Abstract
Objective
Apolipoprotein (apo) A-V is a low abundance protein with a profound influence on plasma triacylglycerol levels. In human populations, single nucleotide polymorphisms and mutations in APOA5 positively correlate with hypertriglyceridemia. As an approach to preventing the deleterious effects of chronic hypertriglyceridemia, apoA-V gene therapy has been pursued.
Methods and Results
Recombinant adeno-associated virus (AAV) 2/8 harboring the coding sequence for human apoA-V or a control AAV2/8 was transduced into hypertriglyceridemic apoa5 (−/−) mice. After injection of 1×1012 viral genome AAV2/8-apoA-V, maximal plasma levels of apoA-V protein were achieved at 3 to 4 weeks, after which the concentration slowly declined. Complementing the appearance of apoA-V was a decrease (50±6%) in plasma triacylglycerol content compared with apoa5 (−/−) mice treated with AAV2/8-β-galactosidase. After 8 weeks the mice were euthanized and plasma lipoproteins separated. AAV2/8-apoA-V–transduced mice displayed a dramatic reduction in very low–density lipoprotein triacylglycerol content. Vector generated apoA-V in plasma associated with both very low–density lipoprotein and high-density lipoprotein fractions.
Conclusion
Taken together, the data show that gene transfer of apoA-V improves the severe hypertriglyceridemia phenotype of apoa5 (−/−) mice. Given the prevalence of hypertriglyceridemia, apoA-V gene therapy offers a potential strategy for maintenance of plasma triacylglycerol homeostasis.
Keywords: apolipoprotein A-V, gene therapy, lipoprotein, triacylglycerol
Apolipoprotein (apo) A-V is a low abundance hepatic-derived protein that was independently identified by comparative sequence analysis of the human and mouse genomes1 and by differential gene expression studies in regenerating rat liver.2 Evidence that apoA-V plays a role in triacylglycerol (TG) metabolism emerged from studies with genetically modified mice developed by Pennacchio et al.1 Human APOA5 transgenic mice had plasma TG levels about one third that of control littermates. However, apoa5 (−/−) mice had an ≈4-fold increase in plasma TG, compared with their wild-type counterparts. In the meantime, genome-wide association studies have consistently identified APOA5 as a gene that affects plasma TG levels.3 Complementing genome-wide association studies are studies of the specific nature and allelic frequency of APOA5 single nucleotide polymorphisms (SNPs) in different human populations. Coding and noncoding SNPs have been identified throughout the APOA5 locus (part of the APOA5-APOA4-APOC3-APOA1 gene cluster on chromosome 11). For example, the −1131T>C SNP, located in the promoter region of APOA5, is associated with hypertriglyceridemia (HTG).4,5 Likewise, the −3A>G SNP, located in the Kozak sequence, has been postulated to reduce apoA-V expression by affecting translation initiation.6 A coding SNP (c.56C>G) that replaces Ser for Trp at position 19 in the apoA-V signal sequence (corresponding to position −4) is postulated to impair translocation to the endoplasmic reticulum.7,8 Another coding SNP, prevalent among Asian subjects (c.553G>T), gives rise to a Gly to Cys amino acid substitution at position 162 in mature apoA-V (corresponding to position 185 in the preprotein). Originally identified in Taiwanese and Chinese populations,9,10 this SNP strongly correlates with TG in Asian-Americans, manifesting a pronounced gene dosage effect.11
In addition to these SNPs, rare mutations in APOA5 have been described that result in premature truncation of this 343-aa protein. Priore Oliva et al12 identified a c.433C>T mutation that introduces a premature stop codon, generating a truncated, Q148X apoA-V. In a similar manner, Marçais et al13 described a Q139X variant apoA-V in a cohort of subjects with HTG. In 2008, Priore Oliva et al14 reported on a human subject who presented with severe HTG and eruptive xanthomas, consistent with chylomicronemia. Sequencing revealed this subject was homozygous for a point mutation in APOA5 (c.289C>T) that converts a glutamine codon at position 97 to a stop codon. No apoA-V was detectable in this patient’s plasma.
In an effort to normalize the HTG that results from a deficiency in apoA-V, intravenous injection studies have been performed in apoa5 (−/−) mice.15 Administration of apoA-V–reconstituted high-density lipoprotein to these mice induced a 60% reduction in plasma TG after 4 hours. This rapid decline was attributed to enhanced catabolism and clearance of very low–density lipoprotein (VLDL). Despite the reduction in plasma TG observed in this experiment, the effect was short-lived. Immunoblot analysis revealed that plasma apoA-V levels decreased rapidly after injection and was virtually absent by 4 hours. In an effort to increase the duration of TG-lowering effect of apoA-V therapy, we performed gene transfer studies. This approach has been successfully used to express apoE and apoA-I in mouse models of atherosclerosis.16–18 Herein, we report that adeno-associated virus (AAV) 2/8–mediated gene transfer of human apoA-V to apoa5 (−/−) mice results in sustained levels of apoA-V protein in plasma along with a significant reduction in plasma TG.
Materials and Methods
AAV2/8 Vector Construction and Preparation
The coding sequence for wild type (WT) human apoA-V was cloned into a replication deficient vector in which expression is under the regulation of the cytomegalovirus (CMV) promoter essentially as described by Grimm et al.19 The desired clones were generated by homologous recombination20 and transformed into E. coli DH5α for large-scale amplification. Recombinant AAV particles were generated by triple plasmid transfection of HEK293 cells with (1) a pseudotyped AAV-multiple cloning site plasmid harboring the coding sequence for WT apoA-V or β-galactosidase (LacZ), (2) a plasmid containing AAV helper genes, and (3) a chimeric construct encoding the AAV2 rep gene fused with an AAV8 cap gene.21 After viral titer determination by real-time polymerase chain reaction (PCR), recombinant virions were isolated by gradient centrifugation and CsCl banding. After dialysis against standard saline, final vector genome (vg) titers were determined by quantitative PCR (qPCR).
