Abstract
Birth weight has been inversely associated with later blood pressure. Firstborns tend to have lower birth weight than their later-born peers, but the long-term consequences remain unclear. The study objective was to investigate differences between firstborn and later-born individuals in early growth patterns, body composition, and blood pressure in Brazilian adolescents. The authors studied 453 adolescents aged 13.3 years from the prospective 1993 Pelotas Birth Cohort. Anthropometry, blood pressure, physical activity by accelerometry, and body composition by deuterium were measured. Firstborns (n = 143) had significantly lower birth weight than later borns (n = 310). At 4 years, firstborns had significantly greater weight and height, indicating a substantial overshoot in catch-up growth. In adolescence, firstborns had significantly greater height and blood pressure and a lower activity level. The difference in systolic blood pressure could be attributed to variability in early growth and that in diastolic blood pressure to reduced physical activity. The magnitude of increased blood pressure is clinically significant; hence, birth order is an important developmental predictor of cardiovascular risk in this population. Firstborns may be more sensitive to environmental factors that promote catch-up growth, and this information could potentially be used in nutritional management to prevent catch-up “overshoot.”
Keywords: birth order, blood pressure, body composition, growth, motor activity
Small size at birth has been associated in many studies with increased blood pressure in adolescence or adulthood (1). Initial research emphasized maternal and fetal malnutrition as a potential important mechanism (2), a hypothesis supported by experimental animal studies (3). However, associations with blood pressure hold across the whole range of birth weight, suggesting that a wide range of factors may be relevant.
Firstborn infants tend to have lower birth weight than later-born infants (4–7). In data from 3 Norwegian cities between 1860 and 1984, there was a substantial increase (approximately 200 g) in birth weight between the first and second pregnancies, followed by a much smaller increase (approximately 30 g) with each succeeding pregnancy (8). Physiologic studies have suggested possible anatomic explanations for the reduced birth weight of firstborns. During a mother’s first pregnancy, structural changes take place in the uterine spiral arteries, increasing blood flow with beneficial effects for fetal growth (9). These changes do not completely disappear following the pregnancy, such that subsequent offspring are from the start of pregnancy exposed to reduced vascular resistance and hence greater uterine blood flow compared with firstborns, promoting fetal growth (9).
The longer-term implications of these birth order associations remain unclear, in part because of inconsistent findings in previous studies and in part because of the possibility that both social and biologic mechanisms may be relevant. In some populations, firstborns remain shorter than later borns in adulthood (10), whereas other studies indicate that firstborns become significantly taller than later borns (11–13). Tanner (11) suggested that firstborn children may benefit from being the only child during their early life, resulting in improved nutrition and greater final size. Given the reduced birth weight of firstborns, this implies a tendency for infant catch-up growth to resolve early growth deficits.
Both low birth weight and rapid weight gain, particularly after the age of 2 years, are independent factors for cardiovascular risk (1, 14, 15), and recently we found that firstborns do indeed have elevated cardiovascular risk in Brazilian young adult men (13). We therefore investigated a second Brazilian birth cohort in order to determine in greater detail associations between birth order and subsequent phenotype. We investigated the association between birth order and 1) birth size, 2) postnatal growth rate, 3) adolescent body size, body composition, and physical activity level, and 4) adolescent blood pressure in the prospective 1993 Pelotas Birth Cohort Study. We tested the hypothesis that firstborns and later borns differ in each of these outcomes, and that these associations are independent of family size.
MATERIALS AND METHODS
Subjects
The 1993 Pelotas Birth Cohort recruited 5,249 individuals (16). Data on early growth at 6, 12, and 48 months were collected in a subsample of 1,272. For this study, we randomly selected 13% of those born in each calendar month of the year. This identified 655 individuals at 1 month of age, of whom 453 with full data at previous time points were successfully located and studied in adolescence. These individuals underwent measurements of body composition by deuterium dilution, physical activity by accelerometry, and blood pressure at 13.3 years. Birth order, based on the number of pregnancies, was obtained by maternal questionnaire at the time of recruitment. Ethics approval was obtained from the Federal University of Pelotas Medical School Ethics Committee.
Anthropometry
Birth weight and length were measured at the hospital by the research team. Weight and length or height at 6, 12, and 48 months were measured at the cohort participant’s household. At the 13.3-year visit, weight and height were again measured.
Body composition and pubertal stage
Body composition in adolescence was measured by using deuterium (17). Briefly, each adolescent was given a drink containing approximately 0.05 g of 99.9% deuterium oxide (2H2O) per kg of body weight. Saliva samples were obtained predose and 4 hours postdose by using absorbent salivettes at least 30 minutes after the last ingestion of food or drink and then stored frozen at −30°C, as described in detail elsewhere (18). The samples were shipped to the United Kingdom for analysis in duplicate with mass spectrometry, by use of the equilibration method (Delta plus XP; Thermofisher Scientific, Bremen, Germany) (19). For calculation of total body water, it was assumed that deuterium oxide dilution space overestimated total body water by a factor of 1.044 (17). Correction was made for dilution of the dose by water intake during the 4-hour equilibration period (17). Values for total body fluid were converted to lean mass (used here synonymously with fat-free mass), by using new reference data for the hydration of lean tissue (20). Fat mass was calculated as the difference of lean mass and weight. Both fat mass and lean mass were then adjusted for height to give the fat mass index and lean mass index, both expressed in the same kg/m2 units as body mass index (21, 22). Pubertal stage was assessed by Tanner staging, by use of line drawings. The 2 scores, each ranging from 1 to 5, were summed and the summed score analyzed.
