Abstract
Background
Offspring to patients with schizophrenia exhibit poorer school performance compared to offspring of non-schizophrenic parents. We aimed to elucidate the mechanisms behind this association.
Methods
We linked longitudinal national population registers in Sweden and compared school performance among offspring to schizophrenic parents to offspring of non-schizophrenic parents (N=1,439,215 with final grades from compulsory school 1988–2006). To investigate the mechanisms, we studied offspring to schizophrenic patients and controls within the same extended families. We investigated genetic effects by stratifying analyses of parent-child associations according to genetic relatedness (half-cousins, full cousins, and half-siblings). Environmental effects were investigated by comparing school performance of offspring to schizophrenic fathers and to schizophrenic mothers, respectively, and by stratifying the analyses according to environmental relatedness while controlling genetic relatedness (paternal and maternal half-cousins, paternal and maternal half-siblings).
Results
Offspring to parents with schizophrenia had poorer overall school performance than unrelated offspring to non-schizophrenic parents (−0.31 SD). Variability in genetic relatedness greatly moderated the strength of the within-family association (β=−0.23 within exposure-discordant half-cousins, β=−0.13 within exposure-discordant full cousins, and β=0.04 within exposure-discordant half-siblings), while no evidence was found that the environment affected offspring school performance.
Conclusions
Genetic factors account for poorer school performance in children of parents with schizophrenia. This supports that cognitive deficits found in individuals with schizophrenia and their relatives might be genetically inherited. Early detection of prodromal signs and impaired functioning of offspring to patients with schizophrenia could lead to earlier and better tailored interventions.
Keywords: school performance, offspring, schizophrenia, genetics, environments
Introduction
Offspring to patients with schizophrenia more often have social and cognitive disadvantages than offspring of non-schizophrenic parents, including lower IQ (David et al. 1997; Kremen et al. 1998; Davidson et al. 1999), greater attention deficits (Lifshitz et al. 1985; Sohlberg, 1985; Erlenmeyer-Kimling & Cornblatt, 1992), higher incidence of speech impairment (DeLisi et al. 1991; Jones et al. 1994), difficulties with social adjustment (Walker et al. 1993; Bearden et al. 2000), and higher risk of schizophrenia (McDonald & Murphy, 2003). Several studies also suggest that children with parents suffering from schizophrenia have lower school competence as rated by peers and teachers (Fisher et al. 1980), lower motivation and more behavior problems (Janes et al. 1983), and poorer mathematical reasoning (Ayalon & Merom, 1985). Researchers have used children of twin studies to investigate the mechanisms for the higher incidence of schizophrenia disorder among offspring to patients with schizophrenia (Gottesman & Bertelsen, 1989). However, these studies were seldom done to specifically investigate the mechanism behind the association between parental schizophrenia and offspring behavior.
One possible causal mechanism underlying the observed association could be adverse family environment. School performance is associated with multiple family environmental factors, including parents’ educational level (Kim, 2004). Schizophrenia is associated with cognitive impairments, social withdrawal and low socioeconomic status (Goldberg & Morrison, 1963; Tandon et al. 2009). Illness-related behaviour might affect family interpersonal relationships and make their children more worried and inattentive in school. Therefore, schizophrenia-associated family environment impairments could worsen offspring school performance.
In contrast, genetic factors shared by parents and offspring might also affect the children’s academic performance. A twin study from the United Kingdom found that 60% of the variation in school performance was explained by genetic influences (Haworth et al. 2008). Thus, offspring’s poorer school performance could be due to genetically influenced traits such as cognitive ability, which are shared by parents with schizophrenia and their children. In fact, numerous studies have tried to identify premorbid markers for schizophrenia (e.g., IQ, attention deficit, or school performance) by studying offspring of individuals with schizophrenia (Niemi et al. 2003). An often implicit assumption in these studies is that the premorbid markers are genetically determined and that the association is due to shared genetic liability (Jaffee & Price, 2007).
In parent-offspring studies, however, researchers cannot disentangle whether the cause of an association is environmental or genetic. Traditionally, the children-of-twin study design has been used to examine the effect from genetic components (Gottesman & Bertelsen, 1989; D'Onofrio et al., 2003). In this design, healthy co-twins of affected twins are studied, and the rates of the disorder in offspring among monozygotic cotwins (MZ) and dizygotic cotwins (DZ) are compared. If genetic influences are negligible, the rate of disorder among offspring to unaffected MZ co-twins should be the same as the rate of disorder among offspring to unaffected DZ co-twins. Conversely, if genetic influences are important, the rate of disorder among offspring to unaffected MZ co-twins should be higher than the rate of disorder among offspring to unaffected DZ co-twins. This is because the only difference between MZ and DZ twins is the genetic relatedness. Recently, the children-of-sibling design has been developed based on the same principle (Rutter et al. 2001; Harden et al. 2007; D'Onofrio et al. 2009a; D'Onofrio et al. 2009b). The intergenerational (parent to offspring) association strength was compared among discordant sibling pairs with decreasing genetic relatedness (full-siblings, half-siblings, full-cousins, half-cousin and unrelated individuals). An increasing trend of association strengths would suggest some role of genetic factors in affecting offspring disorder.
It is well known that offspring of schizophrenic individuals has poorer school performance than offspring of non-schizophrenic parents. However, it is not known whether the poor offspring school performance is due to genetic transmission or environmental factors in families with parental schizophrenia. The aim of the current study was to confirm the association between schizophrenia in parents and offspring school performance and explore the underlying mechanisms behind this association. We linked multiple Swedish longitudinal registers and investigated the mechanism by stratifying the analysis according to genetic and environmental relatedness.
Method
Study population and register linkage
This national cohort study was based on linkage of multiple Swedish longitudinal population-based registers, using the unique national identification number given to all Swedish citizens at birth and to immigrants upon arrival to the country. The registers used were:
The National School Register (NCR)
Education in Sweden is free of charge and compulsory between the ages of 7 and 16 years (9 years). The Swedish National School Register includes grades for all students in each subject from the final year of compulsory school (class 9) since 1988 (Lambe et al. 2006). The register comprises 1,439,215 individuals with grades from 1988 to 2006.
The Multi-Generation Register
This register includes the identity of biological parents of each individual born in Sweden since 1932 or who immigrated to Sweden together with one or both parents before age 18 years. Based on this information, we identified family structures (Multi-Generation Register, 2003).
The Hospital Discharge Register
This Register supplied details of all individual episodes of psychiatric hospitalization in Sweden since 1973 (including data from the few private providers of inpatient care) (The Swedish Hospital Discharge Register, 2005).