Animals
Male apoa5 (−/−) mice,22 aged 4 months, were used in all studies. At 3 months of age, apoa5 (−/−) mice that have been backcrossed onto an FVB background for 10 generations were fasted for 4 hours and screened for plasma TG. Whereas plasma TG values in 33 mice tested ranged from 220 to 1850 mg/dL (average = 907±470 mg/dL), animals with TG values <400 or >1500 mg/dL were excluded. All groups of mice used had a comparable range and average plasma TG values at the start of the experiment. Mice were fed a standard chow diet and maintained on a 12-hour light/dark cycle. A transgenic mouse strain expressing human apoA-V22 was used as positive control. Research was conducted in conformity with the Public Health Service Policy on the Humane Care and Use of Laboratory Animals and was approved by the Children’s Hospital Oakland Research Institute Institutional Animal Care and Use Committee.
Gene Transfer Studies In Vivo
Gene transfer studies were performed in groups of 8 mice. AAV2/8 vectors harboring apoA-V or LacZ were injected into the tail vein (1×1011 or 1×1012 vg per mouse). Blood samples were drawn weekly over the course of 8 weeks. At 8 weeks after transduction, mice were euthanized and tissues harvested. PCR was performed on genomic DNA to amplify the CMV promoter region. To measure human apoA-V mRNA levels in tissue extracts, reverse transcriptase PCR was performed. Liver samples from all treated mice were examined. In addition, genomic DNA and total RNA were extracted from specified tissues of 5 animals transduced with AAV2/8-apoA-V. CMV promoter was quantified by qPCR using genomic DNA as template and apoA-V expression was determined by qPCR using cDNA as template.
Enzyme-Linked Immunosorbent Assay
Plasma apoA-V concentration was measured using an enzyme-linked immunosorbent assay (ELISA) (Human Apolipoprotein A-V ELISA Kit; Millipore, St. Louis, MO). The sensitivity limit of this assay is 1.1 ng/mL.
Immunoblot Analysis
Plasma (2 µL) or liver extracts (60 µg protein) were electrophoresed on 10% acrylamide Bis–Tris gels. Concentrated fractions obtained from fast protein liquid chromatography (FPLC) were electrophoresed on 4% to 20% acrylamide gradient Bis–Tris gels. Size-separated proteins were transferred to a polyvinylidene difluoride membrane and immunoblots processed as described.23 To assess whether antibodies directed against human apoA-V were generated after gene transfer with AAV2/8 constructs, recombinant human apoA-V was electrophoresed on a 10% acrylamide Bis–Tris gel and transferred to a polyvinylidene difluoride membrane. The membrane was probed with plasma collected from mice 8 weeks after transduction with AAV2/8-LacZ and AAV2/8-apoA-V and diluted 1:200, 1:100, 1:50, and 1:20 with phosphate buffered saline. The presence of antihuman apoA-V in plasma samples was detected with horseradish peroxidase–labeled goat antimouse IgG.
Plasma Lipids and Liver-Specific Enzymes
Cholesterol and TG in plasma samples or FPLC fractions were determined by enzyme-based colorimetric assays (Wako, Richmond, VA). Alanine transaminase and aspartate transaminase activities in plasma samples obtained from 5 apoa5 (−/−) mice, 5 AAV2/8-LacZ–transduced apoa5 (−/−) mice, and 5 AAV2/8- apoA-V–transduced apoa5 (−/−) mice were determined on a Roche Diagnostics Cobas Integra 400 Plus instrument (UC Davis Comparative Pathology Laboratory).
FPLC Analysis of Plasma Lipoproteins
Lipoproteins from pooled plasma were separated by FPLC on a Superose 6 10/300 column (GE Healthcare, Pittsburgh, PA). Elution profiles were monitored at 280 nm with collection of 0.25-mL fractions.
Statistical Analysis
Student t test was used to examine statistical differences between AAV2/8-LacZ and AAV2/8-apoA-V–derived samples with values expressed as SEM; P≤0.05 is considered significant. Prism software (GraphPad software, San Diego, CA) was used for statistical analysis.
Results
AAV2/8 Preparation and Characterization
Engineered AAV2/8 vectors were isolated and subjected to analysis by SDS-PAGE. The results indicated the presence of the characteristic capsid proteins, VP1, VP2, and VP3 (data not shown). The lack of contaminating proteins provided evidence of the relative purity of the AAV2/8 preparation. Gene amplification of the LacZ and apoA-V coding sequences using the corresponding AAV2/8 constructs as template confirmed that the virus preparations contained the genes of interest.
