Abstract
Aims
To assess long-term safety and compare neurodevelopmental outcomes in school age children born prematurely who received inhaled nitric oxide or placebo during the first week of life in a randomized, double blind study. Children treated with inhaled nitric oxide had previously been shown to have decreased intraventricular hemorrhage and periventricular leukomalacia as newborns, and decreased cognitive impairment at 2 years1–2.
Methods
Follow-up study of medical outcomes, neurodevelopmental assessment and school readiness in 135 of 167 (81%) surviving premature infants seen at 5.7 ± 1.0 years.
Results
Compared to placebo (n=65), iNO-treated children (n=70) demonstrated no difference in growth parameters, school readiness, or need for subsequent hospitalization. However, iNO-treated children were less likely to have multiple chronic morbidities or technology-dependence (p=0.05). iNO-treated children also had less functional disability (p=0.05).
Conclusions
These results demonstrate the long-term safety of iNO in premature infants. Furthermore, iNO treatment may improve health status by decreasing the incidence of severe ongoing morbidities and technology-dependence, and may also decrease the incidence of educational and community functional disability of premature infants at early school age.
Trial Registration
clinicaltrials.gov Identifier: NCT00152542
Keywords: Nitric oxide inhalation, Prematurity, School-age outcome, Safety
Introduction
With advances in neonatal intensive care medicine, critically ill premature infants born with respiratory distress syndrome (RDS) increasingly survive to school-age and beyond. With this improved survival, there has been an increase in neurodevelopmental impairment and ongoing morbidities.1
In a randomized, double blind, placebo-controlled trial, we previously reported that inhaled nitric oxide (iNO) treatment of premature infants having moderate RDS decreased the incidence of death and chronic lung disease (CLD), as well as decreasing severe (Grades III–IV) intraventricular hemorrhage and periventricular leukomalacia (IVH/PVL).2 At two years corrected age, these iNO-treated infants had improved neurodevelopmental outcomes, primarily due to improved cognitive ability3. After adjusting for neonatal morbidities known to affect neurodevelopmental outcome such as CLD and severe IVH/PVL, improved neurodevelopment in the iNO-treated group persisted. Thus, iNO appears to be neuro-protective in preterm infants with RDS.
We evaluated the long-term safety at school-age in children who have received iNO as premature infants. We also assessed whether the beneficial effects of iNO therapy persisted at school age, using our previously reported multi-dimensional measure of school readiness.
Patients and Methods
Initial Study
The initial study was a randomized, placebo-controlled, single center study of 207 premature infants with RDS requiring mechanical ventilation and surfactant therapy. Treatment with iNO was initiated at a dose of 10 ppm on the first day, followed by 5 ppm for the next six days or until extubation.
Present Study
The study population consisted of children from the original cohort who survived to early school-age. The present protocol was approved by the institutional review board of the University of Chicago and informed parental/legal guardian consent was obtained.
Clinical data were obtained from the medical record. Children were examined in a single visit at early school-age by a general pediatrician and/or a developmental and behavioral specialist unaware of the child’s perinatal course and treatment assignment evaluated. Z scores for height and weight were calculated using the Centers for Disease Control and Prevention (CDC) growth charts 4. Z scores for head circumference were calculated using criteria by Roche5.
Children with abnormalities of posture or tone that impacted on motor control were classified as having one of the cerebral palsy (CP) syndromes. The Gross Motor Function Classification System (GMFCS) 6 was used to classify motor function.
Caretakers completed questionnaires regarding their child’s race, need for rehabilitation/special education services, ongoing morbidities, number of hospitalizations, and socioeconomic status. Socioeconomic status was assigned using Hollingshead Index of Social Position 7. Independent functioning of the child was determined by interview of the caretaker utilizing the Pediatric Functional Independence Measure (also known as the WeeFIM), 8. The NICHQ Vanderbilt Parent Assessment Scale 9 was used as a screen for the presence of pediatric behavioral disorders. Children underwent the Bracken School Readiness Assessment10, the Peabody Picture Vocabulary Test 3rd Ed., 11 and the Beery Test of Visual-Motor Integration 12.
Children with hearing aids were classified as having hearing loss. Children with hearing aids who were unable to communicate were classified deaf. Visual acuity was assessed using Lea Symbols14. Children with corrected visual acuity between 20/60–20/200 were classified visually impaired, and children with acuity worse than 20/200 were classified blind.
