Abstract
Pseudoangiomatous stromal hyperplasia (PASH) is a benign mesenchymal proliferative lesion of the breast. In 2005, only 109 cases had been reported since its initial description in 1986 by Vuitch et al. Our 24 cases represent one of the largest series to be reported from a single institution. We retrospectively reviewed data from 2004 to 2010 of patients diagnosed with PASH by surgical excision or image-guided biopsy. All pathological specimens were reviewed by a single pathologist. The samples were stained for estrogen and progesterone receptors (ER and PR), CD34, and the lymphatic marker D2-40. All but one of 24 (96%) patients presented with breast masses either on imaging or clinically. Fourteen of the 24 patients (58%) were diagnosed on surgical excision, 10 (42%) diagnosed with core needle biopsy, and five (20%) were diagnosed using both techniques. The tumors ranged in size from 0.3 cm to 7.0 cm. All women except two were premenopausal or perimenopausal at diagnosis. Nineteen samples were available for hormonal receptor staining and of these 18 of 19 (95%) were ER or PR positive. PASH was diagnosed in two men, a transgender male on hormones and the other with gynecomastia. The patients’ ages ranged from 18 to 86 years old. In addition to PASH other benign histopathological findings include stromal fibrosis and atypical ductal or lobular hyperplasia. Imaging revealed no distinguishing feature for PASH with benign histology. One patient had synchronous ductal carcinoma in-situ (DCIS). Patients were treated with local excision or observation. This study suggests that PASH is primarily a diagnosis of premenopausal and perimenopausal women. Our series supports a hormonal basis for its development due to the positive staining for hormonal receptors. Management is conservative surgery for larger masses with careful observation being an option in patients not at high risk for breast cancer.
Keywords: breast tumor, PASH, pseudoangiomatous, stromal hyperplasia
Pseudoangiomatous stromal hyperplasia (PASH) is a benign mesenchymal proliferative lesion of the breast that may present clinically as a mass and, from a histopathological point of view must be differentiated from low-grade angiosarcoma and phyllodes tumors. Although its prevalence is difficult to accurately estimate, tumor-forming PASH is a rather uncommon breast lesion and less than 150 cases of tumor-forming PASH were reported from 1986, when the lesion was originally described by Vuitch et al. (1), until 2007 (2). In contrast, focal, non-tumor-forming PASH may be an incidental microscopic finding in up to 23% of breast biopsies (3).
Initially thought to be variants of mammary hamartomas (4), PASH tumors are currently regarded as a benign proliferation of stromal myofibroblasts, which express CD34, vimentin, and at least focally smooth muscle actin, desmin, and bcl-2, but not endothelial markers (CD31, Factor VIII), S100 or cytokeratin. The clinicopathological spectrum of PASH ranges from focal, incidental microscopic findings to clinically and mammographically evident breast masses. It is characterized histologically by interanastamosing angulated and slit-like spaces lined by slender spindle cells and surrounded by dense collagenous stroma. The slits lined by myofribroblastic cells are probably a fixation artifact induced by the retraction of the collagenous stroma, but, although devoid of red blood cells, these slit-like spaces are apt to be mistaken for vascular spaces, hence the potential misdiagnosis as low-grade angiosarcoma. Tumor-forming PASH occurs predominantly in premenopausal women and usually presents clinically as a palpable, mobile, firm, painless intramammary mass. However, occasional cases have been described in postmenopausal women, men, adolescents, and even in pediatric patients (5). Mammographic, ultrasonographic and clinical findings most often lead to a diagnosis of fibroadenoma (6–8).
The pathogenesis is unclear and most recently, the literature postulates that hormonal factors play a role in the development of PASH (9–11). Through this study we hope to add our institutional experience with PASH to the small number of larger case series (2,12–14) reported in the literature to better define the pathological, clinical, and therapeutic approaches to PASH.
METHODS
With the approval of the Investigational Review Boards the surgical pathology data base was searched for all cases diagnosed as PASH on either surgical excisions or image-guided biopsies from 2004 to 2010. Patients’ medical records were retrospectively reviewed for information regarding the patient’s demographics, history of contraceptive use, and/or hormonal replacement therapy, personal and family history of cancer, presentation, clinical, imaging and pathological diagnoses, and treatment. Clinical follow-up, imaging and pathological findings were also recorded, where available. Mammographic and ultrasound imaging were reviewed for all patients in the study.