Gene Transfer of AAV2/8-apoA-V into Mice
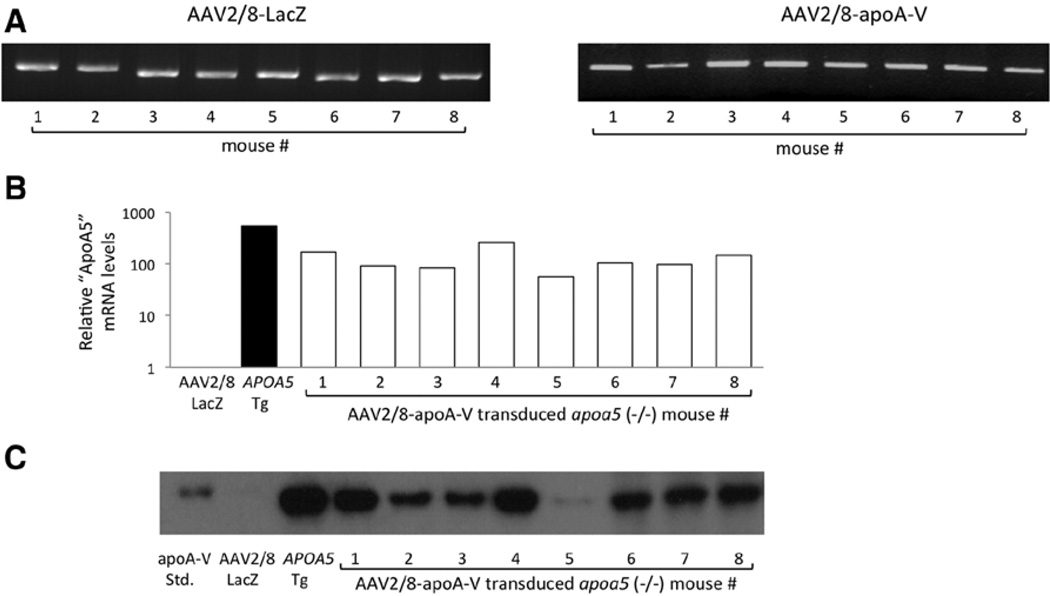
The normal plasma concentration of apoA-V in mice and humans is exceptionally low, ≈ 150 ng/mL.25 Given this, initial gene transfer studies were conducted to define the dose of AAV2/8-apoA-V required to achieve a physiologically relevant plasma concentration of apoA-V. The first experiment used 1×1011 vg, whereas a second study used 1×1012 vg per mouse. Groups of 8 apoa5 (−/−) mice were injected with AAV2/8-LacZ or AAV2/8-apoA-V. Plasma samples were collected weekly for 8 weeks, after which tissues were harvested. Analysis revealed that transduction with 1×1011 vg gave rise to barely detectable levels of apoA-V in plasma along with a modest decrease in plasma TG (data not shown). As a result, all subsequent studies were conducted with animals transduced with 1×1012 vg per mouse. In this case, analysis of liver tissue extracts revealed elevated amounts of the CMV promoter, indicating vector delivery to the target organ (Figure 1A). Furthermore, all livers from AAV2/8-apoA-V–transduced animals, but none from AAV2/8-LacZ–injected animals, expressed apoA-V mRNA (Figure 1B). Relative amounts of apoA-V protein in liver extracts of individual mice were determined by immunoblot analysis (Figure 1C). It is noteworthy that protein expression parallels mRNA expression such that mice with low apoA-V mRNA levels also had low apoA-V protein expression. To assess whether AAV2/8-mediated gene transfer induced detectable liver toxicity, plasma alanine transaminase and aspartate transaminase activity measurements were performed. Alanine transaminase values were 27±3.1, 29±3, and 27±2.3 U/L for apoa5 (−/−) mice, AAV2/8-LacZ apoa5 (−/−) mice, and AAV2/8-apoA-V apoa5 (−/−) mice, respectively. The corresponding values for aspartate transaminase were 44±3, 44±4 and 56±14 U/L.
Figure 1.
Characterization of adeno-associated virus (AAV) 2/8–transduced livers. Eight weeks after administrating AAV2/8-apolipoprotein (apo) A-V or AAV2/8-LacZ (1×1012 vg) into apoa5 (−/−) mice, livers were harvested. A, Gene amplification of cytomegalovirus promoter DNA in liver extracts from each of 8 mice transduced with AAV2/8-LacZ or AAV2/8-apoA-V. B, Histogram depicting apoA-V mRNA expression level in liver tissue extracts from AAV2/8-LacZ, APOA5 transgenic (Tg) and each of 8 AAV2/8-apoA-V–transduced mice. APOA5 mRNA expression was normalized to glyceraldehyde-3-phosphate dehydrogenase expression. C, α-apoA-V immunoblot (left lanes) recombinant human apoA-V standard, AAV2/8-LacZ– injected apoa5 (−/−) mouse liver extract, and human apoA-V Tg mouse liver extract, respectively. Lanes labeled 1 to 8 refer to individual liver extracts from each of 8 AAV2/8-apoA-V–transduced mice.
Vector and apoA-V Content in Other Tissues
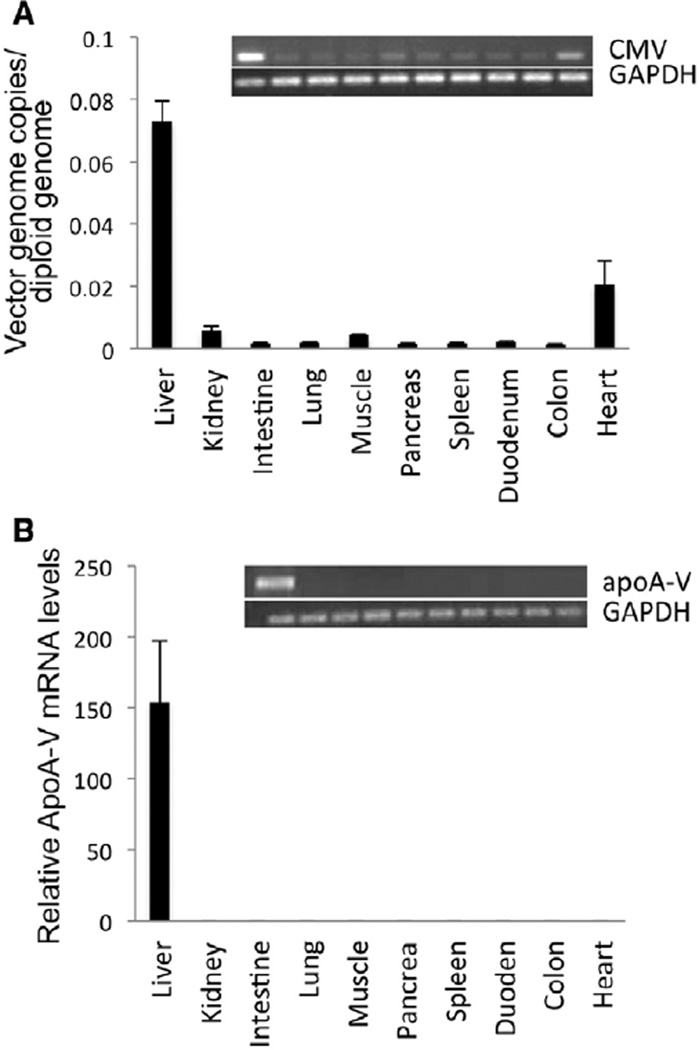
To assess the specificity with which AAV2/8-apoA-V homes to liver tissue, the amount of CMV promoter present in 10 different tissues, obtained 8 weeks after transduction, was determined. Gene amplification of the CMV promoter region in tissue extracts of a representative mouse revealed that liver was the major site of transduction, although CMV promoter DNA was detected in other tissues as well (Figure 2A, inset). To investigate this further, qPCR was performed on tissue extracts obtained from 5 AAV2/8-apoA-V–transduced animals. The resulting histogram (Figure 2A) shows that, although heart tissue contains CMV promoter DNA, liver is the predominant site of transduction. Furthermore, aside from liver and heart, no other tissue examined had appreciable levels of CMV promoter DNA. When apoA-V mRNA levels were measured in tissue extracts of a representative mouse, only liver exhibited a detectable signal (Figure 2B, inset). Subsequent qPCR analysis was performed on cDNAs prepared from tissue extracts of each of 5 AAV2/8-apoA-V–transduced animals. The histogram plot (Figure 2B) reveals that apoA-V mRNA expression in these animals is highly liver specific.