Physical activity
Adolescent physical activity was assessed by using GT1M accelerometers (ActiGraph, Pensacola, Florida). The accelerometer was presented during the initial home interview and placed on the left side of the waist. In addition, an instruction sheet for the accelerometers including a diary was left at the participant’s home at the time of the interview. Participants were instructed to record if they did not wear the monitor for any period >1 hour during the day. Subjects wore the monitors from Wednesday to Monday and were encouraged to wear them 24 hours per day, except when showering, bathing, or swimming.
Blood pressure
Blood pressure was measured while the participant was seated, by using an HEM-629 digital portable wrist monitor (Omron Healthcare, Inc., Bannockburn, Illinois) in combination with a standard cuff size at the beginning and end of the interview (60 minutes apart). This monitor has been validated against a mercury sphygmomanometer in Brazilian adolescents (23). The mean value was used in analyses.
Statistics
Weight and height standard deviation (SD) scores for early growth data were calculated by using World Health Organization (WHO) reference curves. Preliminary analyses indicated differences between firstborns and later borns but not between secondborns and thirdborns, as was the case in a similar prior analysis (13). The analyses therefore compared firstborns with all later borns. Preliminary analyses also considered whether the results changed if mothers aged <18 years or those with a high birth order of ≥4 were excluded. These factors did not alter the findings significantly, and therefore no such exclusions were applied to the full analyses.
Crude differences between firstborn and later-born individuals were assessed by chi-square tests, independent-sample t tests, or Mann-Whitney tests. To take into account other variables, we used regression analysis with a succession of models. Following unadjusted analyses, model 1 adjusted for maternal factors (age, height, body mass index, educational level, family income), as well as offspring sex. Model 2 further adjusted for birth weight z score, weight and length SD scores at 4 years, and the physical activity level at 13.3 years. Conditional growth between birth and 4 years was calculated as recommended by Keijzer-Veen et al. (24), by calculating residuals for the regression of size at 48 months on size at birth for each of weight and length. As preliminary results showed firstborns to have higher maternal height than later borns, we considered the possible interaction between maternal height and birth order for predicting adolescent height, both with and without adjustment for birth size.
In order to separate potential birth order associations from those potentially arising from family size, we reran the analyses separately for firstborns who did or did not have a sibling at 4 years.
RESULTS
Table 1 shows that the subsample included in this analysis (n = 453) is comparable to the remainder of the cohort studied at 13.3 years (n = 5,249, including 1,843 firstborns) in terms of birth order, gender, family income, and maternal age. The subsample was significantly larger in body size at birth. However, this difference was relatively small and unlikely to indicate that our findings cannot be generalized to the whole cohort. Approximately one third (n = 143, 31.6%) of the subjects included in our analyses were firstborns, while 310 were later borns, and 52.3% of the sample were males. Of the firstborns, 47 had siblings by 4 years. Mean height at 13.3 years was 158 cm, mean body mass index was 20.3 kg/m2, mean systolic blood pressure was 111.0 mm Hg, and mean diastolic blood pressure was 68.6 mm Hg.
Table 1.
Description of the Sample in Terms of Birth Order, Sex, Growth Patterns in Early Life, Maternal Age, and Family Income Among Brazilian Firstborn and Later-born Adolescents in the 1993 Pelotas Birth Cohort
| Variable | Study Subset |
Full Cohort |
P Value | ||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | ||
| Categorical | |||||||
| Birth order | 0.10 | ||||||
| Firstborns | 143 | 31.6 | 1,843 | ||||
| Others | 310 | 68.4 | 3,406 | 64.9 | |||
| Sex | 0.24 | ||||||
| Male | 237 | 52.3 | 2,606 | 49.7 | |||
| Female | 216 | 47.7 | 2,642 | 50.3 | |||
| Total | 453 | 100.0 | 5,249 | 100.0 | 1.00 | ||
| Numerical | |||||||
| Maternal age, years | 26.3 (6.2) | 26.0 (6.4) | 0.32 | ||||
| Family income (minimum wages)a | 3.0 (4.0) | 4.3 (5.8) | 0.22 | ||||
| Birth weight z score | −0.18 (1.16) | −0.35 (1.28) | 0.01 | ||||
| Birth length z score | −0.36 (1.22) | −0.53 (1.35) | 0.01 | ||||
Abbreviation: SD, standard deviation.
1.0 Brazilian real = 0.63000 US dollar.
The average weight SD score was below the WHO reference value at birth (mean: −0.18, 95% confidence interval (CI): −0.29, −0.08), but subjects were able to catch up and reach an SD score of 0.31 (95% CI: 0.20, 0.42) at 4 years of age. In terms of the height SD score, individuals were born below the WHO reference (mean: −0.36, 95% CI: −0.47, −0.25) but moved toward the reference mean, reaching −0.12 (95% CI: −0.23, −0.01) at 4 years of age.