Other national registers
The Education Register holds information about the highest level of education obtained by each individual since 1990 (Education in the Swedish population, 1993). The Total Population Register includes the gender and date of birth of each individual since 1961 (Persson & Andersson, 2010).
Using the Multi-Generation Register, individuals were connected to their siblings and cousins via their parents and grandparents. The entire dataset includes 1,439,215 offspring in 810,968 nuclear families nested in 640,035 extended families.
Exposure
Parents were classified to suffer from schizophrenia based on two or more separate hospitalization episodes with a discharge diagnosis of schizophrenia (ICD-8/9: 295, ICD-10: F20, excluding latent schizophrenia [ICD-9: 295.5 and ICD-10: 295F]). Two or more inpatient admissions were chosen as cut-off to increase diagnostic specificity (Lichtenstein et al. 2006). Offspring was considered to be exposed if at least one biological parent fulfilled the criteria above.
Outcome
Grades between 1988 and 1997 were given according to a relative 5-step grade (1–5) in each subject by teachers. A summary grade was calculated as the mean of all grades. From 1998 onwards, each subject was given an absolute mark, and the summary grade was computed based on the 16 best subject grades. The children get their grades when completing the 9th grade; hence usually at age of 15 years old. To use data for the entire period, summary grades were standardized and normalized for each period using Blom transformation (Scorei = Φ−1(Ranki−3/8)/(n+1/4)) (Van den Oord et al. 2000). The normalized score used in analyses represents overall performance of each child.
Covariates
The highest educational level of the parents was obtained from the Education Registry in 2004. Family educational levels were defined as the highest educational level of father and mother. If educational level information was missing for one parent, we used the information from the parent with available data. If information was missing for both parents, family educational level was consequently coded as missing. Offspring gender was obtained from the Total Population Register.
Statistical analysis
To investigate the association between parental schizophrenic disorder and offspring school performance, we used linear regression on unrelated offspring to avoid clustering of data. Parental education and offspring gender were controlled as covariates in the regression.
To estimate mechanisms behind the association, we also investigated within-family effects of having a schizophrenic parent on offspring school performance. Thus, school grades among offspring to parents with schizophrenia were compared to, for example, their cousins (i.e., offspring of the healthy sibling of a patient with schizophrenia). These analyses were thus done in extended families, and we used hierarchical linear modelling (HLM) to adjust for the clustering of data and covariates (Raudenbush & Bry, 2002; Littell et al. 2006; Harden et al. 2007). HLM models provide an effect estimate (β coefficient), which measures the effect of parental schizophrenia on offspring school performance in extended families, thus comparing discordant affected offspring within the same family.
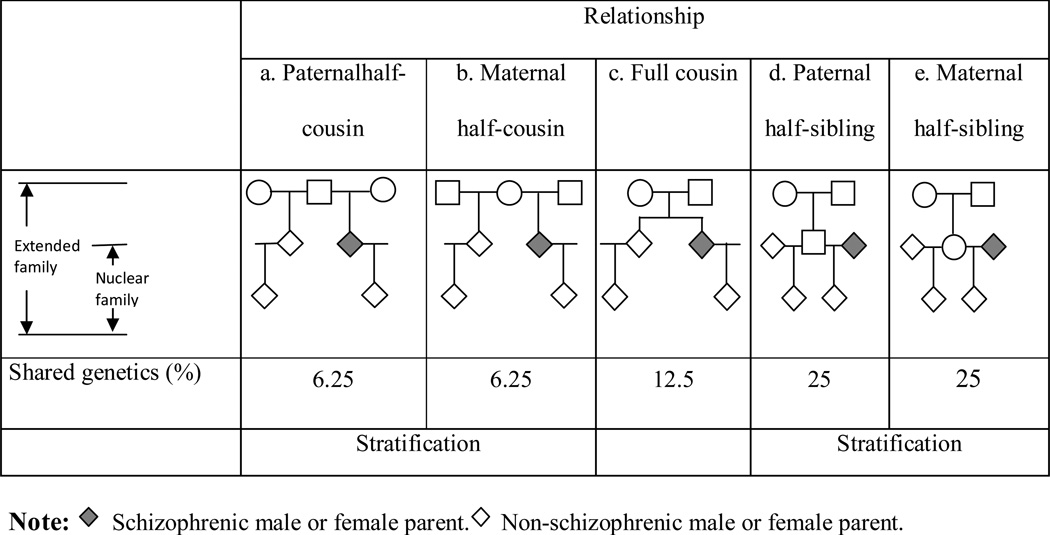
Parents with schizophrenia could affect offspring school performance by passing on genetic risks to their offspring and/or contribute to environmental risks (Plomin et al. 1977). To determine if genetic or environmental factors affected offspring school performance, we compared the within-family intergenerational association (β coefficient) across different family types with different offspring kinship distances. The family types used in this study are displayed in Figure 1. Genetic relatedness is indicated by the average percentage of co-segregating genes for each class of relatives. Paternal half-cousin data included half-cousins who shared the same grandfather but different grandmother in each extended family (Figure 1a). The relationship for maternal half-cousins is depicted in Figure 1b. Full cousin data included full cousins who shared the same grandfather and grandmother in each extended family (Figure 1c). Paternal half-sibling data included paternal half siblings in each extended family who shared the same father but different mother (Figure 1d), and the maternal half-sibling data is depicted in Figure 1e. Since full siblings had the same mother and father, we could not examine the influence of exposure to a parent with schizophrenia in full sibling pairs. Extended family was indexed by the grandmother (paternal half-cousin data were indexed by the grandfather), and nuclear families were indexed by the offspring of grandfather and grandmother. To investigate the role of genetics mechanisms, we explored how the variability in offspring genetic relatedness within extended families moderated the association strength. A bit counterintuitive; if genetic effects are responsible for an observed association, we would expect the within-family effect (β coefficient) to be smaller the closer the genetic relatedness between the compared individuals (offspring exposed to a schizophrenic parent vs. offspring exposed to healthy parents in the same extended family). Since extended families were indexed by the grandmother, the relationship of offspring in one extended family could be maternal half-cousins, full cousin and half siblings. We therefore compared the within-family effect of exposure to parental schizophrenia on offspring school performance (β coefficient) across maternal half-cousins, full cousins and half-siblings (including both paternal and maternal half-siblings) (Figure 1).
Figure 1.
Datasets used when comparing school performance in offspring of parents with schizophrenia with that in relatives at varying genetic distance.