Figure 2.
Adeno-associated virus (AAV) 2/8 tissue distribution after injection. Eight weeks after gene transfer of 1×1012 vg AAV2/8-apolipoprotein (apo) A-V into apoa5 (−/−) mice, indicated tissues were harvested and analyzed for (A) cytomegalovirus (CMV) promoter DNA. Inset shows polymerase chain reaction (PCR) amplification of CMV promoter region and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from genomic DNA from tissue extracts of mouse #4 (see Figure 1C). Lane assignments correspond to specific tissues, as indicated in the corresponding histogram. Histogram depicts quantitative PCR (qPCR) analysis of tissue extracts from 5 individual AAV2/8-apoA-V–transduced mice. Values were calculated as copies of CMV promoter DNA per diploid copy of mouse genomic DNA. B, Tissue distribution of apoA-V mRNA. Inset, PCR amplification of apoA-V cDNA and GAPDH cDNA from tissue extracts of mouse #4. Histogram depicts qPCR analysis of apoA-V mRNA relative to GAPDH in tissue extracts of 5 individual AAV2/8-apoA-V–transduced mice. Values are the mean±SEM (n=5).
Time-Dependent Changes in Plasma apoA-V, TG, and Cholesterol
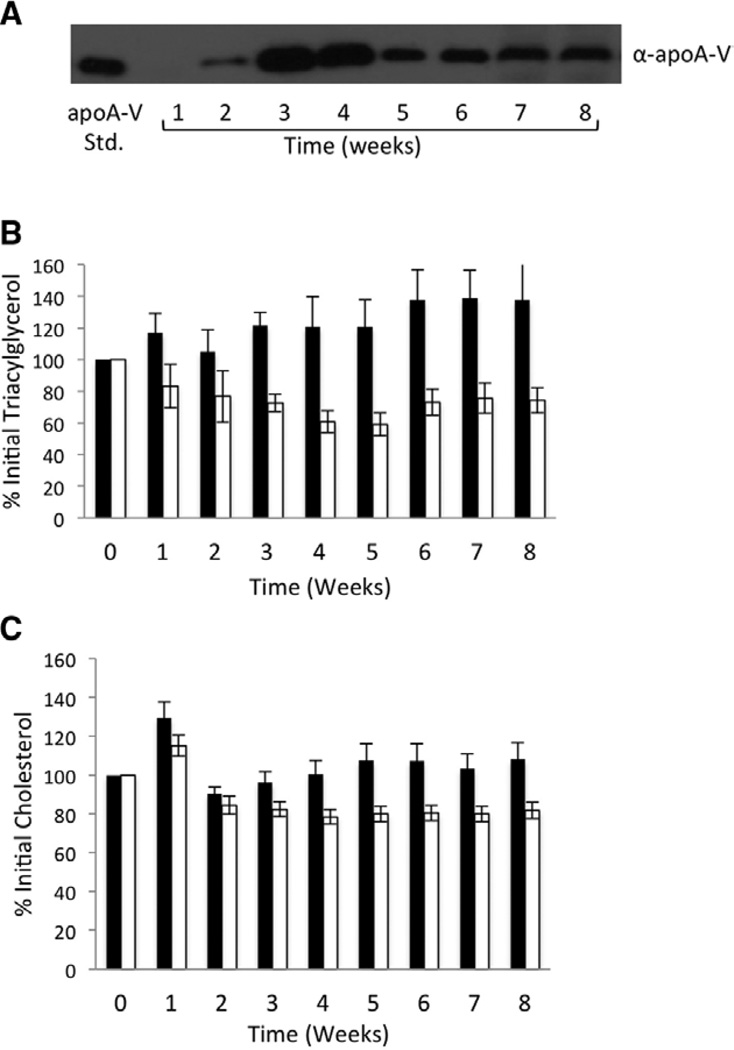
After injection with AAV2/8-apoA-V (1×1012 vg), levels of apoA-V in pooled plasma samples were determined by immunoblot at the end of every week for 8 weeks (Figure 3A). Although apoA-V protein was not detected in plasma after week 1, by week 2, apoA-V was present. In subsequent weeks, plasma apoA-V levels increased steadily, reaching a maximum at weeks 3 to 4. ApoA-V levels peaked at this point and then gradually declined until termination of the experiment. Compared with plasma from AAV2/8-LacZ animals, TG levels declined in AAV2/8-apoA-V–injected animals as apoA-V plasma levels increased. By week 4, when plasma apoA-V levels were the highest, a 50±6% reduction in pooled plasma TG was noted along with a 20±3% decline in cholesterol. In subsequent weeks, the reductions in TG and cholesterol persisted (Figure 3B and 3C), although plasma TG levels rose slightly, consistent with lower plasma apoA-V protein at these time points. In an effort to quantify the relationship between apoA-V expression and plasma TG, ELISA assays were performed on pooled plasma samples obtained from AAV2/8-apoA-V–injected animals. The concentrations of apoA-V observed were in the physiological range found in humans25 at every time point beyond week 1 (Table). When considered together with plasma TG values at these time points, an inverse correlation between apoA-V and TG levels is apparent.
Figure 3.
