Abstract
Objective
The objective of this study was to compare the interictal cortical response to a visual stimulus between migraine with aura (MWA), migraine without aura (MwoA), and control subjects.
Methods
In a prospective case-control study, blood oxygen level-dependent functional magnetic resonance imaging (BOLD fMRI) was used to assess the response to a visual stimulus and arterial spin labeled perfusion MR to determine resting cerebral blood flow. A standardized questionnaire was used to assess interictal visual discomfort.
Results
Seventy-five subjects (25 MWA, 25 MwoA, and 25 controls) were studied. BOLD fMRI response to visual stimulation within primary visual cortex was greater in MWA (3.09±0.15%) compared to MwoA (2.36±0.13%, p=0.0008) and control subjects (2.47±0.11%, p=0.002); responses were also greater in the lateral geniculate nuclei in MWA. No difference was found between MwoA and control groups. Whole brain analysis showed that increased activation in MWA was confined to the occipital pole. Regional resting cerebral blood flow did not differ between groups. MWA and MwoA subjects had significantly greater levels of interictal visual discomfort compared to controls (p=0.008 and p=0.005, respectively), but this did not correlate with BOLD response.
Conclusions
Despite similar interictal symptoms of visual discomfort, only MWA subjects have cortical hyperresponsiveness to visual stimulus, suggesting a direct connection between cortical hyperresponsiveness and aura itself.
Keywords: Migraine with aura, cortical hyperresponsiveness, hyperexcitability, visual sensitivity
Introduction
Light sensitivity is a common complaint in patients with migraine, both during headache and also in the headache-free interictal period. This clinical observation has been confirmed by systematic behavioral studies demonstrating lower discomfort thresholds for visual stimulation and an increase in visual illusions and distortions in migraineurs (1–3). A number of investigations have reported a neural correlate of these interictal symptoms, finding larger occipital cortex responses to light stimulation in migraineurs. This has been observed primarily with electrophysiological techniques such as surface-evoked potentials and transcranial magnetic stimulation (4,5).
In contrast to the physiological mechanisms underlying the acute cortical alterations seen during migrainous aura, which have long been ascribed to cortical spreading depression and elegantly mapped with functional magnetic resonance imaging (fMRI) (6), the mechanisms underlying interictal cortical hyperresponsiveness remain uncertain. There is considerable evidence that a defect in neural habituation to repetitive stimuli contributes to hyperresponsiveness (4,7). Furthermore, the precise neuroanatomic localization of the cortical hyperresponsiveness seen in migraine is poorly defined, largely because the majority of available data is based on electrophysiological techniques that have relatively poor spatial resolution. Only small cohorts have been studied using functional neuroimaging, such as blood oxygen level-dependent (BOLD) MRI, with high spatial resolution (4,5). The present study used multi-modality MRI to examine the cortical response to light stimulation in a large cohort of subjects with migraine, both with (MWA) and without aura (MwoA), as well as matched controls. We examined the within-group relationship between BOLD fMRI measures of light-evoked responses, and both an imaging measure of baseline resting metabolic activity (resting cerebral perfusion assessed with arterial spin-labeled (ASL) perfusion MRI), and a behavioral measure of light sensitivity (8,9). Using whole brain analysis, we explored the precise neuroanatomic location of the cortical response. Our principal finding is that visual hyperresponsiveness is a specific phenomenon restricted to MWA and seen in the occipital pole in striate cortex and the lateral geniculate nuclei. This increased cortical response is not explained by differences in resting cerebral blood flow (CBF) and appears independent of interictal clinical symptoms of visual discomfort.