One way to test the importance of the environment in families with schizophrenia patients, is to compare school performance of offspring who are exposed to different load of schizophrenia associated family environment. As a result, we compared mean school performance between unrelated offspring to schizophrenic mothers and to schizophrenic fathers, respectively. The assumption is that since the mother has traditionally been the primary caregiver in Sweden (Kate, 1991), the family environment would be more detrimental if the mother (as compared to the father) had a diagnosis of schizophrenia. Therefore, if family environments play an important role in determining offspring school performance, offspring to a schizophrenic mother would perform worse than offspring to a schizophrenic father. Another way to test the importance of a family environment is to compare relatives who differ in environmental relatedness. If family environments are important, we would expect the difference between exposed offspring and their unexposed relatives in school performance to change if the environment relatedness of the relatives changes. We therefore contrasted the association strength between paternal and maternal half-sibling pairs and between paternal and maternal half-cousin pairs, respectively (Figure 1 – symbolized with “stratification”). This was because both paternal and maternal half-sibling share 25% genetics, but the family environmental similarity is lower between paternal half-sibling than between maternal half-sibling. The same logic applies to the comparison between paternal and maternal half-cousins. The rationale is that a vast majority (91%) of children in Sweden continue to live with their mother after parental divorce or separation (Fakta om den svenska familjen, 1994). Therefore, although the genetic relatedness of paternal half-sibling pairs is the same as genetic relatedness of maternal half-sibling pairs (25%), the family environment would be more similar between maternal half-sibling pairs (as compared to paternal half-sibling pairs) since they most often stayed with the same mother, while paternal half-siblings were brought up by different mothers. The assumption is that maternal half-sibling pairs are more similar in behaviour and characteristics as compared to paternal half-sibling pairs since they are brought up by the same mother. Therefore, if environment plays an important role in the association, variability in environment similarity should moderate association strength. In other word, the within-family intergenerational association (β coefficient) should be different between paternal half-siblings (paternal half-cousins) and maternal half-siblings (maternal half-cousins) if family environment plays an important role.
Results
Demographic sample characteristics are shown in Table 1. The proportion of all offspring with final grades from compulsory school who had one or more parents with schizophrenia was 0.25%. The effect size of the influence of parental schizophrenia on offspring school performance was −0.31 (p<0.0001) before controlling for covariates and −0.18 (p<0.0001) after controlling for covariates (Appendix Table A1), suggesting that offspring to schizophrenic parents had a mean overall grade 0.31 standard deviations lower than those to non-schizophrenic parents. In agreement with previous studies (for reviews, see Claudia et al. 2008), our results showed that men performed poorer academically than women (average grades) at school (Appendix Table A1).
Table 1.
Characteristics of offspring to parents with and without schizophrenia in Sweden.
| Variable | Unexposed offspring (N=1,439,215 |
Exposed offspring (N=3,654) |
P-value | ||
|---|---|---|---|---|---|
| Gender | 0.164 | ||||
| Male | 736,513 | 51.2% | 1,912 | 52.3% | |
| Female | 702,702 | 48.8% | 1,742 | 47.7% | |
| Highest parental education | <0.001 | ||||
| < 9 years of education | 32,212 | 2.2% | 143 | 3.9% | |
| 9 years of education | 74,879 | 5.2% | 375 | 10.3% | |
| 1–2 years upper secondary education | 476,646 | 33.1% | 1,470 | 40.2% | |
| 3 year secondary education less than 3 years post | 229,922 | 16.0% | 502 | 13.7% | |
| secondary education | 264,529 | 18.4% | 488 | 13.4% | |
| 3+ years post-secondary education | 332,325 | 23.1% | 598 | 16.4% | |
| Post-graduate education | 25,086 | 1.7% | 37 | 1.0% | |
| Data missing | 3,616 | 0.3% | 41 | 1.1% | |
Descriptive statistics of offspring in each of the comparison group (Figure 1) is showed in Table 2. As can be seen from the Table all offspring has a mean grade that is negative. This is because offspring with cousins has a slightly lower mean value than offspring without cousins. Also, the mean school performance of unexposed offspring were not exactly the same, indicating that the baseline school performance in different family types were different. More specifically, children from extended families with only full cousins had considerably higher mean value of their grades compared to the other family groups. The education levels of the spouse of schizophrenia patients were similar across comparison groups.