Effect of adeno-associated virus (AAV) 2/8 gene transfer on plasma lipid and apolipoprotein (apo) A-V content. Apoa5 (−/−) mice (8 mice per group) were transduced with AAV2/8-apoA-V or AAV2/8-LacZ (1×1012 vg each). A, α-apoA-V immunoblot of recombinant apoA-V standard (left) and pooled plasma samples from AAV2/8-apoA-V–transduced mice obtained each week for 8 weeks. B, Triacylglycerol content in plasma from 8 AAV2/8-LacZ–transduced animals (filled bars) and 8 AAV2/8-apoA-V–transduced animals (open bars). C, Cholesterol content in plasma from AAV2/8-LacZ–transduced animals (filled bars) and AAV2/8-apoA-V–transduced animals (open bars). Values reported as percent change vs week 0 values as mean±SEM (n=8).
Table.
Effect of Apolipoprotein (apo) A-V on Plasma Triacylglycerol
| AAV2/8-apoA-V Plasma Sample | apoA-V (ng/mL)* | TG (mg/dL)† |
|---|---|---|
| Pre injection | ND | 1098±300 |
| Week 1 | 11.5 | 903±500 |
| Week 2 | 151 | 827±500 |
| Week 3 | 450 | 585±200 |
| Week 4 | 462 | 535±200 |
| Week 5 | 311 | 634±300 |
| Week 6 | 305 | 812±300 |
| Week 7 | 291 | 828±300 |
| Week 8 | 285 | 843±300 |
ELISA was performed on plasma samples pooled from 8 animals; values represent the average of duplicate determinations. AAV indicates adeno-associated virus; apo, apolipoprotein; ND, not detected; and TG, triacylglycerol.
Triacylglycerol assays were performed on plasma samples from individual animals; values represent the mean±SEM (n=8).
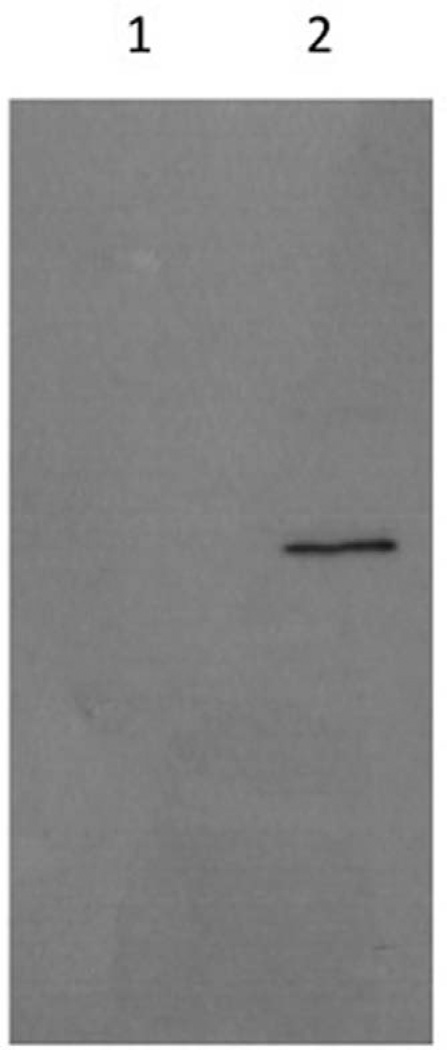
A question that arises, however, is why TG levels in AAV2/8-apoA-V–transduced animals do not decline to values normally observed in WT mice. One possibility that must be considered is that human apoA-V and murine apoA-V share only 71% sequence identity and 78% similarity at the amino acid level.1 As such, interactions of human apoA-V with murine receptors/binding partners may not be optimal, resulting in an attenuated response. Alternatively, it is possible that expression of human apoA-V in the mouse results in generation of antibodies that recognize human apoA-V. To examine this, plasma was obtained from apoa5 (−/−) mice 8 weeks after gene transfer of AAV2/8-apoA-V or AAV2/8-LacZ and used to probe an immunoblot of recombinant human apoA-V (Figure 4). Plasma collected from AAV2/8-apoA-V– transduced mice, but not that from AAV2/8-LacZ–transduced mice, showed positive immunoreactivity. On dilution of the AAV2/8-apoA-V–injected mouse plasma, a corresponding decrease in apoA-V immunoreactivity was observed (data not shown). Given this result, it is conceivable that the gradual decline in plasma apoA-V concentration (Table) that begins 5 weeks after transduction may be ascribed, in part, to the generation of antibodies directed against human apoA-V. To evaluate whether the decline in plasma apoA-V with time may occur as a result of defective liver processing/secretion of this protein, the apoA-V content of liver extracts and plasma of AAV2/8-apoA-V–transduced mice was examined. The results revealed that intracellular concentrations of apoA-V are roughly equivalent to those in plasma (data not shown), consistent with previous findings in lysates and media of hepatoma cells transiently transfected with human apoA-V.24
Figure 4.
Human apolipoprotein (apo) A-V immunoblot. Recombinant human apoA-V was separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and probed with plasma collected from AAV2/8-LacZ mice (Lane 1) and AAV2/8-apoA-V (Lane 2) mice, 8 weeks after infection.