All hematoxylin and eosin-stained sections from all cases identified were reviewed by a single pathologist with interest in breast pathology. The diagnosis of PASH was confirmed and, when present, coexistent epithelial changes were noted and classified as proliferative and non-proliferative fibrocystic changes, gynecomastia-like changes, columnar cell changes, atypical hyperplasia, and in situ or invasive ductal or lobular carcinoma. Cases with sufficient tissue remaining in the paraffin blocks were immunostained with a panel of antibodies directed against ER (clone SP1), PR (clone 1E2), CD34 (clone QBEND-10), all from Ventana Medical Systems, Inc., Tucson, Arizona and against D2-40 (clone D2-40, Biocare, Concord, California), to identify lymphatic endothelium. All immunoperoxidase stains were performed on a Ventana NexES® automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ) with adequate positive and negative controls.
All patients’ identifiers were kept confidential.
RESULTS
Twenty-four patients, 22 women and 2 men with a pathological diagnosis of PASH were identified in the study period; all diagnoses were confirmed on review.
All but one of 24 (96%) patients presented with breast masses either on imaging or clinically. One patient presented with masses in both breasts and one axilla. Fourteen of the 24 patients (58%) were diagnosed on surgical excision of a breast mass, 10 (42%) were diagnosed with core needle biopsy and five (20%) were diagnosed with both techniques. The tumors ranged in size from 0.6 cm to 7.0 cm with the smallest tumor occurring in an 86-year-old woman, Figure 1. All but two women were premenopausal or perimenopausal at diagnosis and the two men were 43 and 46 years old. The patients’ ages ranged from 18 to 86 years as shown in Figure 2. On presentation, breast masses were more prevalent on the left side than on the right or both breasts (62.5%, 25%, and 12.5%, respectively). One third of the patients presented with pain or focal tenderness in the affected breast. Two patients (8%) presented with non-bloody nipple discharge.
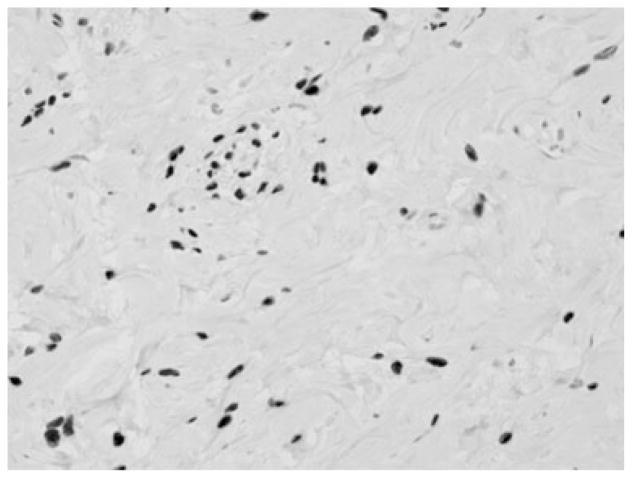
Figure 1.
PASH: ER positive nuclear staining of stromal cells lining the slit-like spaces (magnification ×40).
Figure 2.
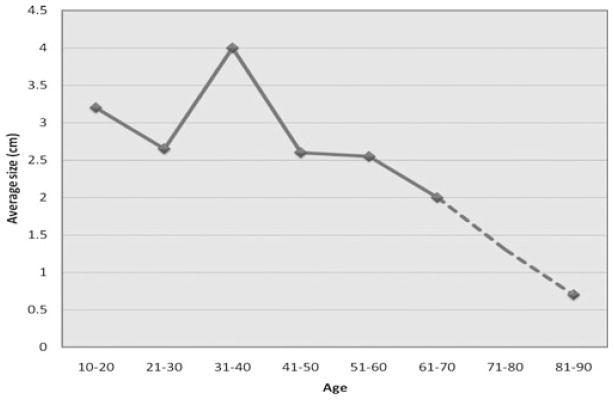
Average tumor size relative to patients’ ages.