Ongoing morbidities were classified as none/mild (e.g. eczema, obesity, seasonal allergies, or mild asthma not requiring controller medication), chronic (e.g. asthma requiring controller medications, failure to thrive requiring nutritional supplements, and epilepsy), or multiple chronic/technology dependence (e.g. ventriculoperitoneal shunt, gastrostomy tube, or tracheostomy).
Based on standardized scores, children were assigned one of four school-readiness levels15. The criterion for school readiness was a level of greater than 2. Children who could not be evaluated in person were assessed by phone questionnaire of the caretaker.
Statistical Analyses
Continuous and normally distributed baseline characteristics such as birth weight and gestational age were compared between the iNO and the placebo group using the two sample t test. Ordinal baseline characteristics such as Apgar score and skewed distributed variables such as oxygenation index were compared between the two groups using the Wilcoxon Rank-Sum test. Categorical baseline variables were compared using Fisher’s exact test. Similarly, we compared the outcome variables at age school-age between the two groups using Wilcoxon Rank-Sum tests for ordinal outcomes and Fisher’s exact tests for categorical outcomes.
Results
Early school age (mean age 5.7 ± 1.0 year) outcome data were obtained for 135 of the 167 (81%) surviving children from the original cohort (Fig. 1). The 32 children lost to follow-up had similar birth weights (1136 ± 380 grams, p=0.12) and gestational ages (27.9 ± 2.7 weeks, p=0.44) as those assessed. As previously reported, phone interviews allowed unequivocal assessment of developmental status of the five children who could not be assessed in person16.
Figure 1.
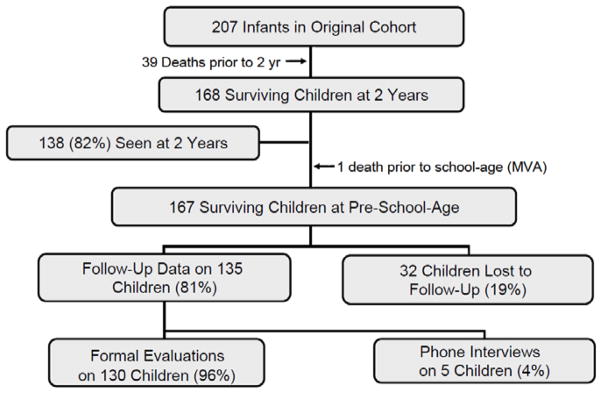
Flow diagram showing the number of patients involved at various stages of the study.
Of children assessed, 70 (51.8%) had received iNO. Neither the mean age at follow-up nor the rates of follow-up significantly differed between children who received iNO (79.6%) and those who received placebo (82.3%). The clinical characteristics of the children and maternal demographics at the time of enrollment in the initial study did not differ significantly between the two follow-up groups (Table 1). The Hollingshead Indices of Social Position were not significantly different between the two groups (p=0.89). Consistent with our original report, the group receiving iNO had a decreased incidence of severe IVH/PVL (p=0.04).
Table 1.