Lipoprotein and Apo Distribution Studies
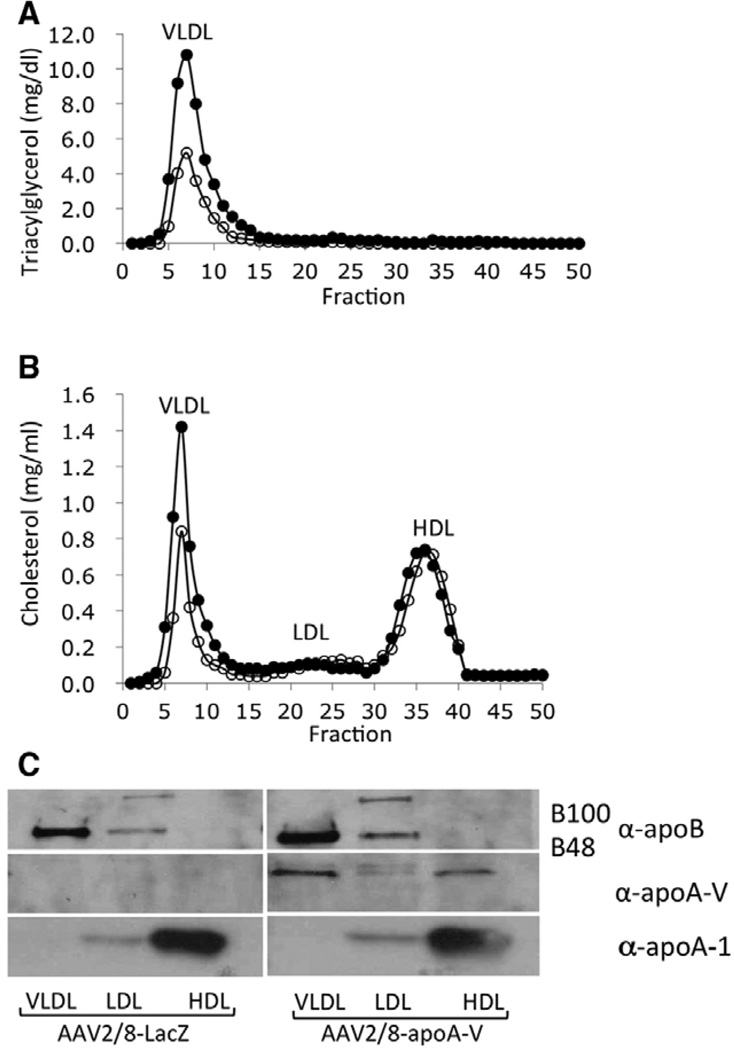
Eight weeks after injection of AAV2/8-apoA-V or AAV2/8-LacZ into apoa5 (−/−) mice, animals were euthanized and blood collected. To separate individual lipoprotein classes, pooled plasma samples were subjected to FPLC (Figure 5A and 5B). Lipid analysis of the separated lipoprotein fractions revealed that reductions in TG and cholesterol observed in AAV2/8-apoA-V–injected mice occurred exclusively in the VLDL fraction, whereas the LDL and high-density lipoprotein fractions remained unchanged. Immunoblot analysis of fractionated lipoproteins (Figure 5C) indicated that apoA-V associates primarily with VLDL, with a small amount on high-density lipoprotein. As expected, no apoA-V was detected in plasma of mice transduced with AAV2/8-LacZ. Furthermore, apoB was confined to the VLDL and LDL fractions, whereas nearly all apoA-I was present in the high-density lipoprotein fraction. No differences in apoB or apoA-I lipoprotein distribution were noted between AAV2/8-LacZ and AAV2/8-apoA-V–infected apoa5 (−/−) mouse plasmas.
Figure 5.
Effect of adeno-associated virus (AAV) 2/8 gene transfer on lipid and apolipoprotein distribution. Plasma samples collected 8 weeks after injection of AAV2/8- apolipoprotein (apo) A-V or AAV2/8-LacZ (1×1012 vg) were pooled (from 8 animals) and subjected to fast protein liquid chromatography to separate lipoproteins. A, Triacylglycerol content in lipoprotein fractions from AAV2/8-LacZ mouse plasma (filled circles) and AAV2/8-apoA-V mouse plasma (open circles). B, Cholesterol content in lipoprotein fractions from AAV2/8-LacZ mouse plasma (filled circles) and AAV2/8-apoA-V mouse plasma (open circles). C, A 4% to 20% acrylamide gradient Bis–Tris gel was used for immunoblot analysis of lipoprotein fractions probed with antibodies directed against apoB, apoA-V, and apoA-I. HDL indicates high-density lipoprotein; LDL, low-density lipoprotein; and VLDL, very low–density lipoprotein.
Discussion
Genome-wide association studies and characterization of prevalent APOA5 SNPs reveal the importance of apoA-V as a regulator of plasma TG homeostasis. When considered together with the fact that circulating levels of apoA-V in human plasma are exceptionally low (≈ 150 ng/mL25), apoA-V is an ideal candidate for gene therapy. The hypothesis tested in the present study is whether AAV2/8-mediated gene transfer of apoA-V will result in sustained plasma levels of apoA-V that reverse/reduce the HTG phenotype of apoa5 (−/−) mice. By extension, it is anticipated that improved TG control in human populations that carry specific APOA5 SNPs or rare mutations associated with HTG will decrease their dyslipidemia.
The present study provides evidence for a dose–response effect of apoA-V on plasma TG levels. Whereas gene transfer of 1×1011 vg AAV2/8-apoA-V gave rise to barely detectable levels of apoA-V and a modest decrease in plasma TG, injection of 1×1012 vg resulted in physiological levels of apoA-V in plasma and a 50±6% reduction in plasma TG. The effect of apoA-V on plasma TG levels persisted for at least 8 weeks. Given results obtained by others using this gene transfer strategy,26 it is conceivable that long-term expression of apoA-V from the AAV2/8 vector is possible. With regard to this issue, data generated in the present study showed a gradual decline in plasma apoA-V concentration beginning 5 weeks after transduction with AAV2/8-apoA-V. This may be partly explained by the generation of antibodies against human apoA-V in these mice. The presence of antibodies may also contribute to the observation that, despite the fact that human apoA-V was expressed at physiological levels, plasma TG levels did not return to those seen in WT mice. Insofar as human apoA-V may not display full biological activity in the mouse owing to species differences, it may be anticipated that an even more robust decrease in plasma TG concentration would occur if human apoA-V is transduced into human subjects harboring mutations or polymorphisms in APOA5 that give rise to HTG. Furthermore, although human apoA-V expression in mice leads to an immune response and generation of antibodies in plasma that recognize recombinant human apoA-V, we do not anticipate this would occur in humans expressing a human apoA-V transgene. The observation that AAV2/8 homes to the liver after injection fits well with the fact that apoA-V is expressed exclusively in this tissue.2
Likewise, we found that plasma activities of enzymes that serve as markers of hepatic toxicity were comparable between control mice and AAV2/8-transduced mice. Given that the average values were well within the normal range for these enzymes in mouse plasma (17–77 U/L for alanine transaminase and 54–298 U/L for aspartate transaminase) indicates that AAV2/8 gene transfer caused minimal liver damage. One of the novel features of apoA-V is its dual existence as a secreted protein and a lipid droplet–associated protein in hepatocytes.27 Based on this, it is conceivable that inefficient or delayed secretion of apoA-V from hepatocytes may alter TG trafficking or secretion.28 On examination of the relative distribution of human apoA-V between plasma and liver tissue, however, no evidence of hepatic apoA-V overaccumulation was obtained, suggesting the transgene is processed normally.