In Table 2, we present the distribution of the maternal variables and the child’s sex according to birth order. Subjects born to older mothers were less likely to be firstborns (P < 0.001). Also, the mean maternal age was 23.2 years among firstborns and 27.7 years among the others (P < 0.001). The likelihood of being a firstborn was not statistically associated with offspring sex, family income, or maternal height. However, the mothers of firstborns were 1.4 (95% CI: 0.1, 2.8) cm taller than mothers of later borns (P = 0.04), had completed on average 1.4 (95% CI: 0.7, 2.1) more years of education (P < 0.001), and had 0.8 (95% CI: −1.6, −0.1) kg/m2 lower body mass index (P = 0.03). Tertile analysis showed that the association between maternal height and offspring height appeared to vary by birth order. Compared with those in the lowest tertile for maternal height, those in the second and third tertiles had 5.4 (95% CI: 2.7, 8.5) and 8.1 (95% CI: 5.2, 11.1) cm, respectively, if the child was firstborn and 3.5 (95% CI: 1.4, 5.7) and 6.3 (95% CI: 4.3, 8.7) cm, respectively, if the child was a later born. These birth-order differences remained similar, although the absolute values were slightly reduced, if birth weight was held constant. However, the interaction between maternal height and birth order in relation to adolescent height was not statistically significant (P = 0.53).
Table 2.
Description of the Covariates (Sex, Maternal Age, Socioeconomic Status) According to Birth Order (Firstborns vs. Others) Among Brazilian Adolescents in the 1993 Pelotas Birth Cohort
| Variable | Firstborns |
Others |
P Value | ||
| % | Mean (SD) | % | Mean (SD) | ||
| Sex | 0.063a | ||||
| Male | 58.7 | 49.4 | |||
| Female | 41.3 | 50.7 | |||
| Maternal age, years | <0.001a | ||||
| <20 | 29.4 | 5.8 | |||
| 20–34 | 65.7 | 81.0 | |||
| ≥35 | 4.9 | 13.2 | |||
| Maternal age, years | 23.2 (5.9) | 27.7 (5.8) | <0.001b | ||
| Quartile of family income | 0.20a | ||||
| 1 (poorest) | 22.1 | 29.4 | |||
| 2 | 25.0 | 24.1 | |||
| 3 | 25.7 | 26.8 | |||
| 4 (wealthiest) | 27.1 | 19.8 | |||
| Family income, Brazilian realc | 4.0 (4.0) | 3.7 (4.1) | 0.10d | ||
| Quartile of maternal height | 0.24a | ||||
| 1 (shortest) | 24.1 | 30.3 | |||
| 2 | 27.7 | 28.3 | |||
| 3 | 24.1 | 24.8 | |||
| 4 (tallest) | 24.1 | 16.6 | |||
| Maternal height, cm | 161.0 (7.3) | 159.6 (6.5) | 0.04b | ||
| Maternal schooling, years | <0.001a | ||||
| 0–4 | 14.7 | 33.3 | |||
| 5–8 | 51.8 | 46.6 | |||
| ≥9 | 33.6 | 20.1 | |||
| Maternal schooling, years | 7.6 (3.3) | 6.2 (3.6) | <0.001d | ||
| Maternal BMI, kg/m2 | 0.275 | ||||
| <18.5 | 10.7 | 8.8 | |||
| 18.5–24.9 | 71.4 | 65.3 | |||
| 25.0–29.9 | 14.3 | 19.2 | |||
| ≥30.0 | 3.6 | 6.7 | |||
| Maternal BMI, kg/m2 | 22.2 (3.8) | 23.1 (3.9) | 0.034b | ||
Abbreviations: BMI, body mass index; SD, standard deviation.
Chi-square test.
t test.
1.0 Brazilian real = 0.63000 US dollar.
Mann-Whitney test.