Clinical Characteristics and Demographics of the Follow-up Cohort
| Placebo (n=65) | iNO (n=70) | P | |
|---|---|---|---|
| Patient characteristic | |||
| Birth weight, g | 1002±403 | 1028±381 | 0.70 |
| Gestational age, weeks | 27.4 ± 2.7 | 27.6±2.6 | 0.71 |
| Age at follow-up, years | 5.6±1.1 | 5.7±0.9 | 0.43 |
| Male sex, n (%) | 32 (49) | 35 (50) | 1.0 |
| Initial oxygenation index | |||
| Median | 7.1 | 6.1 | 0.24 |
| Interquartile range | 4.5–15.0 | 3.7–12.3 | |
| 1-minute Apgar score | |||
| Median | 5 | 5 | 0.53 |
| Interquartile range | 4–6 | 3.5–6 | |
| 5-minute Apgar score | |||
| Median | 7 | 7 | 0.56 |
| Interquartile range | 6–8 | 7–8 | |
| Antenatal corticosteroids, n (%) | 37/65 (57) | 41/68 (60) | 0.73 |
| Postnatal corticosteroids>7days, n (%) | 7/64 (11) | 8/69 (11) | 1.0 |
| Surfactant, no. of doses | 2.3±0.9 | 2.2±0.9 | 0.44 |
| Neonatal Morbidities, n (%) | |||
| Chronic lung disease | 35 (54) | 28 (40) | 0.12 |
| Severe intraventricular hemorrhage or periventricular leukomalacia | 16 (25) | 7 (10) | 0.04 |
| Hollingshead Index of Social Position, n (%) | |||
| Level 1–2 | 7 (11) | 9 (13) | 0.89 |
| Level 3 | 22 (34) | 19 (27) | |
| Level 4 | 16 (24) | 21 (30) | |
| Level 5 | 20 (31) | 21 (30) | |
| Maternal Race (self-reported) | |||
| Black | 47 (72) | 48 (69) | 0.66 |
| White | 9 (14) | 14 (20) | |
| Other | 9 (14) | 8 (11) |
Plus-minus values are means ± standard deviation
Long-term Safety of iNO
To understand whether iNO treatment during the initial hospitalization for prematurity increases the risk of adverse outcomes during the first five years of life, we assessed children along three axes: somatic growth, need for re-hospitalization and neurodevelopment, as reflected by school readiness scores.
Although z scores for median weight (−0.45) and head circumference (−1.47) in all children were significantly lower than term infants, scores did not differ between study groups. These data indicate that iNO-treated premature infants (median weight 19.3 kg) did not have increased rates of growth failure compared with placebo-treated premature infants (median weight 18.2 kg).
Fewer than half of all infants, regardless of treatment status, were hospitalized during the 5–6 years following initial discharge. Of those children, half were hospitalized once. The distribution of the number of hospitalizations in the iNO-treated group was not different from that of the placebo-treated group (p=0.94).
To assess whether iNO treatment adversely impacted neurodevelopmental outcomes, we asked whether the number of children developmentally ready for school differed between treatment groups. To assess school readiness, we employed our multidimensional assessment of neurodevelopment15. As previously reported, two-thirds of children were scored at Levels 3 and 415. Children treated with iNO had similar distributions of school-readiness to the children who received placebo (p=0.87). In addition, there were no significant differences between groups in developmental assessment components or the incidences of cerebral palsy, hearing impairment, or visual impairment (Table 2). Taken together, these data indicate that iNO treatment during prematurity adversely affects neither physical development nor neurodevelopment at early school age, supporting its safety for use in premature infants.
Table 2.
School-Readiness Levels of the Follow-Up Cohort at Early School-Age
| Placebo N=65 | iNO n=70 | P | |
|---|---|---|---|
| Bracken* | |||
| >85 | 32 | 36 | 0.60 |
| 70–84 | 14 | 15 | |
| <70 | 18 | 16 | |
| PPVT-III | |||
| >85 | 26 | 34 | 0.47 |
| 70–84 | 23 | 16 | |
| <70 | 14 | 16 | |
| VMI | |||
| >85 | 37 | 44 | 0.33 |
| 70–84 | 15 | 12 | |
| <70 | 13 | 11 | |
| WeeFIM | |||
| >85 | 49 | 56 | 0.36 |
| 70–84 | 8 | 5 | |
| <70 | 8 | 7 | |
| GMFCS | |||
| No Cerebral Palsy | 59 | 63 | 0.77 |
| Level 1–2 | 2 | 1 | |
| Level 3 | 1 | 0 | |
| Level 4 | 1 | 2 | |
| Level 5 | 0 | 2 | |
| Hearing | |||
| No Problems | 60 | 67 | 0.44 |
| Requires Hearing Aids (can hear and speak) | 5 | 1 | |
| Requires Hearing Aids (no language) | 0 | 2 | |
| Vision | |||
| No Problems | 55 | 54 | 0.35 |