Twenty-three of the 24 patients had mammograms and one patient 30 years old had only ultrasound available for review. A total of 27 lesions were seen on these studies. Imaging revealed most masses to be isodense and oval or round (12), followed by asymmetry (6), no visualization due to dense parenchyma (4), architectural distortion (1), and complex fluid collection (1). On mammography there were varied histological features, although none suggested malignancy. Ultrasound likewise revealed most masses to be homogeneous and oval or round (10) and heterogeneous oval or round (5) with well circumscribed margins. There were 8 patients with follow-up images ranging from 6 to 60 months that were found to have stable masses and 10 patients had no follow-up. Two patients had changes in the size of their masses: 1.9–2.9 in 4 years and 1.4–3.8 in 8 years. The 10 patients’ lack of follow-up was due to either complete excision or failure to return.
On gross evaluation, the tissue was primarily well circumscribed, rubbery, and tan-yellow in color with evidence of surrounding fibrofatty tissue. Pathology from each case reviewed was significant for findings of PASH as well as another benign entity, most commonly stromal fibrosis and benign proliferative disease; one case was associated with atypical ductal hyperplasia (ADH) and two with atypical lobular hyperplasia (ALH). In one patient, the tumoral PASH was associated with multiple foci of DCIS- grade3, with comedo, solid, cribiform, and macropapillary growth pattern.
Although the majority of the patients were treated with surgical excision, many were treated with close monitoring and repeat mammography 6 months after diagnosis. On follow-up of mammograms after surgical excision, there was no evidence of recurrence of PASH. Family history of 20 female patients revealed that 40% had a family history of breast cancer. Three of the four (75%) patients with high risk pathology (ADH, ALH, or DCIS) are included in the group of women with a family history of breast cancer (Table 1). Nineteen of the 22 female samples had enough available tissue and underwent immunohistochemical (IHC) staining. IHC stains were positive for ER (Fig. 1) and PR receptors within the stromal cells lining the slit-like spaces in 95% of samples assessed (Table 2). The cells lining the slit-like spaces characteristic of PASH were all confirmed to be stromal myofibrobasts since all samples stained were positive for CD34. The D2-40 stain, which indicates the presence of lymphatic endothelium was negative in 17/18 (94%) of samples.
Table 1.
Associated Malignancy Potential. Patients Presenting with PASH and at High Risks for Developing Malignant Lesion
| Risk | Patients (n) |
|---|---|
| ADH | 2 (1) |
| ALH | 1 (<1) |
| DCIS | 1 (<1) |
| Family history (n = 20) | |
| Breast cancer positive | 8 (40) |
| Breast cancer negative | 12 (60) |
Values within parenthesis are expressed in percentage.
ADH, atypical ductal hyperplasia; ALH, atypical lobular hyperplasia; PASH, Pseudoangiomatous stromal hyperplasia.
Table 2.
IHC stain for ER, PR, and D2-40
| Stain | Number of cases with no staining in stroma (%) | Number of cases with positive staining in stroma
|
Total number of cases | ||
|---|---|---|---|---|---|
| Occasional (%) | Focally frequent (%) | Frequent (%) | |||
| ER | 4 (22.2) | 10 (55.6) | 1 (5.6) | 3 (16.7) | 18 |
| PR | 7 (36.8) | 5 (26.3) | 2 (10.5) | 5 (26.3) | 19 |
| D2-40 | 17 (94.4) | 1 (5.6) | 18 | ||
ER, estrogen receptors; PR, progesterone receptors.