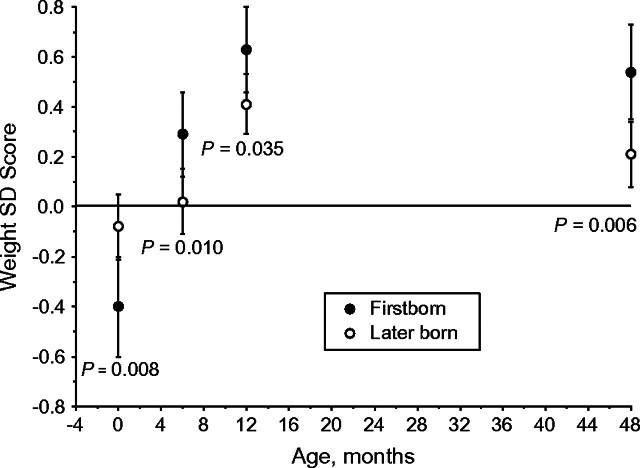
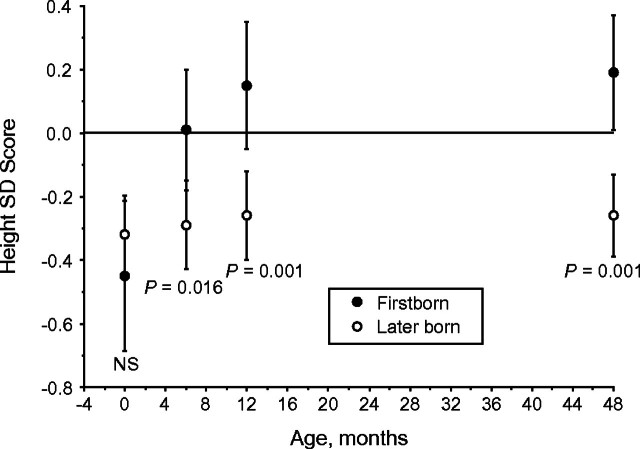
Firstborns were born significantly smaller than later borns (Δ in birth weight SD scores = −0.31, 95% CI: −0.54, −0.08) (P = 0.008) and presented significantly greater catch-up within the first year of life, particularly within the first 6 months (Figure 1). At 4 years of age, firstborns presented a mean weight SD score of 0.29 (95% CI: 0.05, 0.52) greater than later borns (P = 0.006). In terms of height (Figure 2), firstborns were born shorter than later borns, although not significantly so, but showed substantial catch-up growth within the first year of life, such that they were already significantly taller than later borns by 1 year of age, as well as above the reference mean. At 4 years of age, firstborns were 0.41 SD score (95% CI: 0.17, 0.64) taller than later borns (P < 0.001).
Figure 1.
Weight standard deviation (SD) score of the World Health Organization Child Growth Standards and 95% confidence interval at birth and at 6, 12, and 48 months in Brazilian firstborn and later-born adolescents in the 1993 Pelotas Birth Cohort.
Figure 2.
Height standard deviation (SD) score of the World Health Organization Child Growth Standards and 95% confidence interval at birth and at 6, 12, and 48 months in Brazilian firstborn and later-born adolescents in the 1993 Pelotas Birth Cohort. NS, nonsignificant.
Table 3 shows that, in the unadjusted analyses, firstborns remained taller at 13.3 years than later borns, as well as presenting significantly higher blood pressure values. No significant crude differences were observed in terms of body mass index, sum of skinfolds, physical activity, or body composition indicators. Firstborns were also significantly more advanced in terms of pubertal stage: firstborns: 7.8 (95% CI: 7.5, 8.1); later borns: 7.4 (95% CI: 7.2, 7.6) (P = 0.05).
Table 3.
Outcomes at 13.3 Years According to Birth Order Among Brazilian Adolescents in the 1993 Pelotas Birth Cohort
| Variable | Mean (SD) |
P Value | |
| Firstborns (n = 143) | Others (n = 310) | ||
| Height, cm | 159.6 (7.8) | 157.0 (8.5) | 0.002a |
| BMI, kg/m2 | 20.3 (3.3) | 20.3 (4.0) | 0.834a |
| Sum of skinfolds, mm | 25.4 (12.0) | 25.4 (13.7) | 0.998a |
| Accelerometry, 1,000 counts | 384 (142) | 401 (149) | 0.274b |
| Accelerometry, minutes/week MVPA | 485 (218) | 515 (218) | 0.132b |
| Systolic blood pressure, mm Hg | 113.4 (13.9) | 109.8 (14.0) | 0.011a |
| Diastolic blood pressure, mm Hg | 70.7 (12.0) | 67.7 (10.8) | 0.009a |
| Lean mass, kg | 39.3 (7.4) | 38.6 (7.0) | 0.307a |
| Fat mass, kg | 11.9 (7.1) | 11.8 (7.9) | 0.937a |
| Lean mass index, kg/m2 | 15.4 (1.9) | 15.6 (1.9) | 0.452a |
| Fat mass index, kg/m2 | 4.7 (2.7) | 4.8 (3.0) | 0.792a |
Abbreviations: BMI, body mass index; MVPA, moderate-to-vigorous physical activity; SD, standard deviation.
t test.
Mann-Whitney test.
After adjustment for maternal covariates and sex, the association of birth order with height and blood pressure persisted (Table 4). With further adjustment for birth weight z score, conditional weight and length at 4 years, and accelerometry counts, the associations between birth order and either height or blood pressure were diluted, and the confidence intervals included the null value. If only adjustment for conditional weight or length at 4 years were made, the increment in height for firstborns disappeared (Δ = 0 cm, 95% CI: −1.2, 1.2) (P = 0.99), whereas the difference in blood pressure remained significant (diastolic blood pressure: Δ = 1.9 mm Hg, 95% CI: 0.4, 3.4) (P = 0.01) or borderline significant (systolic blood pressure: Δ = 1.9 mm Hg, 95% CI: −0.3, 4.1) (P = 0.08). If birth weight SD scores were added to the model with size at 4 years, the birth order association with systolic blood pressure lost further significance although not magnitude (Δ = 1.9 mm Hg, 95% CI: −0.6, 4.3) (P = 0.14), whereas that for diastolic blood pressure remained significant (Δ = 1.9 mm Hg, 95% CI: 0.2, 3.5) (P = 0.03). Only in the final model 2 (Table 4), when physical activity was also included, did the birth order difference in diastolic blood pressure lose significance. There were no significant differences between birth order groups in lean mass or fat mass in crude or adjusted models. Our analyses therefore indicate that birth order affects blood pressure through the impact of early growth and physical activity, although differently so for the 2 blood pressure components.