| Glasses or strabismus | 4 | 11 | |
| Visually impaired (can see objects) | 4 | 4 | |
| Blindness (no functional vision) | 2 | 1 |
Bracken: Bracken School Readiness Assessment; PPVT-III: Peabody Picture Vocabulary Test 3rd Ed; VMI: Beery Test of Visual-Motor Integration; GMFCS: Gross Motor Function Classification System
Efficacy of iNO
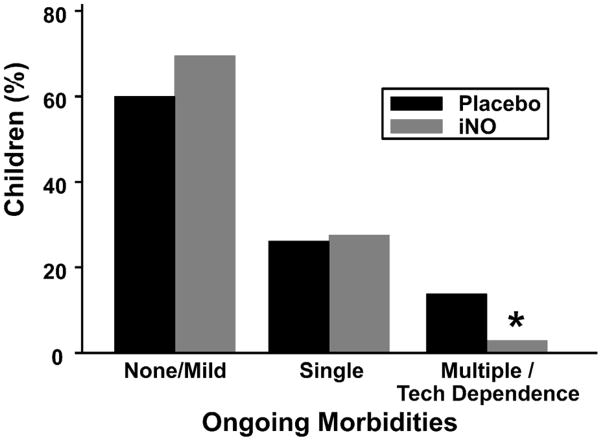
To assess whether beneficial effects of iNO treatment during prematurity persisted to early school age, we first compared the incidences of chronic morbidities between groups. There were no significant differences between groups in the percentage of children having one or no chronic morbidities. Importantly, however, significantly fewer iNO-treated infants had multiple chronic morbidities or were dependent on technology compared with placebo-treated infants (Fig. 2). Because the presence of multiple chronic morbidities or dependence on technology strongly reduces the likelihood of school readiness15, these data suggest that, by decreasing the total morbidity burden, iNO may decrease the number of premature children requiring additional societal resources.
Figure 2. Ongoing morbidities of study patients at school-age.
*P=0.05.
To assess whether iNO-treatment improved more subtle neuro-developmental outcomes at school age, we compared performances on the WeeFim, a component of our multi-dimensional neurodevelopmental battery. Scores on the WeeFim of less than 80% signify significant educational and community disability. Because children with school readiness level 1 were so profoundly disabled, we restricted our analysis to children having school readiness levels of 2–4. Five placebo-treated children had WeeFim scores of less than 80%. In contrast, no iNO-treated child fell into this group (p=0.05). These data suggest that some improved neurodevelopment in iNO-treated infants persists at early school age.
Discussion
In this longitudinal follow-up study of iNO- or placebo-treated premature infants, we found that iNO neither impairs somatic growth, increases hospitalizations nor worsens neurodevelopmental outcomes at school age. These data indicate that iNO treatment in premature infants is safe. Furthermore, fewer iNO-treated infants had multiple chronic morbidities. More iNO-treated infants also had improved functional outcomes compared with placebo-treated infants.
Several randomized placebo-controlled studies in addition to ours have reported improved short-term pulmonary and/or neurologic outcomes following iNO therapy 2, 17–19, although a recent multi-center trial of iNO in premature infants having extremely mild respiratory disease failed to find improved short-term outcomes20. Follow-up studies of these infants have yielded conflicting results: in our study population, neurodevelopmental outcomes were improved at two years corrected age3. In contrast, despite decreased CLD following late-onset iNO treatment in the NO-CLD study21, investigators failed to find differences in neurodevelopment in iNO-treated infants at two years of age22. Nonetheless, in the Kinsella trial23, investigators found that, at one year corrected age, iNO-treated infants weighing 750–999g had a lower rate of death or neurodevelopmental impairment compared with placebo-treated infants24. This improved outcome occurred in the group in which decreased IVH/PVL/ventriculomegaly had been previously reported23.
Because previous therapies in premature infants have provided short-term benefits at the expense of neurodevelopmental impairment, (most notably dexamethasone therapy25), assessment of the five year safety of iNO treatment is essential. While the sub-population of premature infants who benefit from iNO therapy has yet to be precisely determined, our findings that iNO-treated premature infants do not differ from placebo-treated infants in somatic growth, hospitalization rates or overall neurodevelopmental outcomes provide reassurance of the safety of iNO treatment in premature infants with moderate RDS. These findings should help facilitate future studies intended to better elucidate optimal iNO treatment regimens in this population.