DISCUSSION
Pseudoangiomatous stromal hyperplasia is a rare but benign breast lesion presenting mostly in premenopausal women. It may be found incidentally in routine biopsies performed for benign or malignant diseases of the breast. The presentation of masses in all of our patients is quite rare based on our literature review. This may be due to the fact that pathologists in our institution did not diagnose PASH when benign or malignant lesions may have explained the mass lesion. Another rather unusual finding of this study is the diagnosis of PASH in two men. One male patient was transgender and receiving exogenous hormones at the time of diagnosis and the other male patient had gynecomastia. Gynecomastia has been found to be associated with microscopic foci of PASH in a relatively high proportion of cases. These findings provide additional evidence that an aberrant reactivity of myofibroblasts to endogenous or exogenous hormones is probably an important factor in the development of PASH. The contention that elevated hormonal levels may contribute to the development of PASH is also supported by the fact that the PASH occurred mostly in hormonally active, premenopausal and perimenopausal women (Fig. 2). Another argument for the contribution of estrogen and progesterone to the etiology of PASH is the fact that the smallest PASH masses were seen in menopausal women and the largest ones were in women expected to be at the height of hormonal activity (Fig. 3). It is of note that 95% of the PASH tumors in our study stained positive for ER and/or PR receptors. Most of the reported cases in the literature demonstrate the presence of progesterone receptor activity and weak to no estrogen receptor activity (15–17). As the previous studies on estrogen reactivity were performed in the 1990s, it is likely that the fact that we found at least focal estrogen expression in the majority of cases of PASH tested may be explained by improved tissue fixation times, more sensitive antibodies and use of a standardized, automated testing platform. The literature also reports PASH in the setting of post-menopausal women on hormone replacement therapy (1,15) as well as men with gynecomastia (18,19), which also supports a hormonal basis to these lesions. In addition, there are reports of at least partial response of large or symptomatic PASH to hormonal manipulation (16) or tamoxifen therapy (9,10).
Figure 3.
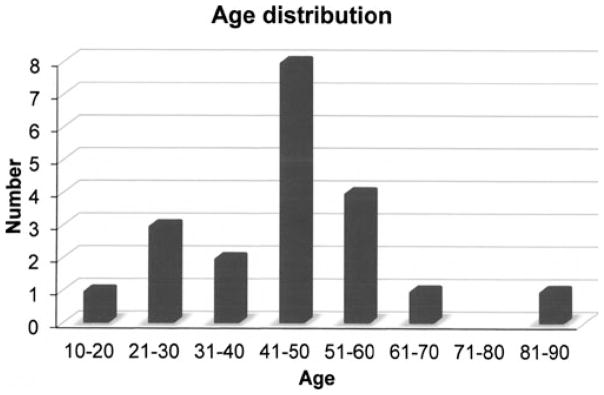
PASH presenation by age.
The imaging of these PASH lesions on mammography and ultrasound indicated that most presented as oval or round well circumscribed masses. The masses were usually homogeneous, although some were heterogeneous without features of malignancy. The lesion with DCIS had a distinguishing feature that was asymmetry with amorphous calcifications. All the other patients with high-risk lesion associated with their PASH had no imaging features that would distinguish them from benignity.
For those patients with family histories of breast cancers 75% of their relatives were first degree and 25% were second degree. All first degree relatives were maternally related and of the two second degree relatives, one was maternally related and the other had no record as to whether it was a maternal or paternal relative with only ‘grandmother’ being listed.
Although PASH is a benign entity it must be histopathologically differentiated from low-grade angiosarcoma (Fig. 4), with which it can be confused, on low magnification, because the slit-like channels lined by spindle characteristic of PASH may resemble endothelial cells (1). Angiosarcomas are rare malignant tumors that arise from endothelial cells lining vascular channels and the breast is one of the most common sites of its occurrence. When presenting in younger premenopausal women they usually form palpable masses (20) and require histopathological differentiation from PASH. Low-grade angiosarcoma is identified by the presence of anastomosing vascular channels containing red blood cells that invade the surrounding breast tissue and are not associated with a collageneous stroma. The treatment of angiosarcoma is mastectomy, although the literature indicates that breast conserving therapy for small, grade one primary tumors may be appropriate (20). In addition, chemotherapy may reduce the local recurrence rate (20). The differentiation of the two entities is based on histology with IHC staining support for CD31 and Factor VIII, angiosarcoma being CD31 and Factor VIII positive, whereas PASH is negative for these antibodies.
Figure 4.
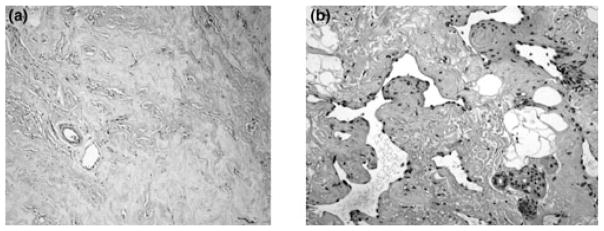
Pathological comparisons between PASH and angiosarcoma. (a) PASH. Interlobular stromal expansion of irregular spacing of the mammary lobules. Slit-like spaces which are angulated and often anastomosing. There are no erythrocytes and cells are lined by attenuated myofibroblasts in a background of hyalinized stroma.(Mag. 10X). (b) Low-grade angiosarcoma. Slit spaces contain erythrocytes and the nuclei of the lining cells are malignant. There is hyalinization of stroma in this tumor.