Table 2.
Descriptive statistics of patients with schizophrenia, their relatives, and offspring in each comparison group.
| Relationship | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comparison groups | a. Paternal half-cousin |
b. Maternal half-cousin |
c. Full cousin | d. Paternal half-sibling |
e. Maternal half-sibling |
|||||
| Offspring exposure status | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No |
| Number of offspring | 352 | 629 | 265 | 407 | 2923 | 6179 | 427 | 505 | 488 | 597 |
| Mean offspring grades | −0.47 | −0.19 | −0.55 | −0.36 | −0.23 | −0.05 | −0.38 | −0.45 | −0.42 | −0.42 |
| - Confidence intervals) | −.57, −.37 | |
−0.27 | −0.11 |
−0.66 | −0.45 |
−0.45 | −0.27 |
−0.26 | −0.19 |
−0.07 | −0.02 |
−0.47 | −0.29 |
−0.53 | −0.36 |
−0.50 | −0.34 |
−0.51 | −0.34 |
| Mean education level of schizophrenic parents (CI) | 2.83 2.69 | 2.97 |
- | 2.65 2.52 | 2.79 |
- | 3.13 3.08 | 3.18 |
- | 2.80 2.67 | 2.92 |
- | 2.83 2.69 | 2.97 |
- |
| Mean education level of non-schizophrenic parents (CI) | 3.22 3.07 | 3.38 |
3.32 3.22 | 3.42 |
3.26 3.07 | 3.45 |
3.18 3.08 | 3.29 |
3.28 3.22 | 3.33 |
3.52 3.49 | 3.56 |
3.35 3.21 | 3.49 |
3.38 3.28 | 3.49 |
3.34 3.20 | 3.46 |
3.29 3.19 | 3.38 |
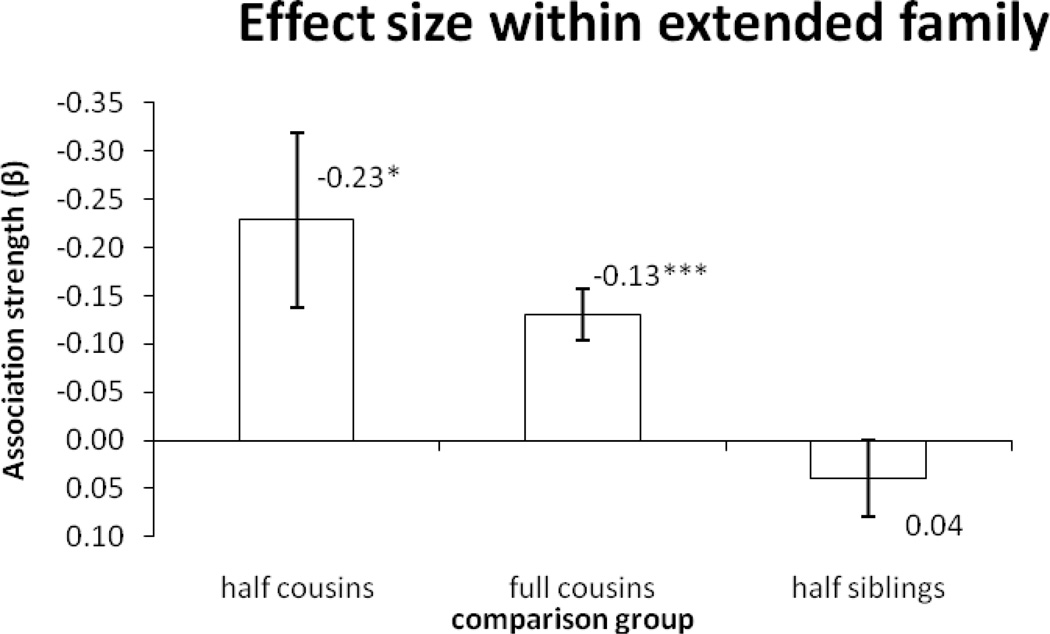
To investigate the effect of genetic factors in mediating the observed effect on offspring school performance, we stratified the analysis by genetic relatedness. HLM was used to adjust for covariates and the clustering of data so that the average effect of parental schizophrenia to offspring school performance (i.e, the within-family β coefficient) could be examined. These details of the results are showed in Appendix Table A2, and summarized in Figure 2. Within extended families, the association between parental schizophrenia and offspring school performance was strongest when comparing differentially exposed half-cousins (β=−0.23, p=0.01, N=65,126), followed by full cousins (β=−0.13, p<0.0001, N=917,678), and vanished when comparing half-siblings (β=0.04, p=0.31, N=203,506), indicating that offspring school performance became more similar as the genetic relatedness of offspring increased. Thus, the results suggest that change in genetic relatedness within extended families greatly moderates the association between parental schizophrenia and offspring school performance. A similar trend was also observed when offspring gender and/or parental education were not included as covariates (Appendix Table A3).
Figure 2.
Associations between parental schizophrenic disorder and offspring school performance. Comparison of half cousins, full cousins and half siblings.
Note: * p<0.05. *** p<0.0001. The estimates denote fixed effect sizes.
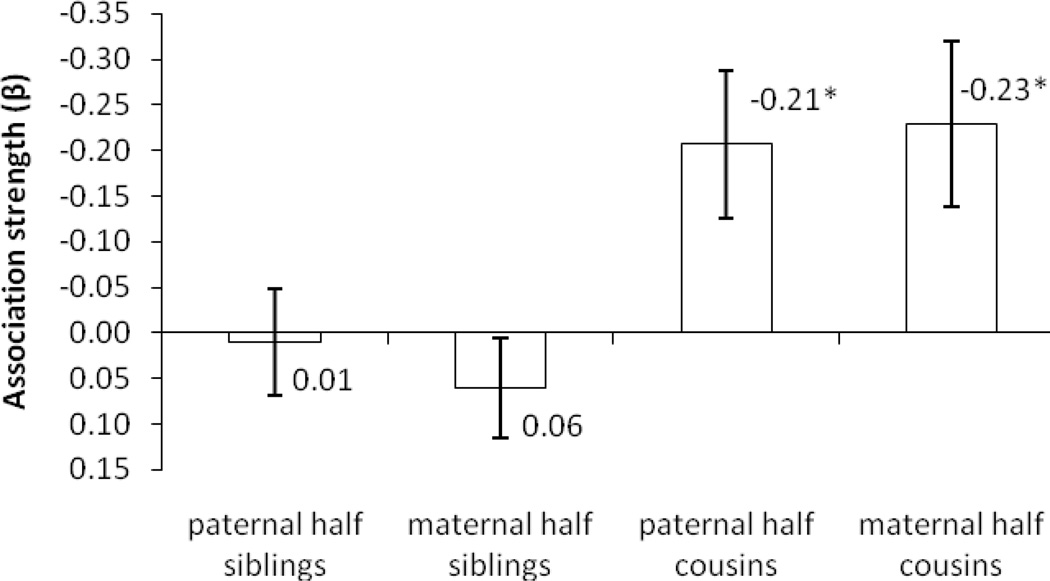
Next, we examined the effect of family environment on offspring school performance in two ways. (1) We compared the mean school performance between offspring with schizophrenic father and those with schizophrenic mother. If environment played an important role, the offspring with schizophrenic mother would have worse school performance than offspring with schizophrenic father. We found no significant difference (mean difference=0.015 SD, p=0.70, N=2739) (Appendix Table A4). (2) We stratified the association analysis according to offspring environmental similarity while controlling for genetic relatedness. It was achieved by comparing the strength of the associations (β coefficient) between paternal half-sibling and maternal half-sibling pairs and by comparing the association between paternal half-cousin and maternal half-cousin pairs (see Methods, statistical analysis). As showed in Figure 3, there was no significant association between schizophrenia in parents and school performance in offspring neither within differentially exposed paternal half-sibling (β=0.01, p=0.83, N=98,166) nor within differentially exposed maternal half-sibling pairs (β=0.06, p=0.27, N=105,340) (Appendix Table A5). The strength of the association was equal for paternal half-cousins (β=−0.21, p=0.01, N=68,371) and maternal half-cousin (β=−0.23, p=0.01, N=65,126) (Figure 3; Appendix Table A6). Thus, in all the comparisons where we investigated the effect of the family environment, the results suggested that variability in offspring environmental similarity did not affect the association strength when the genetic relatedness was held constant. Altogether, we found no evidence that family environment substantially affected offspring school performance, lending little support to the hypothesis that the family environment played an important role for the association.
Figure 3.
Association between parental schizophrenic disorder and offspring school performance. Comparison of paternal and maternal half cousins.
Note: * p<0.05. The estimates denote fixed effect sizes.
Table A6.