Table 4.
Adjusted Analyses for Outcomes at 13.3 Years According to Birth Order Among Brazilian Adolescents in the 1993 Pelotas Birth Cohorta
| Variable | Unadjusted |
Model 1b
|
Model 2c
|
||||||
| Firstborns |
P Value | Firstborns |
P Value | Firstborns |
P Value | ||||
| Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI | ||||
| Height, cm | 2.6 | 1.0, 4.2 | <0.01 | 2.1 | 0.5, 3.8 | 0.01 | 0.4 | −0.9, 1.7 | 0.57 |
| BMI, kg/m2 | −0.1 | −0.8, 0.7 | 0.83 | 0.1 | −0.7, 0.9 | 0.80 | −0.2 | −0.9, 0.5 | 0.67 |
| Sum of skinfolds, mm | 0.0 | −2.6, 2.6 | 1.00 | 0.9 | −1.8, 3.7 | 0.51 | 0.2 | −2.7, 3.1 | 0.91 |
| Accelerometry, 1,000 counts | −17.3 | −48.8, 14.2 | 0.28 | −22.6 | −55.3, 10.2 | 0.18 | −16.9d | −47.6, 13.8 | 0.28 |
| Accelerometry, minutes/week MVPA | −30 | −77, 17 | 0.21 | −39 | −88, 10 | 0.12 | −38d | −85, 8 | 0.11 |
| Systolic blood pressure, mm Hg | 3.5 | 1.2, 5.7 | <0.01 | 2.5 | 0.4, 4.7 | 0.02 | 2.0 | −0.7, 4.7 | 0.14 |
| Diastolic blood pressure, mm Hg | 2.0 | 0.7, 3.4 | <0.01 | 2.1 | 0.7, 3.6 | 0.01 | 1.5 | −0.3, 3.2 | 0.10 |
| Lean mass, kg | 0.77 | −0.71, 2.26 | 0.31 | 0.10 | −1.43, 1.63 | 0.90 | −0.66 | −2.30, 0.97 | 0.43 |
| Fat mass, kg | 0.06 | −1.53, 1.66 | 0.94 | 0.57 | −1.07, 2.21 | 0.50 | −0.10 | −1.71, 1.50 | 0.90 |
Abbreviations: BMI, body mass index; CI, confidence interval; MVPA, moderate-to-vigorous physical activity.
Reference group: nonfirstborn.
Model 1: adjusted for confounders (maternal age, maternal height, maternal BMI, smoking during pregnancy, family income, and sex).
Model 2: adjusted for model 1 variables plus z score of birth weight, conditional weight and length at 48 months, and physical activity (counts) at 13.3 years.
Not adjusted for physical activity (counts) at 13.3 years.
When the analyses for height and blood pressure were repeated separately for firstborns with or without siblings at 4 years, the difference between firstborns and later borns was slightly reduced in those with siblings in terms of height (with sibling: Δ = 2.0 cm, 95% CI: −0.5, 4.6 (P = 0.12); no sibling: Δ = 2.6 cm, 95% CI: 0.7, 4.4 (P = 0.007)) and diastolic blood pressure (with sibling: Δ = 2.5 mm Hg, 95% CI: −1.0, 6.0 (P = 0.16); no sibling: Δ = 3.6 mm Hg, 95% CI: 1.0, 6.1 (P = 0.006)) but much reduced for systolic blood pressure (with sibling: Δ = 0.7 mm Hg, 95% CI: −1.5, 2.8 (P = 0.54); no sibling: Δ = 2.3 mm Hg, 95% CI: 0.8, 3.0 (P = 0.003)). At 4 years, firstborns with no sibling were also taller (height SD score = 0.2, 95% CI: 0.0, 0.4) than those with siblings (height SD score = 0.1, 95% CI: −0.3, 0.4), although the difference was not significant. However, none of the interactions (between birth order and sibling presence in relation to height, systolic blood pressure, or diastolic blood pressure) was significant.
DISCUSSION
The smaller birth size in firstborns shown here has been observed in not only humans but also other mammals such as mice (25), sheep (26), and seals (27). However, this early growth deficit was more than compensated for such that, by 6 months, the firstborns had become heavier and taller than later borns, with a height difference of 2.6 cm persisting at 13.3 years. In turn, these early slow-fast growth patterns were associated with greater blood pressure in firstborns, but not with differences in body mass index or fatness.
Our analyses indicate that these birth order associations are not due to confounding by maternal factors or the sex of the offspring. The mothers of firstborns were 1.5 cm taller. Hence, it is possible that minor differences in growth potential might have become magnified during the window for catch-up growth. Nevertheless, birth order differences in height and blood pressure remained at 2–3 mm Hg, after adjustment for sex and maternal height. In general, differences of such magnitude are clinically important (28).