In our study we reported positivity for CD34 in all cases of PASH examined which is consistent with the findings in the literature and supports the myofibroblastic stromal origin of these lesions. In contrast, we found D2-40 staining in only one of the cases examined, which further supports the theory that these lesions are not variants of lymphangiomas and the lining cells are not ‘attenuated lymphatic endothelial cells’ (21). The one specimen that stained weakly positive for D2-40 was otherwise pathologically consistent with PASH. To our knowledge this is the first study to use the lymphatic channels marker to study PASH lesions.
All patients in this series that showed high risk (atypia) or premalignant epithelial lesions (DCIS) in addition to PASH had family history of breast cancer. As noted in Table 1, 40% (8/20) of women with PASH for whom data were available had a family history of breast cancer and 38% (3/8) of them had PASH lesions associated with high risk or premalignant lesions. The one high-risk patient with no associated family history had atypical lobular hyperplasia.
Based on the reports in the literature, if the diagnosis of PASH is made on core biopsy, surgical excision may not be indicated and close observation with serial mammography to assess interval growth is adequate treatment. It was recommended by the surgeon that lesions greater than 2 cm be excised otherwise it was the decision of the patient to have the benign tumor removed from her breast or followed up with observation. To date, there is only one reported case suggesting a malignant transformation of a PASH lesion (22) and only rare cases have been reported where PASH was associated with malignancy (12) or DCIS, as we found in one of the patients included in this series. The presence of such neoplastic epithelial lesions in association with PASH may be coincidental as a large study showed that the risk of developing cancer is actually smaller in women with PASH than in those without PASH (13).
This study suggests that PASH is primarily a diagnosis of premenopausal and perimenopausal women. There is strong clinical evidence that there is a hormonal basis for the development of PASH. Patients treated with conservative surgery or observations seem to do very well without increased risk of malignancy. However, there is some cause for caution in patients that have a strong family history of breast cancer and we suggest excision as opposed to observation for this group of patients.
References
- 1.Vuitch MF, Rosen PP, Erlandson RA. Pseudoangiomatous hyperplasia of mammary stroma. Hum Pathol. 1986;17:185–91. doi: 10.1016/s0046-8177(86)80292-1. [DOI] [PubMed] [Google Scholar]
- 2.Wieman SM, Landercasper J, Johnson JM, et al. Tumoral pseudoangiomatous stromal hyperplasia of the breast. Am Surg. 2008;74:1211–4. [PubMed] [Google Scholar]
- 3.Ibrahim RE, Sciotto CG, Weidner N. Pseudoangiomatous hyperplasia of mammary stroma. Some observations regarding its clinicopathologic spectrum. Cancer. 1989;63:1154–60. doi: 10.1002/1097-0142(19890315)63:6<1154::aid-cncr2820630619>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 4.Fisher CJ, Hanby AM, Robinson L, Millis RR. Mammary hamartoma – a review of 35 cases. Histopathology. 1992;20:99–106. doi: 10.1111/j.1365-2559.1992.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 5.Shehata BM, Fishman I, Collings MH, et al. Pseudoangiomatous stromal hyperplasia of the breast in pediatric patients: an underrecognized entity. Pediatr Dev Pathol. 2009;12:450–4. doi: 10.2350/08-09-0528.1. [DOI] [PubMed] [Google Scholar]
- 6.Hargaden GC, Yeh ED, Georgian-Smith D, et al. Analysis of the mammographic and sonographic features of pseudoangiomatous stromal hyperplasia. AJR Am J Roentgenol. 2008;191:359–63. doi: 10.2214/AJR.07.2479. [DOI] [PubMed] [Google Scholar]