Hierarchical linear modelling of the effect of parental schizophrenia on offspring school performance in paternal and maternal half-cousin datasets.
| Model 1. Paternal half-cousin comparisona | |||
|---|---|---|---|
| Effect | Estimate | Standard error | P-value |
| Intercept | −1.08 | 0.015 | <.0001 |
| Exposure | |||
| within extended family | −0.21 | 0.081 | 0.010 |
| between extended family | −0.22 | 0.085 | 0.009 |
| Education | |||
| within extended family | 0.20 | 0.004 | <.0001 |
| between extended family | 0.28 | 0.003 | <.0001 |
| Gender | |||
| Male | −0.37 | 0.007 | <.0001 |
| Female | 0.00 | ||
| Model 2. Maternal half-cousin comparisonb | |||
| Intercept | −1.08 | 0.015 | <.0001 |
| Exposure | |||
| within extended family | −0.23 | 0.091 | 0.012 |
| between extended family | −0.25 | 0.097 | 0.011 |
| Education | |||
| within extended family | 0.18 | 0.004 | <.0001 |
| between extended family | 0.27 | 0.004 | <.0001 |
| Gender | |||
| Male | −0.37 | 0.007 | <.0001 |
| Female | 0.00 | ||
Note:
Based on the sample including 68,371offspring.
Based on the sample including 65,126 offspring.
Discussion
In agreement with previous studies, we found that offspring to patients with schizophrenia exhibited poorer school performance compared with offspring to parents with no diagnosis (Fisher et al. 1980; Janes et al. 1983; Ayalon & Merom, 1985). Importantly, we also studied the putative mechanisms for this association; our data suggests that the association between parental schizophrenia and poorer offspring academic performance is primarily due to genetic factors.
Parents could affect offspring behavior through environmental or genetic mechanisms. To disentangle the effects of genetic and environmental influences, we stratified the association analyses according to genetic and environmental relatedness. Genetic factors greatly moderated the association; the effect size of exposure to a schizophrenic parent on offspring school performance dropped from −0.23 to −0.13 when genetic relatedness increased from 6.25% in half-cousins to 12.5% in full cousins, and the effect disappeared when genetic relatedness reached 25% in half-sibling (Figure 2).Thus, there was strong evidence for genetic contributions to the association. However, environmental similarity between exposed offspring and unexposed offspring might increase together with genetic relatedness due to gene-environment correlation (Plomin, 1977). As a result, based on these results alone, we cannot exclude the possibility that the observed genetic effects were mediated by environmental effects.
To test if family environments were important, we stratified the analysis according to environmental relatedness while holding genetic relatedness constant. We found no significant effect neither in paternal half-siblings nor in maternal half-siblings, and the association strength was only marginally different between paternal half-cousins and maternal half-cousins. In addition, we found no significant difference in mean school performance between offspring to schizophrenic mother and offspring to schizophrenic father, despite that offspring to schizophrenic mothers were exposed to a more detrimental family environment. Altogether, these results gave little evidence for family environmental influences on the association, and supported the interpretation that the intergenerational association between parental schizophrenia and offspring academic performance was mainly mediated by genetic effects. An unexpected result was that the school performances in offspring to schizophrenia-discordant half-sibling were basically the same (β=0.04, p=0.31), when we would have expected poorer school performance among the offspring to the affected sibling. Even though the confidence intervals around these estimate are relatively wide and congruent with a true effect, the lack of effect have probably been influenced by the low performance in offspring to half-siblings compared to other types of offspring (Table 2). Assortive mating, known to exist among patients with schizophrenia (Parnas, 1988; Lichtenstein, 2006), could also influence the association. Thus, possible genetic effect could have been masked by assortative mating and the strong selection effects among half-siblings.
School performance is a complex trait determined by multiple environmental and genetic factors (Lemelin et al. 2007). It has previously been studied as a premorbid marker for later development of schizophrenia with inconsistent results. A Dutch twin study suggested that underperformance at school was one of the first prodromal signs of schizophrenia (Van Oel et al. 2002), and a Swedish study reported that poor school performance was strongly associated with risk of schizophrenia (MacCabe et al. 2008). In contrast, two Finnish studies found no association between poor school performance and schizophrenia development (Isohanni et al. 1998; Cannon et al. 1999). Our results suggest that academic underperformance of offspring to parents with schizophrenia, who are at higher risk of developing schizophrenia, are due primarily to genetic factors. Thus, poor school performance is one of the initial signs in the development of schizophrenia, and could possibly be considered an endophenotype in this process (Allen et al. 2009).
Our study had several strengths. Particularly, we employed a population-based design to reduce potential selection bias. We also used stratification within a quasi-experimental study design framework to pull apart presumably co-occurring genetic and environmental risks associated with poor offspring school performance. Our study also has some potential limitations. The prevalence of offspring with at least one parent with schizophrenia was only 0.25% in our study; lower than the 1% schizophrenia prevalence usually reported (Tandon et al. 2008). One possibility is that the fertility of schizophrenia patients is lower than for non-schizophrenic individuals (Nanko & Moridaira, 1993), and our use of a strict criterion for schizophrenia classification, requiring at least two separate inpatient episodes involving a schizophrenia diagnosis (Lichtenstein et al. 2006). In our study, we found decreasing associations with increasing genetic relatedness within extended families, suggesting genetic influences of the intergenerational association between parental schizophrenia and offspring school performance. However, it is not known whether the genetic effect was driven by those offspring that later would develop schizophrenia or could be attributed to the majority of the offspring. A long-term follow-up study would be necessary to elucidate this relationship. It has been observed that assortative mating exists among schizophrenia patients (Parnas, 1988; Lichtenstein, 2006). Assortative mating could overestimate the intergeneration association since the offspring might in that case be exposed to more environment and/or genetic exposure, even though it wouldn’t have changed the pattern of results. Our study design cannot prove that family environment have no effect on offspring school performance. Since we found little support to the hypothesis that environmental effects were important for the intergenerational transmission, however, the most probable interpretation was that the environment did not play a substantial role for poorer school performance in offspring to parents with schizophrenia. We used offspring graduating from compulsory school during two time periods (1988–1997 and 1998–2006) with different grade systems. We standardized school grades for each period with Blom transformation and assumed no major temporal trends. We tested if the association was similar in the two time periods, and found neither an effect of the time period nor an interaction effect between time period and parental schizophrenia on offspring school performance (p>.20; data not shown), thus validating our approach. Finally, offspring of schizophrenic parents did not represent all families with a schizophrenic individual in Sweden, because fewer schizophrenic compared to non-schizophrenic individuals have children. However, since parents with more severe symptoms (and likely stronger impact on offspring performance) are even less likely to have children, our estimate of the association between parental schizophrenia and offspring school performance was probably conservative.