With further adjustment for early growth patterns and physical activity, the associations of birth order with height practically disappeared. This is not surprising as the literature strongly suggests that adolescent and adult height deficits are largely explained by growth patterns in early childhood (29). Associations between birth order and blood pressure were reduced by about 0.5 mm Hg after such adjustment, and the confidence interval of these differences included the null value. This indicates that these factors partly contribute to the mechanisms whereby birth order is associated with later blood pressure. Early growth appeared more important for systolic blood pressure, and activity patterns seemed more important for diastolic blood pressure. Further work is required to understand these contrasting findings in more detail. We previously observed reduced activity levels in firstborns in this cohort at 11 years and suggested that the number of siblings might explain this association (30).
Several other studies have shown firstborns to have a tendency to overcompensate in catch-up growth and to become taller by adolescence and adulthood (11–13). Whether the smaller birth size of small-for-gestational-age infants tracks into later life is strongly dependent on the magnitude of early catch-up during the first 6–12 months (31), in other words, a “critical window.” This might account for the findings of Hermanussen et al. (10), who found that firstborns remained shorter than their peers in adulthood. The opportunity for catch-up may have been diminished in this population studied in the aftermath of World War II, causing early deficits to track into adulthood.
Elsewhere, early catch-up growth has been associated with increased adiposity in firstborns. Firstborns had greater central adiposity by 5 years in the Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) cohort (7) and increased weight and body mass index in teenage girls from Poland (32). Birth order enhanced associations of socioeconomic status with central adiposity in young adult males from the Philippines (33). In adults, increased total and central adiposity was observed in firstborn Bengali women (12) and Brazilian men (13). In our study, however, birth order differences in adiposity were not apparent. This is consistent with several recent studies in developing countries, where infant catch-up appears beneficial for later height and lean mass but much weaker in association with adiposity (34, 35), whereas in industrialized populations, rapid growth is associated with later adiposity (36, 37) and obesity (38, 39). Such differences may also be due to variation in the duration of catch-up growth.
Other studies have suggested family size associations with growth. Among Da-an boys of Taiwan, boys without sisters were ∼2.5 cm taller and ∼3.5 kg heavier than those with 1 or 2 sisters (40). A study of Cairo children showed that boys in smaller family sizes were taller and heavier than those of larger family sizes, but with no specific birth-order association (41). In the ALSPAC cohort, family size was negatively related to height, so that compared with children lacking siblings, those with 4 siblings had 0.9 cm lower birth length and 3.1 cm reduced height at age 10 years (42). In our study, firstborns without a sibling achieved greater height by 4 years and had the highest values for height and blood pressure at 13.3 years. However, these differences were not significant, and the association with family size appears much less important than that with early growth patterns for explaining the birth-order difference in height and systolic blood pressure.
The strengths of the study include the prospective nature of the early growth data, the relatively large sample size, and the objective measurements of body composition and physical activity level. The main limitations comprise the definition of birth order according to previous pregnancies and the lack of information about siblings. However, our definition of “firstborn” would tend to a conservative estimate of any differences, because some individuals may have been classified as secondborn despite the previous pregnancy’s not persisting long enough to affect uterine physiology. A second limitation is that we did not adjust our study for multiple comparisons. However, we believe this approach is acceptable, as previously published studies have reported associations of birth order with all the outcomes we considered here.
Our findings are important in understanding the pathways whereby early growth patterns are associated with later degenerative disease. There is little reason to assume that maternal nutritional status itself varies substantially across successive pregnancies in this population, where undernutrition is practically nonexistent. Many components of maternal phenotype (e.g., height, uterine volume) are relatively consistent across the reproductive career and reflect early maternal growth and development. Other components of phenotype may change modestly across pregnancies, such as body weight and adipose tissue distribution (43, 44). The primary shift in fetal growth pattern occurs between the first and second pregnancies (8) and appears attributable to changes in uterine vascular function (9) rather than to drastic changes in maternal nutritional status. The reduced birth size of firstborns therefore appears due to reduced access to maternal resources rather than the inadequacy of those resources.
Our findings therefore do not support the predictive adaptive response hypothesis (45), which attributes associations between early growth and later metabolic phenotype to proactive “anticipation” of future breeding conditions. It is difficult to see how or why firstborns should generate different predictions of future environmental conditions than their later-born peers, given a common maternal phenotype. Instead, our findings are consistent with the maternal capital hypothesis (46), and they indicate that firstborns have reduced access to the maternal nutritional supply during pregnancy, resulting in their smaller size at birth. Firstborns can undergo rapid compensatory growth during infancy but at a cost of long-term associations with blood pressure. The greater sensitivity of firstborns to factors promoting catch-up could be addressed in nutritional management to avoid catch-up “overshoot.”
Acknowledgments
Author affiliations: Childhood Nutrition Research Centre, Institute of Child Health, University College London, London, United Kingdom (Jonathan C. K. Wells); and Postgraduate Program in Epidemiology, Federal University of Pelotas, Pelotas, Brazil (Pedro C. Hallal, Felipe F. Reichert, Samuel C. Dumith, Ana M. Menezes, Cesar G. Victora).
Funding for this study was contributed by both the Wellcome Trust and the Medical Research Council.
Conflict of interest: none declared.
