(Suppl. 1):539–543. doi: 10.1016/0167-4943(96)86996-2. [DOI] [PubMed] [Google Scholar]
- 20.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 21.Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin. 2001;17:219–237. doi: 10.1016/s0749-0704(05)70161-5. [DOI] [PubMed] [Google Scholar]
- 22.Harrois A, Huet O, Duranteau J. Alterations of mitochondrial function in sepsis and critical illness. Curr Opin Anaesthesiol. 2009;22:143–149. doi: 10.1097/ACO.0b013e328328d1cc. [DOI] [PubMed] [Google Scholar]
- 23.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Stroke Council. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 24.Radhakrishnan J, Wang S, Ayoub IM, Kolarova JD, Levine RF, Gazmuri RJ. Circulating levels of cytochrome c after resuscitation from cardiac arrest: a marker of mitochondrial injury and predictor of survival. Am J Physiol Heart Circ Physiol. 2007;292:H767–H775. doi: 10.1152/ajpheart.00468.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karanjia N, Geocadin RG. Post-cardiac arrest syndrome: update on brain injury management and prognostication. Curr Treat Options Neurol. 2011;13:191–203. doi: 10.1007/s11940-011-0112-2. [DOI] [PubMed] [Google Scholar]
- 26.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 27.Hack CE, De Groot ER, Felt-Bersma RJ, et al. Increased plasma levels of interleukin-6 in sepsis. Blood. 1989;74:1704–1710. [PubMed] [Google Scholar]
- 28.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 29.Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 30.Damas P, Ledoux D, Nys M, et al. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 1992;215:356–362. doi: 10.1097/00000658-199204000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lelli JL, Drongowski RA, Gastman B, Remick DG, Coran AG. Effects of coenzyme Q10 on the mediator cascade of sepsis. Circ Shock. 1993;39:178–187. [PubMed] [Google Scholar]
- 32.Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10:208–212. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- 33.Van Leeuwen HJ, Heezius ECJM, Dallinga GM, Van Strijp JAG, Verhoef J, Van Kessel KPM. Lipoprotein metabolism in patients with severe sepsis. Crit Care Med. 2003;31:1359. doi: 10.1097/01.CCM.0000059724.08290.51. [DOI] [PubMed] [Google Scholar]