- 7.Celliers L, Wong DD, Bourke A. Pseudoangiomatous stromal hyperplasia: a study of the mammographic and sonographic features. Clin Radiol. 2010;65:145–9. doi: 10.1016/j.crad.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Jones KN, Glazebrook KN, Reynolds C. Pseudoangiomatous stromal hyperplasia: imaging findings with pathologic and clinical correlation. AJR Am J Roentgenol. 2010;195:1036–42. doi: 10.2214/AJR.09.3284. [DOI] [PubMed] [Google Scholar]
- 9.Pruthi S, Reynolds C, Johnson RE, Gisvold JJ. Tamoxifen in the management of pseudoangiomatous stromal hyperplasia. Breast J. 2001;7:434–9. doi: 10.1046/j.1524-4741.2001.07611.x. [DOI] [PubMed] [Google Scholar]
- 10.Seltzer MH, Kintiroglou M. Pseudoangiomatous hyperplasia and response to tamoxifen therapy. Breast J. 2003;9:344. doi: 10.1046/j.1524-4741.2003.09426.x. [DOI] [PubMed] [Google Scholar]
- 11.AbdullGaffar B. Pseudoangiomatous stromal hyperplasia of the breast. Arch Pathol Lab Med. 2009;133:1335–8. doi: 10.5858/133.8.1335. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira M, Albarracin CT, Resetkova E. Pseudoangiomatous stromal hyperplasia tumor: a clinical, radiologic and pathologic study of 26 cases. Mod Pathol. 2008;21:201–7. doi: 10.1038/modpathol.3801003. [DOI] [PubMed] [Google Scholar]
- 13.Degnim AC, Frost MH, Radisky DC, et al. Pseudoangiomatous stromal hyperplasia and breast cancer risk. Ann Surg Oncol. 2010;17:3269–77. doi: 10.1245/s10434-010-1170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gresik CM, Godellas C, Aranha GV, Rajan P, Shoup M. Pseudoangiomatous stromal hyperplasia of the breast: a contemporary approach to its clinical and radiologic features and ideal management. Surgery. 2010;148:752–7. doi: 10.1016/j.surg.2010.07.020. discussion 757–8. [DOI] [PubMed] [Google Scholar]
- 15.Anderson C, Ricci A, Jr, Pedersen CA, Cartun RW. Immunocytochemical analysis of estrogen and progesterone receptors in benign stromal lesions of the breast. Evidence for hormonal etiology in pseudoangiomatous hyperplasia of mammary stroma. Am J Surg Pathol. 1991;15:145–9. doi: 10.1097/00000478-199102000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Powell CM, Cranor ML, Rosen PP. Pseudoangiomatous stromal hyperplasia (PASH). A mammary stromal tumor with myofibroblastic differentiation. Am J Surg Pathol. 1995;19:270–7. doi: 10.1097/00000478-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Zanella M, Falconieri G, Lamovec J, Bittesini L. Pseudoangiomatous hyperplasia of the mammary stroma: true entity or phenotype? Pathol Res Pract. 1998;194:535–40. doi: 10.1016/S0344-0338(98)80042-3. [DOI] [PubMed] [Google Scholar]
- 18.Badve S, Sloane JP. Pseudoangiomatous hyperplasia of male breast. Histopathology. 1995;26:463–6. doi: 10.1111/j.1365-2559.1995.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 19.Milanezi MF, Saggioro FP, Zanati SG, Bazan R, Schmitt FC. Pseudoangiomatous hyperplasia of mammary stroma associated with gynaecomastia. J Clin Pathol. 1998;51:204–6. doi: 10.1136/jcp.51.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR Am J Roentgenol. 2008;190:533–8. doi: 10.2214/AJR.07.2909. [DOI] [PubMed] [Google Scholar]
- 21.Damiani S, Eusebi V, Peterse JL. Malignant neoplasms infiltrating pseudoangiomatous’ stromal hyperplasia of the breast: an unrecognized pathway of tumour spread. Histopathology. 2002;41:208–15. doi: 10.1046/j.1365-2559.2002.01443.x. [DOI] [PubMed] [Google Scholar]
- 22.Nassar H, Elieff MP, Kronz JD, Argani P. Pseudoangiomatous stromal hyperplasia (PASH) of the breast with foci of morphologic malignancy: a case of pash with malignant transformation? Int J Surg Pathol. 2008;18:564–9. doi: 10.1177/1066896908320835. [DOI] [PubMed] [Google Scholar]