Glossary
Abbreviations
- ALSPAC
Avon Longitudinal Study of Pregnancy and Childhood
- CI
confidence interval
- SD
standard deviation
- WHO
World Health Organization
References
- 1.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18(7):815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey KM, Forrester T, Barker DJ, et al. Maternal nutritional status in pregnancy and blood pressure in childhood. Br J Obstet Gynaecol. 1994;101(5):398–403. doi: 10.1111/j.1471-0528.1994.tb11911.x. [DOI] [PubMed] [Google Scholar]
- 3.Kawamura M, Itoh H, Yura S, et al. Undernutrition in utero augments systolic blood pressure and cardiac remodeling in adult mouse offspring: possible involvement of local cardiac angiotensin system in developmental origins of cardiovascular disease. Endocrinology. 2007;148(3):1218–1225. doi: 10.1210/en.2006-0706. [DOI] [PubMed] [Google Scholar]
- 4.Karn MN, Penrose LS. Birth weight and gestation time in relation to maternal age, parity and infant survival. Ann Eugen. 1951;16(2):147–164. [PubMed] [Google Scholar]
- 5.Roberts DF, Tanner RE. Effects of parity on birth weight and other variables in a Tanganyika Bantu sample. Br J Prev Soc Med. 1963;17(4):209–215. doi: 10.1136/jech.17.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcox MA, Chang AM, Johnson IR. The effects of parity on birthweight using successive pregnancies. Acta Obstet Gynecol Scand. 1996;75(5):459–463. doi: 10.3109/00016349609033354. [DOI] [PubMed] [Google Scholar]
- 7.Ong KK, Ahmed ML, Emmett PM, et al. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg M. Birth weights in three Norwegian cities, 1860–1984. Secular trends and influencing factors. Ann Hum Biol. 1988;15(4):275–288. doi: 10.1080/03014468800009751. [DOI] [PubMed] [Google Scholar]
- 9.Khong TY, Adema ED, Erwich JJ. On an anatomical basis for the increase in birth weight in second and subsequent born children. Placenta. 2003;24(4):348–353. doi: 10.1053/plac.2002.0922. [DOI] [PubMed] [Google Scholar]
- 10.Hermanussen M, Hermanussen B, Burmeister J. The association between birth order and adult stature. Ann Hum Biol. 1988;15(2):161–165. doi: 10.1080/03014468800009581. [DOI] [PubMed] [Google Scholar]
- 11.Tanner JM. Foetus into Man—Physical Growth From Conception to Maturity. Ware, United Kingdom: Castlemead; 1989. [Google Scholar]
- 12.Ghosh JR, Bandyopadhyay AR. Income, birth order, siblings, and anthropometry. Hum Biol. 2006;78(6):733–741. doi: 10.1353/hub.2007.0012. [DOI] [PubMed] [Google Scholar]
- 13.Siervo M, Horta BL, Stephan BC, et al. First-borns carry a higher metabolic risk in early adulthood: evidence from a prospective cohort study [electronic article] PLoS One. 2010;5(11):e13907. doi: 10.1371/journal.pone.0013907. (doi:10.1371/journal.pone.0013907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker DJ. Fetal and Infant Origins of Adult Disease. London, United Kingdom: BMJ Publishing Group; 1992. [Google Scholar]
- 15.Dulloo AG. Thrifty energy metabolism in catch-up growth trajectories to insulin and leptin resistance. Best Pract Res Clin Endocrinol Metab. 2008;22(1):155–171. doi: 10.1016/j.beem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Victora CG, Hallal PC, Araújo CL, et al. Cohort profile: the 1993 Pelotas (Brazil) Birth Cohort Study. Int J Epidemiol. 2008;37(4):704–709. doi: 10.1093/ije/dym177. [DOI] [PubMed] [Google Scholar]
- 17.Wells JC, Fuller NJ, Dewit O, et al. Four-component model of body composition in children: density and hydration of fat-free mass and comparison with simpler models. Am J Clin Nutr. 1999;69(5):904–912. doi: 10.1093/ajcn/69.5.904. [DOI] [PubMed] [Google Scholar]
- 18.Reichert FF, Menezes AM, Kingdom Wells JC, et al. A methodological model for collecting high-quality data on physical activity in developing settings—the experience of the 1993 Pelotas (Brazil) Birth Cohort Study. J Phys Act Health. 2009;6(3):360–366. doi: 10.1123/jpah.6.3.360. [DOI] [PubMed] [Google Scholar]
- 19.Chomtho S, Fewtrell MS, Jaffe A, et al. Evaluation of arm anthropometry for assessing pediatric body composition: evidence from healthy and sick children. Pediatr Res. 2006;59(6):860–865. doi: 10.1203/01.pdr.0000219395.83159.91. [DOI] [PubMed] [Google Scholar]
- 20.Wells JC, Williams JE, Chomtho S, et al. Pediatric reference data for lean tissue properties: density and hydration from age 5 to 20 y. Am J Clin Nutr. 2010;91(3):610–618. doi: 10.3945/ajcn.2009.28428. [DOI] [PubMed] [Google Scholar]
- 21.VanItallie TB, Yang MU, Heymsfield SB, et al. Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr. 1990;52(6):953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 22.Wells JC. A critique of the expression of paediatric body composition data. Arch Dis Child. 2001;85(1):67–72. doi: 10.1136/adc.85.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menezes AM, Dumith SC, Noal RB, et al. Validity of a wrist digital monitor for blood pressure measurement in comparison to a mercury sphygmomanometer. Arq Bras Cardiol. 2010;94(3):345–349. 365–370. [PubMed] [Google Scholar]