Although most children of schizophrenic parents do not develop clinically significant cognitive problems, our results support genetically determined early deficits or impaired development of cognitive functioning in these children (Reichenberg et al. 2010). This could have practical implications for school, mental health, and social services. A substantiated decrease in cognitive performance during adolescence among children with a severely mentally ill parent or other family member might motivate assessment and monitoring also of other prodromal signs (Salokangas & McGlashan, 2008). Early detection of prodromal signs and impaired functioning followed by appropriate interventions could, for instance, include collaboration between child and adult psychiatric services, educational assistance in school, and family support from the social services.
Acknowledgments
This work was supported by NIH grant 061817-01A1, quasi-experimental studies of early risk factors for severe psychopathology. The authors wish to acknowledge Swedish Research Council, Swedish Council for Working Life and Social Research. The authors are grateful to Dr Chia Kee Seng for his helpful comments and suggestions on the manuscript.
Appendix
Table A1.
Regression analysis of the effect of parental schizophrenia on offspring school performance.
| Model 1: Crude model without adjusting for covariatesa | |||
|---|---|---|---|
| Effect | Estimate | Standard Error |
P-value |
| Intercept | 0.08 | 0.001 | <.0001 |
| Exposure | −0.31 | 0.019 | <.0001 |
| Model 2: Model adjusted for covariatesa | |||
| Intercept | 1.09 | 0.008 | <.0001 |
| Exposure | −0.18 | 0.017 | <.0001 |
| Gender | |||
| Male | −0.39 | 0.002 | <.0001 |
| Female | 0 | ||
| Family Education | |||
| <9 years of education | −1.25 | 0.010 | <.0001 |
| 9 years of education | −1.30 | 0.010 | <.0001 |
| 1–2 years upper secondary education | −1.14 | 0.009 | <.0001 |
| 3 year secondary education | −0.86 | 0.009 | <.0001 |
| less than 3 years post secondary education | −0.65 | 0.009 | <.0001 |
| 3+ years post-secondary education | −0.35 | 0.009 | <.0001 |
| post graduate education | 0 | ||
Note:
Based on the sample including 631,358 unrelated offspring.
Table A2.
Hierarchical linear modelling of the effect of parental schizophrenia on offspring school performance in half-cousin, full cousin and half-sibling datasets.
| Model 1. Half-cousin comparisona | |||
|---|---|---|---|
| Effect | Estimate | Standard Error | P-value |
| Intercept | −1.08 | 0.015 | <.0001 |
| Exposure | |||
| within extended family | −0.23 | 0.091 | 0.012 |
| between extended family | −0.25 | 0.097 | 0.011 |
| Education | |||
| within extended family | 0.18 | 0.004 | <.0001 |
| between extended family | 0.27 | 0.004 | <.0001 |
| Gender | |||
| Male | −0.37 | 0.007 | <.0001 |
| Female | 0.00 | ||
| Model 2. Full cousin comparison b | |||
| Intercept | −0.84 | 0.003 | <.0001 |
| Exposure | |||
| within extended family | −0.13 | 0.026 | <.0001 |
| between extended family | −0.24 | 0.029 | <.0001 |
| Education | |||
| within extended family | 0.19 | 0.001 | <.0001 |
| between extended family | 0.31 | 0.001 | <.0001 |
| Gender | |||
| Male | −0.40 | 0.002 | <.0001 |
| Female | 0 | ||
| Model 3. Half sibling-comparison c | |||
| Intercept | −1.91 | 0.009 | <.0001 |
| Exposure | |||
| within extended family | 0.04 | 0.040 | 0.310 |
| between extended family | −0.09 | 0.052 | 0.098 |
| Education | |||
| within extended family | 0.12 | 0.003 | <.0001 |
| between extended family | 0.28 | 0.002 | <.0001 |
| Gender | |||
| Male | −0.36 | 0.004 | <.0001 |
| Female | 0 | ||
Note:
Based on the sample including 65,126 offspring.
Based on the sample including 917,678 offspring.
Based on the sample including 203,506 offspring.
Table A3.
Hierarchical linear modelling of the effect of parental schizophrenia on offspring school performance in half-cousin, full cousin and half-sibling datasets using different combination of covariates.
| Model 1. Half-cousin comparisons | ||||||
|---|---|---|---|---|---|---|
| Covariates selections | ||||||
| No covariates | Gender | With interactiona | ||||
| Estimate | P-value | Estimate | P-value | Estimate | P-value | |
| Intercept | −0.22 | <.0001 | −0.77 | <.0001 | −1.81 | <.0001 |
| Exposure | ||||||
| within extended family | −0.29 | 0.002 | −0.29 | 0.002 | 0.17 | 0.561 |
| between extended family | −0.36 | 0.001 | −0.36 | 0.001 | −0.25 | 0.011 |
| Education | ||||||
| within extended family | - | - | - | - | 0.18 | 0.004 |
| between extended family | - | - | - | - | 0.27 | <.0001 |
| Gender | ||||||
| Male | - | - | −0.37 | <.0001 | −0.37 | <.0001 |
| Female | - | - | 0 | - | 0 | - |
| Interaction (offspring gender × parental schizophrenia status) | - | - | - | - | −0.27 | 0.153 |
| Model 2. Full-cousin comparison | ||||||
| Intercept | 0.07 | <.0001 | −0.51 | <.0001 | −1.64 | <.0001 |
| Exposure | ||||||
| within extended family | −0.22 | <.0001 | −0.21 | <.0001 | −0.01 | 0.921 |
| between extended family | −0.37 | <.0001 | −0.37 | <.0001 | −0.24 | <.0001 |
| Education | ||||||
| within extended family | - | - | - | - | 0.19 | <.0001 |
| between extended family | - | - | - | - | 0.31 | <.0001 |
| Gender | ||||||
| Male | - | - | −0.39 | <.0001 | −0.40 | <.0001 |
| Female | - | - | 0 | - | 0 | - |
| Interaction (offspring gender × parental schizophrenia status) | - | - | - | - | −0.08 | 0.145 |
| Model 3. Half-sibling comparison | ||||||
| Intercept | −0.31 | <.0001 | −0.84 | 0.006 | −1.89 | <.0001 |
| Exposure | ||||||
| within extended family | −0.01 | 0.844 | 0.00 | 0.972 | 0.02 | 0.888 |
| between extended family | −0.15 | <.0001 | −0.16 | <.0001 | −0.06 | 0.073 |
| Education | ||||||
| within extended family | - | - | - | - | 0.17 | <.0001 |
| between extended family | - | - | - | - | 0.31 | <.0001 |
| Gender | ||||||
| Male | - | - | −0.36 | <.0001 | − 0.36 | <.0001 |
| Female | - | - | 0 | - | 0 | - |
| Interaction (offspring gender × parental schizophrenia status) | - | - | - | - | 0.018 | 0.834 |
Note:
The model adjusted for offspring gender, parental education level and an interaction term with offspring gender and exposure status.