At two years of age, iNO-treated premature infants in our study had approximately half the risk of cognitive impairment exhibited by the placebo-treated group3. That this marked difference in cognitive impairment was not apparent at school age indicates that factors outside of the neonatal period, such as the home environment and socioeconomic status, become increasingly important as infants acquire more complex repertoires of skills and behaviors. For example, low socioeconomic status has roughly four times the negative impact on school readiness compared with that of severe IVH/PVL or CLD15. It is also possible that the battery of neurodevelopmental assessments we used may be insufficiently sensitive to detect cognitive differences between iNO- and placebo-treated children. Studies of school performance at older ages may be more useful to detect subtle differences in neurodevelopment.
The iNO-induced decreases in neurodevelopmental impairment we observed at two years corrected age were statistically independent of the decreased incidences of both severe IVH/PVL or CLD3. This observation raises the possibility that iNO acts directly on the developing brain, possibly through delivery of NO to brain vasculature (reviewed by Shechter and Gladwin28). Providing iNO to cerebrovascular beds supplying vulnerable pre-oligodendrocytes and neurons, therefore, could result in both improved blood flow during hypoxic episodes common during the initial hospitalization, perhaps overcoming hypoxia-induced eNOS down-regulation29. In our study, infants received iNO for one week, irrespective of gestational age and, hence, the stage of brain development. It is possible, therefore, that longer iNO treatment provided throughout critical periods of brain development may further improve neurodevelopmental outcomes in this at-risk population.
Acknowledgments
This work benefited from the assistance of the following individuals: Danielle Zageris, Emily Msall, Scott Schreiber, Larry Gray, MD, and Jennifer Park, MA.
Funding/Support: Dr. Patrianakos-Hoobler was supported in part by the American Academy of Pediatrics Resident Research Grant. Dr Marks was supported by R01 NS056313 from the NINDS. Dr. Schreiber was supported by an investigator-initiated grant from INO Therapeutics/IKARIA.
Footnotes
Contributors’ list:
Dr. Patrianakos-Hoobler was contributed to protocol development, was responsible for patient screening, enrollment, and outcome assessment and contributed to preliminary data analysis and the writing of the manuscript.
Dr. Marks contributed to protocol development and final data analyses and was responsible for writing the manuscript.
Dr. Msall was primarily responsible for protocol development, patient screening, enrollment, outcome assessment, as well as contributing to preliminary data analysis and the writing of the manuscript.
Dr. Huo was primarily responsible for final data analyses and contributed to the writing of the manuscript.
Dr. Schreiber supervised the design and execution of the study, contributed to the final data analyses and writing the manuscript.
Conflicts of Interest: Drs. Schreiber and Marks report receiving speakers’ honoraria from INO Therapeutics/IKARIA.
Previous Presentations: Portions of these data were presented at the 2007 Pediatric Academic Societies Conference in Toronto in May, 2007, the 2007 American Academy of Cerebral Palsy and Developmental Medicine Conference in Vancouver, BC in October, 2007, the 2008 American Academy of Cerebral Palsy and Developmental Medicine Conference in Atlanta, Georgia in September 2008, and the 2008 Pediatric Academic Societies Conference in Hawaii in May 2008.
References
- 1.Doyle LW, Anderson PJ. Improved neurosensory outcome at 8 years of age of extremely low birthweight children born in Victoria over three distinct eras. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2005;90:F484–F8. doi: 10.1136/adc.2004.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. New England Journal of Medicine. 2003;349:2099–107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 3.Mestan KL, Marks JD, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. New England Journal of Medicine. 2005;353:23–32. doi: 10.1056/NEJMoa043514. [DOI] [PubMed] [Google Scholar]
- 4.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC growth charts for the United States: Methods and development. Vital and Health Statistics. 2000;2002:11. [PubMed] [Google Scholar]
- 5.Roche AF, Mukherjee D, Guo SM, Moore WM. Head circumference reference data: birth to 18 years. Pediatrics. 1987;79:706–12. [PubMed] [Google Scholar]