- 24.Keijzer-Veen MG, Euser AM, van Montfoort N, et al. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol. 2005;58(12):1320–1324. doi: 10.1016/j.jclinepi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Reading AJ. Effects of parity and litter size on the birth weight of inbred mice. J Mammal. 1966;47(1):111–114. [PubMed] [Google Scholar]
- 26.Hyatt MA, Keisler DH, Budge H, et al. Maternal parity and its effect on adipose tissue deposition and endocrine sensitivity in the postnatal sheep. J Endocrinol. 2010;204(2):173–179. doi: 10.1677/JOE-09-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowen WD, Oftedal OT, Boness DJ, et al. The effect of maternal age and other factors on birth mass in the Harbor seal. Can J Zool. 1994;72(1):8–14. [Google Scholar]
- 28.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Prospective Studies Collaboration. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 29.Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Maternal and Child Undernutrition Study Group. Lancet. 2008;371(9609):340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hallal PC, Victora CG, Wells JC, et al. Physical inactivity: prevalence and associated variables in Brazilian adults. Med Sci Sports Exerc. 2003;35(11):1894–1900. doi: 10.1249/01.MSS.0000093615.33774.0E. [DOI] [PubMed] [Google Scholar]
- 31.Ong KK. Catch-up growth in small for gestational age babies: good or bad? Curr Opin Endocrinol Diabetes Obes. 2007;14(1):30–34. doi: 10.1097/MED.0b013e328013da6c. [DOI] [PubMed] [Google Scholar]
- 32.Koziel S, Kolodziej H. Birth order and BMI in teenage girls. Coll Antropol. 2001;25(2):555–560. [PubMed] [Google Scholar]
- 33.Dahly DL, Adair LS. Does lower birth order amplify the association between high socioeconomic status and central adiposity in young adult Filipino males? Int J Obes (Lond) 2010;34(4):751–759. doi: 10.1038/ijo.2009.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Stein AD, Barnhart HX, et al. Associations between prenatal and postnatal growth and adult body size and composition. Am J Clin Nutr. 2003;77(6):1498–1505. doi: 10.1093/ajcn/77.6.1498. [DOI] [PubMed] [Google Scholar]
- 35.Sachdev HS, Fall CH, Osmond C, et al. Anthropometric indicators of body composition in young adults: relation to size at birth and serial measurements of body mass index in childhood in the New Delhi birth cohort. Am J Clin Nutr. 2005;82(2):456–466. doi: 10.1093/ajcn.82.2.456. [DOI] [PubMed] [Google Scholar]
- 36.Chomtho S, Wells JC, Williams JE, et al. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;87(6):1776–1784. doi: 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 37.Ekelund U, Ong K, Linné Y, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83(2):324–330. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 38.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95(8):904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 39.Stettler N, Kumanyika SK, Katz SH, et al. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr. 2003;77(6):1374–1378. doi: 10.1093/ajcn/77.6.1374. [DOI] [PubMed] [Google Scholar]
- 40.Floyd B. Heights and weights of Da-an boys: did sisters really make a difference? J Biosoc Sci. 2005;37(3):287–300. doi: 10.1017/s0021932004006674. [DOI] [PubMed] [Google Scholar]
- 41.el-Nofely A. The influence of some socio-demographic factors on body weight and stature of 6-18 years old boys from Cairo, Egypt. Anthropol Anz. 1985;43(3):245–256. [PubMed] [Google Scholar]
- 42.Lawson DW, Mace R. Sibling configuration and childhood growth in contemporary British families. Int J Epidemiol. 2008;37(6):1408–1421. doi: 10.1093/ije/dyn116. [DOI] [PubMed] [Google Scholar]
- 43.Lassek WD, Gaulin SJ. Changes in body fat distribution in relation to parity in American women: a covert form of maternal depletion. Am J Phys Anthropol. 2006;131(2):295–302. doi: 10.1002/ajpa.20394. [DOI] [PubMed] [Google Scholar]
- 44.Wells JC, Griffin L, Treleaven P. Independent changes in female body shape with parity and age: a life-history approach to female adiposity. Am J Hum Biol. 2010;22(4):456–462. doi: 10.1002/ajhb.21017. [DOI] [PubMed] [Google Scholar]
- 45.Gluckman PD, Hanson MA, Spencer HG. Predictive adaptive responses and human evolution. Trends Ecol Evol. 2005;20(10):527–533. doi: 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Wells JC. Maternal capital and the metabolic ghetto: an evolutionary perspective on the transgenerational basis of health inequalities. Am J Hum Biol. 2010;22(1):1–17. doi: 10.1002/ajhb.20994. [DOI] [PubMed] [Google Scholar]