Table A4.
Comparison of school performance between offspring with a schizophrenic mother and offspring with a schizophrenic father, respectively.
| Number of offspring |
Mean school performance (SD) | Standard Error | |
|---|---|---|---|
| Offspring with schizophrenic mother |
1073 | −0.220 | 0.031 |
| Offspring with schizophrenic father |
1666 | −0.235 | 0.024 |
| Mean difference | 0.015 | ||
| p valuea | 0.70 | ||
| p value (adjusted)b | 0.76 | ||
Note:
t test.
Adjusted for offspring gender and parental education in linear regression.
Table A5.
Hierarchical linear modelling of the effect of parental schizophrenia on offspring school performance in paternal and maternal half-sibling datasets.
| Model 1. Paternal half-sibling comparisona | |||
|---|---|---|---|
| Effect | Estimate | Standard Error | P value |
| Intercept | −1.93 | 0.013 | <.0001 |
| Exposure | |||
| within extended family | 0.01 | 0.058 | 0.833 |
| between extended family | −0.13 | 0.072 | 0.073 |
| Education | |||
| within extended family | 0.13 | 0.004 | <.0001 |
| between extended family | 0.29 | 0.003 | <.0001 |
| Gender | |||
| Male | −0.36 | 0.006 | <.0001 |
| Female | 0 | ||
| Model 2. Maternal half-sibling comparisonb | |||
| Intercept | −1.88 | 0.013 | <.0001 |
| Exposure | |||
| within extended family | 0.06 | 0.055 | 0.268 |
| between extended family | −0.05 | 0.074 | 0.509 |
| Education | |||
| within extended family | 0.10 | 0.005 | <.0001 |
| between extended family | 0.26 | 0.002 | <.0001 |
| Gender | |||
| Male | −0.35 | 0.005 | <.0001 |
| Female | 0 | ||
Note:
Based on the sample including 98,166 offspring.
Based on the sample including 105,340 offspring.
Footnotes
Declaration of Interest None.
References
- Allen A, Griss M, Folley B, Hawkins K, Pearlson G. Endophenotypes in schizophrenia: a selective review. Schizophrenia Research. 2009;109:24–37. doi: 10.1016/j.schres.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon M, Merom H. The teacher interview. Schizophrenia Bulletin. 1985;11:117–120. doi: 10.1093/schbul/11.1.117. [DOI] [PubMed] [Google Scholar]
- Bearden C, Rosso I, Hollister J, Sanchez L, Hadley T, Cannon T. A prospective cohort study of childhood behavioral deviance and language abnormalities as predictors of adult schizophrenia. Schizophrenia Bulletin. 2000;26:395–410. doi: 10.1093/oxfordjournals.schbul.a033461. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones P, Huttunen M, Tanskanen A, Huttunen T, Rabe-Hesketh S, Murray R. School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Archives of General Psychiatry. 1999;56:457–463. doi: 10.1001/archpsyc.56.5.457. [DOI] [PubMed] [Google Scholar]
- Claudia B, Thomas A, Anne M. Gender inequalities in education. Annual Review of Sociology. 2008;34:319–337. [Google Scholar]
- D'Onofrio B, Goodnight J, Van Hulle C, Rodgers J, Rathouz P, Waldman I, Lahey B. A quasi-experimental analysis of the association between family income and offspring conduct problems. Journal of Abnormal Child Psychology. 2009a;37:415–429. doi: 10.1007/s10802-008-9280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio B, Goodnight J, Van Hulle C, Rodgers J, Rathouz P, Waldman I, Lahey B. Maternal age at childbirth and offspring disruptive behaviors: testing the causal hypothesis. Journal of Child Psychology and Psychiatry. 2009b;50:1018–1028. doi: 10.1111/j.1469-7610.2009.02068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio B, Turkheimer E, Eaves L, Corey L, Berg K, Solaas M, Emery R. The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. Journal of Child Psychology and Psychiatry. 2003;44:1130–1144. doi: 10.1111/1469-7610.00196. [DOI] [PubMed] [Google Scholar]
- David A, Malmberg A, Brandt L, Allebeck P, Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychological Medicine. 1997;27:1311–1323. doi: 10.1017/s0033291797005680. [DOI] [PubMed] [Google Scholar]
- Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. American Journal of Psychiatry. 1999;156:1328–1335. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- DeLisi L, Boccio A, Riordan H, Hoff A, Dorfman A, McClelland J, Kushner M, Van Eyl O, Oden N. Familial thyroid disease and delayed language development in first admission patients with schizophrenia. Psychiatry Research. 1991;38:39–50. doi: 10.1016/0165-1781(91)90051-p. [DOI] [PubMed] [Google Scholar]
- Education in the Swedish population. Reports on statistical co-ordination in Swedish. Örebro, Sweden: Statistics Sweden; 1993. [Google Scholar]
- Erlenmeyer-Kimling L, Cornblatt B. A summary of attentional findings in the New York High-Risk Project. Journal of Psychiatric. 1992;26:405–426. doi: 10.1016/0022-3956(92)90043-n. [DOI] [PubMed] [Google Scholar]
- Fakta om Den svenska familjen. Demografiska rapporter. Örebro, Sweden: Statistics Sweden; 1994. [Google Scholar]
- Fisher L, Kokes R, Harder D, Jones J. Child Competence and psychiatric risk. VI. Summary and integration of findings. Journal of Nervous and Mental Disease. 1980;168:353–355. doi: 10.1097/00005053-198006000-00006. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Morrison S. Schizophrenia and social class. British Journal of Psychiatry. 1963;109:785–802. doi: 10.1192/bjp.109.463.785. [DOI] [PubMed] [Google Scholar]
- Gottesman I, Bertelsen A. Confirming unexpressed genotypes for schizophrenia. Risks in the offspring of Fischer's Danish identical and fraternal discordant twins. Archives of General Psychiatry. 1989;46:867–872. doi: 10.1001/archpsyc.1989.01810100009002. [DOI] [PubMed] [Google Scholar]
- Harden K, Lynch S, Turkheimer E, Emery R, D'Onofrio B, Slutske W, Waldron M, Statham D, Martin N. A behavior genetic investigation of adolescent motherhood and offspring mental health problems. Journal of Abnormal Psychology. 2007;116:667–683. doi: 10.1037/0021-843X.116.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth C, Dale P, Plomin R. A Twin Study into the Genetic and Environmental Influences on Academic Performance in Science in nine-year-old Boys and Girls. International Journal of Science Education. 2008;30:1003–1025. doi: 10.1080/09500690701324190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isohanni I, Järvelin M, Nieminen P, Jones P, Rantakallio P, Jokelainen J, Isohanni M. School performance as a predictor of psychiatric hospitalization in adult life. A 28-year follow-up in the Northern Finland 1966 Birth Cohort. Psychological Medicine. 1998;28:967–974. doi: 10.1017/s0033291798006928. [DOI] [PubMed] [Google Scholar]