Apo5 (−/−) mice lack apoA-V altogether and manifest chronic HTG. The ability of apoA-V gene transfer to significantly decrease plasma TG levels in apoa5 (−/−) mice opens the door to future gene transfer studies with apoA-V variants designed to evaluate their ability to ameliorate the HTG phenotype. Using this approach it should be possible to test hypotheses related to the structural basis of apoA-V effects on plasma TG levels. To our knowledge, this is the first study that results in sustained expression of physiological levels of apoA-V up to 8 weeks after gene transfer with no evidence of liver damage. Previous adenovirus gene transfer study into WT mice resulted in an acute, supraphysiological apoA-V expression.29 As a result, neither human apoA-V transgenic1,22 mice nor adenovirus studies reported earlier are well suited to study TG-lowering effects of apoA-V.
An important question arising from gene transfer studies of apoA-V relates to whether increasing its plasma concentration may be antiatherogenic. Whereas TG itself does not have a direct role in plaque formation, an indirect role in disease progression may occur through its association with other genetically regulated components.27 Thus, APOA5 polymorphisms that correlate with elevated plasma TG are likely to influence the atherosclerotic process. Indeed, Mansouri et al30 provided evidence that apoA-V has an atheroprotective role in combined dyslipidemic (ie, increased plasma TG and cholesterol), apoE2 knock-in mice. When these mice were crossed with human apoA-V transgenic mice, a 2-fold reduction in aortic lesion area was observed, compared with apoE2 knock-in mice. Subsequently, Grosskopf et al31 evaluated the effect of apoA-V on atherosclerosis in apoE (−/−) mice. Overexpression of apoA-V in apoe (−/−) mice led to a significant decrease in VLDL and remnant lipoproteins, together with a 70% reduction in aortic lesion area. Overexpression of apoA-V decreased TG secretion, enhanced TG-rich lipoprotein clearance from plasma, and led to a decrease in proatherogenic cytokines. Based on this, it seems reasonable to consider that human subjects harboring APOA5 SNPs that promote HTG would benefit from expression of apoA-V. In summary, the present studies demonstrate that gene transfer of AAV2/8-apoA-V into apoa5 (−/−) mice results in physiological levels of apoA-V in plasma that are accompanied by significant reductions in plasma TG.
Acknowledgments
We thank Lee Ying for assistance with animal experiments.
Sources of Funding
This work was supported by an American Heart Association Postdoctoral Fellowship Award (12POST12030008) and the National Institutes of Health (HL64159). J.B.S. acknowledges support from the Danish VKR and IMK Almene Foundations.
Footnotes
Disclosures
None.
References
- 1.Pennacchio LA, Olivier M, Hubacek JA, Cohen JC, Cox DR, Fruchart JC, Krauss RM, Rubin EM. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 2.van der Vliet HN, Sammels MG, Leegwater AC, Levels JH, Reitsma PH, Boers W, Chamuleau RA. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J Biol Chem. 2001;276:44512–44520. doi: 10.1074/jbc.M106888200. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson SK, Heeren J, Olivecrona G, Merkel M. Apolipoprotein A-V; a potent triglyceride reducer. Atherosclerosis. 2011;219:15–21. doi: 10.1016/j.atherosclerosis.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Johansen CT, Wang J, Lanktree MB, et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat Genet. 2010;42:684–687. doi: 10.1038/ng.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pennacchio LA, Olivier M, Hubacek JA, Krauss RM, Rubin EM, Cohen JC. Two independent apolipoprotein A5 haplotypes influence human plasma triglyceride levels. Hum Mol Genet. 2002;11:3031–3038. doi: 10.1093/hmg/11.24.3031. [DOI] [PubMed] [Google Scholar]
- 6.Charriere S, Bernard S, Aqallal M, Merlin M, Billon S, Perrot L, Le Coquil E, Sassolas A, Moulin P, Marcais C. Association of APOA5-1131T>C and S19W gene polymorphisms with both mild hypertriglyceridemia and hyperchylomicronemia in type 2 diabetic patients. Clin Chim Acta. 2008;394:99–103. doi: 10.1016/j.cca.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Palmen J, Smith AJ, Dorfmeister B, Putt W, Humphries SE, Talmud PJ. The functional interaction on in vitro gene expression of APOA5 SNPs, defining haplotype APOA52, and their paradoxical association with plasma triglyceride but not plasma apoAV levels. Biochim Biophys Acta. 2008;1782:447–452. doi: 10.1016/j.bbadis.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Talmud PJ, Martin S, Taskinen MR, Frick MH, Nieminen MS, Kesäniemi YA, Pasternack A, Humphries SE, Syvänne M. APOA5 gene variants, lipoprotein particle distribution, and progression of coronary heart disease: results from the LOCAT study. J Lipid Res. 2004;45:750–756. doi: 10.1194/jlr.M300458-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Kao JT, Wen HC, Chien KL, Hsu HC, Lin SW. A novel genetic variant in the apolipoprotein A5 gene is associated with hypertriglyceridemia. Hum Mol Genet. 2003;12:2533–2539. doi: 10.1093/hmg/ddg255. [DOI] [PubMed] [Google Scholar]