- 6.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 7.Hollingshead AdB. Two factor index of social position. New Haven: Hollingshead; 1957. [Google Scholar]
- 8.Msall ME, DiGaudio K, Rogers BT, et al. The Functional Independence Measure for Children (WeeFIM): Conceptual Basis and Pilot Use in Children With Developmental Disabilities. Clin Pediatr (Phila) 1994;33:421–30. doi: 10.1177/000992289403300708. [DOI] [PubMed] [Google Scholar]
- 9.Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. J Pediatr Psychol. 2003;28:559–67. doi: 10.1093/jpepsy/jsg046. [DOI] [PubMed] [Google Scholar]
- 10.Bracken B. Bsra-Bracken School Readiness Assessment Administration Manual. San Antonio, TX: PsychCorp Harcourt Assessment; 2002. [Google Scholar]
- 11.Dunn LM, Dunn LM. Peabody Picture Vocabulary Test (PPVT-III) 3. Circle Pines, MN: American Guidance Services; 1997. [Google Scholar]
- 12.Beery KE, Beery NA. Beery-Buktenica Developmental Test of Visual-Motor Integration (Beery VMI) 3. NCS Pearson; 2004. [Google Scholar]
- 13.Schopler E, Reichler R, DeVellis R, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS) J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 14.Hyvarinen L, Nasanen R, Laurinen P. New visual acuity test for pre-school children. Acta Ophthalmol (Copenh) 1980;58:507–11. doi: 10.1111/j.1755-3768.1980.tb08291.x. [DOI] [PubMed] [Google Scholar]
- 15.Patrianakos-Hoobler AI, Msall ME, Marks JD, Huo D, Schreiber MD. Risk Factors Affecting School Readiness in Premature Infants With Respiratory Distress Syndrome. Pediatrics. 2009;124:258–67. doi: 10.1542/peds.2008-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrianakos A, Msall M, Marks JD, Huo D, Schreiber MD. Risk factors affecting school-readiness for premature infants. Pediatrics. 2009;124:258–67. doi: 10.1542/peds.2008-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinsella JP, Cutter GR, Walsh WF, et al. Early Inhaled Nitric Oxide Therapy in Premature Newborns with Respiratory Failure. N Engl J Med. 2006;355:354–64. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 18.Ballard RA, Truog WE, Cnaan A, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–53. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 19.Hibbs AM, Walsh MC, Martin RJ, et al. One-year respiratory outcomes of preterm infants enrolled in the Nitric Oxide (to prevent) Chronic Lung Disease trial. J Pediatr. 2008;153:525–9. doi: 10.1016/j.jpeds.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercier JC, Hummler H, Durrmeyer X, et al. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet. 2010;376:346–54. doi: 10.1016/S0140-6736(10)60664-2. [DOI] [PubMed] [Google Scholar]
- 21.Ballard RA, Truog WE, Cnaan A, et al. Inhaled Nitric Oxide in Preterm Infants Undergoing Mechanical Ventilation. New England Journal of Medicine. 2006;355:343–53. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 22.Walsh MC, Hibbs AM, Martin CR, et al. Two-year neurodevelopmental outcomes of ventilated preterm infants treated with inhaled nitric oxide. J Pediatr. 2010;156:556–61. e1. doi: 10.1016/j.jpeds.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinsella JP, Cutter GR, Walsh WF, et al. Early Inhaled Nitric Oxide Therapy in Premature Newborns with Respiratory Failure. New England Journal of Medicine. 2006;355:354–64. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 24.Watson RS, Clermont G, Kinsella JP, et al. Clinical and economic effects of iNO in premature newborns with respiratory failure at 1 year. Pediatrics. 2009;124:1333–43. doi: 10.1542/peds.2009-0114. [DOI] [PubMed] [Google Scholar]
- 25.Stark AR. Inhaled NO for Preterm Infants -- Getting to Yes? N Engl J Med. 2006;355:404–6. doi: 10.1056/NEJMe068129. [DOI] [PubMed] [Google Scholar]
- 26.Patrianakos-Hoobler AI, Msall M, Huo D, Marks JD, Plesha-Troyke S, Schreiber MD. Predicting school readiness from neurodevelopmental assessments at age 2 years after respiratory distress syndrome in infants born preterm. Dev Med Child Neurol. 2009:52. doi: 10.1111/j.1469-8749.2009.03343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon TJ, Moon RE, Luschinger BP, et al. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–7. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 28.Schechter AN, Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. N Engl J Med. 2003;348:1483–5. doi: 10.1056/NEJMcibr023045. [DOI] [PubMed] [Google Scholar]
- 29.Marks JD, Schreiber MD. Inhaled nitric oxide and neuroprotection in preterm infants. Clin Perinatol. 2008;35:793–807. viii. doi: 10.1016/j.clp.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]