- Jaffee S, Price T. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12:432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes C, Weeks D, Worland J. School behavior in adolescent children of parents with mental disorder. Journal of Nervous and Mental Disease. 1983;171:234–240. doi: 10.1097/00005053-198304000-00005. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Kate F. Motherhood, fatherhood. The legal balance. Family Matters. 1991;30:34–37. [Google Scholar]
- Kim HJ. Family resources and children’s academic performance. Children and Youth Services Review. 2004;6:529–536. [Google Scholar]
- Kremen W, Buka S, Seidman L, Goldstein J, Koren D, Tsuang M. IQ decline during childhood and adult psychotic symptoms in a community sample: a 19-year longitudinal study. American Journal of Psychiatry. 1998;155:672–677. doi: 10.1176/ajp.155.5.672. [DOI] [PubMed] [Google Scholar]
- Lambe M, Hultman C, Torrång A, Maccabe J, Cnattingius S. Maternal smoking during pregnancy and school performance at age 15. Epidemiology. 2006;17:524–530. doi: 10.1097/01.ede.0000231561.49208.be. [DOI] [PubMed] [Google Scholar]
- Lemelin J, Boivin M, Forget-Dubois N, Dionne G, Séguin J, Brendgen M, Vitaro F, Tremblay R, Pérusse D. The genetic-environmental etiology of cognitive school readiness and later academic achievement in early childhood. Child Development. 2007;78:1855–1869. doi: 10.1111/j.1467-8624.2007.01103.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Björk C, Hultman C, Scolnick E, Sklar P, Sullivan P. Recurrence risks for schizophrenia in a Swedish national cohort. Psychological Medicine. 2006;36:1417–1425. doi: 10.1017/S0033291706008385. [DOI] [PubMed] [Google Scholar]
- Lifshitz M, Kugelmass S, Karov M. Perceptual-motor and memory performance of high-risk children. Schizophrenia Bulletin. 1985;11:74–84. doi: 10.1093/schbul/11.1.74. [DOI] [PubMed] [Google Scholar]
- Littell R, Milliken G, Stroup W, Wolfinger R, Schnabenberger O. SAS for mixed models. SAS Press; 2006. [Google Scholar]
- MacCabe J, Lambe M, Cnattingius S, Torrång A, Björk C, Sham P, David A, Murray R, Hultman C. Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Psychological Medicine. 2008;38:1133–1140. doi: 10.1017/S0033291707002048. [DOI] [PubMed] [Google Scholar]
- McDonald C, Murphy K. The new genetics of schizophrenia. Psychiatric Clinics of North America. 2003;26:41–63. doi: 10.1016/s0193-953x(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Multi-generation Register. A description of contents and quality. Örebro, Sweden: Statistics Sweden; 2003. [Google Scholar]
- Nanko S, Moridaira J. Reproductive rates in schizophrenic outpatients. Acta Psychiatrica Scandinavica. 1993;87:400–404. doi: 10.1111/j.1600-0447.1993.tb03395.x. [DOI] [PubMed] [Google Scholar]
- Niemi L, Suvisaari J, Tuulio-Henriksson A, Lönnqvist J. Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophrenia Research. 2003;60:239–258. doi: 10.1016/s0920-9964(02)00234-7. [DOI] [PubMed] [Google Scholar]
- Persson L, Andersson G. Human Fertility Database Documentation:Sweden. Sweden: Statistics Sweden; 2010. [Google Scholar]
- Parnas J. Assortative mating in schizophrenia: results from the Copenhagen High-Risk Study. Psychiatry. 1988;51:58–64. doi: 10.1080/00332747.1988.11024380. [DOI] [PubMed] [Google Scholar]
- Persson L, Andersson G. Human fertility database documentation:Sweden. Sweden: Statistics Sweden; 2010. [Google Scholar]
- Plomin R, DeFries J, Loehlin J. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Raudenbush S, Bry A. Hierarchical Linear Models – Applications and Data Analysis Methods. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe R, Murray R, Poulton R, Moffitt T. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. American Journal of Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Pickles A, Murray R, Eaves L. Testing hypotheses on specific environmental causal effects on behavior. Psychological Bulletin. 2001;127:291–324. doi: 10.1037/0033-2909.127.3.291. [DOI] [PubMed] [Google Scholar]
- Salokangas R, McGlashan T. Early detection and intervention of psychosis. A review. Nordic Journal of Psychiatry. 2008;62:92–105. doi: 10.1080/08039480801984008. [DOI] [PubMed] [Google Scholar]
- Sohlberg S. Personality and neuropsychological performance of high-risk children. Schizophrenia Bulletin. 1985;11:48–60. doi: 10.1093/schbul/11.1.48. [DOI] [PubMed] [Google Scholar]
- Tandon R, Keshavan M, Nasrallah H. Schizophrenia, "just the facts" what we know in 2008. 2. Epidemiology and etiology. Schizophrenia Research. 2008;102:1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah H, Keshavan M. Schizophrenia, "just the facts" 4. Clinical features and conceptualization. Schizophrenia Research. 2009;110:1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- The Swedish Hospital Discharge Register. Stockholm: National Board of Health and Welfare, Centre for Epidemiology. 2005. [Accessed June]. http://www.sos.se/epc/english/ParEng.htm#Publications. [Google Scholar]
- Van den Oord E, Simonoff E, Eaves L, Pickles A, Silberg J, Maes H. An evaluation of different approaches for behavior genetic analyses with psychiatric symptom scores. Behavior Genetics. 2000;30:1–18. doi: 10.1023/a:1002095608946. [DOI] [PubMed] [Google Scholar]
- Van Oel C, Sitskoorn M, Cremer M, Kahn R. School performance as a premorbid marker for schizophrenia: a twin study. Schizophrenia Bulletin. 2002;28:401–414. doi: 10.1093/oxfordjournals.schbul.a006949. [DOI] [PubMed] [Google Scholar]
- Walker E, Grimes K, Davis D, Smith A. Childhood precursors of schizophrenia: facial expressions of emotion. American Journal of Psychiatry. 1993;150:1654–1660. doi: 10.1176/ajp.150.11.1654. [DOI] [PubMed] [Google Scholar]