- 10.Tang Y, Sun P, Guo D, Ferro A, Ji Y, Chen Q, Fan L. A genetic variant c.553G > T in the apolipoprotein A5 gene is associated with an increased risk of coronary artery disease and altered triglyceride levels in a Chinese population. Atherosclerosis. 2006;185:433–437. doi: 10.1016/j.atherosclerosis.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Pullinger CR, Aouizerat BE, Movsesyan I, Durlach V, Sijbrands EJ, Nakajima K, Poon A, Dallinga-Thie GM, Hattori H, Green LL, Kwok PY, Havel RJ, Frost PH, Malloy MJ, Kane JP. An apolipoprotein A-V gene SNP is associated with marked hypertriglyceridemia among Asian-American patients. J Lipid Res. 2008;49:1846–1854. doi: 10.1194/jlr.P800011-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priore Oliva C, Pisciotta L, Li Volti G, Sambataro MP, Cantafora A, Bellocchio A, Catapano A, Tarugi P, Bertolini S, Calandra S. Inherited apolipoprotein A-V deficiency in severe hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 2005;25:411–417. doi: 10.1161/01.ATV.0000153087.36428.dd. [DOI] [PubMed] [Google Scholar]
- 13.Marçais C, Verges B, Charrière S, Pruneta V, Merlin M, Billon S, Perrot L, Drai J, Sassolas A, Pennacchio LA, Fruchart-Najib J, Fruchart JC, Durlach V, Moulin P. Apoa5 Q139X truncation predisposes to late-onset hyperchylomicronemia due to lipoprotein lipase impairment. J Clin Invest. 2005;115:2862–2869. doi: 10.1172/JCI24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Priore Oliva C, Carubbi F, Schaap FG, Bertolini S, Calandra S. Hypertriglyceridaemia and low plasma HDL in a patient with apolipoprotein A-V deficiency due to a novel mutation in the APOA5 gene. J Intern Med. 2008;263:450–458. doi: 10.1111/j.1365-2796.2007.01912.x. [DOI] [PubMed] [Google Scholar]
- 15.Shu X, Nelbach L, Weinstein MM, Burgess BL, Beckstead JA, Young SG, Ryan RO, Forte TM. Intravenous injection of apolipoprotein A-V reconstituted high-density lipoprotein decreases hypertriglyceridemia in apoav−/− mice and requires glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1. Arterioscler Thromb Vasc Biol. 2010;30:2504–2509. doi: 10.1161/ATVBAHA.110.210815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris JD, Schepelmann S, Athanasopoulos T, Graham IR, Stannard AK, Mohri Z, Hill V, Hassall DG, Owen JS, Dickson G. Inhibition of atherosclerosis in apolipoprotein-E-deficient mice following muscle transduction with adeno-associated virus vectors encoding human apolipoprotein-E. Gene Ther. 2002;9:21–29. doi: 10.1038/sj.gt.3301615. [DOI] [PubMed] [Google Scholar]
- 17.Kitajima K, Marchadier DH, Miller GC, Gao GP, Wilson JM, Rader DJ. Complete prevention of atherosclerosis in apoE-deficient mice by hepatic human apoE gene transfer with adeno-associated virus serotypes 7 and 8. Arterioscler Thromb Vasc Biol. 2006;26:1852–1857. doi: 10.1161/01.ATV.0000231520.26490.54. [DOI] [PubMed] [Google Scholar]
- 18.Lebherz C, Sanmiguel J, Wilson JM, Rader DJ. Gene transfer of wild-type apoA-I and apoA-I Milano reduce atherosclerosis to a similar extent. Cardiovasc Diabetol. 2007;2:6–15. doi: 10.1186/1475-2840-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grimm D, Pandey K, Nakai H, Storm TA, Kay MA. Liver transduction with recombinant adeno-associated virus is primarily restricted by capsid serotype not vector genotype. J Virol. 2006;80:426–439. doi: 10.1128/JVI.80.1.426-439.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelbach L, Shu X, Konrad RJ, Ryan RO, Forte TM. Effect of apolipoprotein A-V on plasma triglyceride, lipoprotein size, and composition in genetically engineered mice. J Lipid Res. 2008;49:572–580. doi: 10.1194/jlr.M700281-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Beckstead JA, Oda MN, Martin DD, Forte TM, Bielicki JK, Berger T, Luty R, Kay CM, Ryan RO. Structure–function studies of human apolipoprotein A-V: a regulator of plasma lipid homeostasis. Biochemistry. 2003;42:9416–9423. doi: 10.1021/bi034509t. [DOI] [PubMed] [Google Scholar]
- 24.Shu X, Chan J, Ryan RO, Forte TM. Apolipoprotein A-V association with intracellular lipid droplets. J Lipid Res. 2007;48:1445–1450. doi: 10.1194/jlr.C700002-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien PJ, Alborn WE, Sloan JH, Ulmer M, Boodhoo A, Knierman MD, Schultze AE, Konrad RJ. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin Chem. 2005;51:351–359. doi: 10.1373/clinchem.2004.040824. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda M, Bishop DF, Fowkes M, Cheng SH, Gan L, Desnick RJ. AAV8-mediated gene therapy prevents induced biochemical attacks of acute intermittent porphyria and improves neuromotor function. Mol Ther. 2010;18:17–22. doi: 10.1038/mt.2009.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma V, Ryan RO, Forte TM. Apolipoprotein A-V dependent modulation of plasma triacylglycerol: a puzzlement. Biochim Biophys Acta. 2012;1821:795–799. doi: 10.1016/j.bbalip.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao X, Forte TM, Ryan RO. Influence of apolipoprotein A-V on hepatocyte lipid droplet formation. Biochem Biophys Res Commun. 2012;427:361–365. doi: 10.1016/j.bbrc.2012.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaessen SF, Dallinga-Thie GM, Ross CJ, Splint LJ, Castellani LW, Rensen PC, Hayden MR, Schaap FG, Kuivenhoven JA. Plasma apolipoprotein AV levels in mice are positively associated with plasma triglyceride levels. J Lipid Res. 2009;50:880–884. doi: 10.1194/jlr.M800551-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansouri RM, Baugé E, Gervois P, Fruchart-Najib J, Fiévet C, Staels B, Fruchart JC. Atheroprotective effect of human apolipoprotein A5 in a mouse model of mixed dyslipidemia. Circ Res. 2008;103:450–453. doi: 10.1161/CIRCRESAHA.108.179861. [DOI] [PubMed] [Google Scholar]
- 31.Grosskopf I, Shaish A, Afek A, Shemesh S, Harats D, Kamari Y. Apolipoprotein A-V modulates multiple atherogenic mechanisms in a mouse model of disturbed clearance of triglyceride-rich lipoproteins. Atherosclerosis. 2012;224:75–83. doi: 10.1016/j.atherosclerosis.2012.04.011. [DOI] [PubMed] [Google Scholar]