Methods
Subjects
Participants were recruited both from the neurology clinic at the University of Pennsylvania and by advertisements in the wider University of Pennsylvania community. Participants were eligible for inclusion if they were 25–50 years old, had a diagnosis of MWA or MwoA using International Classification of Headache Disorders criteria, or were headache-free healthy controls (HCs) (10). All participants were screened and examined by a single study neurologist (BC) to ensure that they met inclusion/exclusion criteria. This analysis is a sub-study of an ongoing larger MRI study, the Anatomy and Cerebral Hemodynamic Evaluation of Migraine (ACHE-M) study, comparing patients with migraine to HCs. For the present study, patients were recruited between 2008 and 2011. All MWA participants from the overall study during this period with imaging data suitable for analysis were included. MwoA and control participants were randomly selected from the overall cohort in an iterative manner until optimal age- and sex-matching with MWA participants was achieved. This subject selection was conducted prior to any analysis being performed. Study participation concluded with the completion of the MRI, and follow-up for future occurrence of headache after the immediate scanning period was not performed. The study was approved by the University of Pennsylvania Institutional Review Board and all participants provided written informed consent.
Image acquisition
MRI scans were acquired at 3 Tesla using a Siemens Trio and an eight-channel Siemens head coil. BOLD fMRI data were collected using gradient-echo echoplanar imaging with 160 volumes at repetition time (TR)=3 sec, echo time (TE)=30 msec and 3×3×3mm isotropic voxels in 44 axial slices. Anatomical images were acquired using a T1-weighted magnetization prepared rapid gradient echo (3D MPRAGE) at 1×1×1mm resolution with 160 slices, TR=1.62 s, TE=3.09 msec, inversion time (TI)=950 msec, field of view (FOV)=250 mm, and flip angle (FA)=15°. A single, “scout” echoplanar volume was also acquired close to the time of the anatomical image to facilitate coregistration. For each subject, in the same scan session, a pulsed-continuous arterial spin labeled (pCASL) perfusion MRI sequence was used to measure resting cerebral blood flow (CBF), and consisted of 30 label/control pairs with 3.4×3.4×6mm nominal resolution in 16 axial slices using gradient-echo echolanar imaging with TR=4 sec, TE=18 msec, post-labeling delay time=1200 msec, and labeling time=1125 msec. ASL MRI was conducted with minimal ambient lighting while the subject rested with eyes open. All scans were performed interictally, that is, when subjects were free of complaints of headache.
Visual stimulus for BOLD fMRI
Stimuli were generated on an Apple MacBook using Matlab (Mathworks Inc) augmented by mgl (http://justingardner.net/doku.php/mgl/overview) and Psychtoolbox 4 (http://psychtoolbox.org) display routines, and presented on a rear-projection Mylar screen using a liquid crystal display (LCD) projector equipped with a long-throw lens. Subjects viewed the screen through a head coil mounted mirror (124.25 cm distant from the screen) mounted over the eyes. While the subject maintained central fixation, a 5 Hz flickering square-wave checkerboard (0.5 cycles per degree fundamental spatial frequency) was presented in 15 sec, randomly ordered blocks either on the subject's left, right or bilateral visual field. A blank period of no visual stimulation was also presented randomly for 15 sec between the different blocks of visual stimulation. In the analyses presented here, contralateral and bilateral visual stimulation were combined and compared to periods without visual stimulation; responses from ipsilateral stimulation are not reported here.
The stimulus had a maximal eccentricity in any direction of 10 degrees visual angle. The order of blocks was fixed across subjects. Luminance modulation between the white and black checks was 1750 cd/m2. To ensure task compliance, subjects performed an attentional task focusing on a fixation dot and pressed a button each time the dot changed color.
Image analysis
BOLD fMRI data
The echoplanar scout image from each subject was co-registered to its anatomical image (ANTS toolkit http://picsl.upenn.edu/ANTS/) and used as the target for six-parameter, least squares rigid body realignment. The BOLD time series data were then sinc-interpolated in time to correct for the interleaved slice acquisition sequence. The stimulus responses were modeled with separate square-wave covariates for left, right, and bilateral hemifield stimulation, convolved with a population average hemodynamic response using a general linear model (11). Because of occasional errors in the timing of the onset of the stimulus relative to the onset of MRI scanning, the set of covariates for every subject was temporally shifted by sinc-interpolation to maximize model fits for the average signal within the 10° eccentricity, V1 region of interest. Nuisance covariates included effects of scan, global signals, and spikes (periods of raw signal deviation greater than two standard deviations from the mean). The beta effect for each covariate (expressed as percentage signal change) was derived for each subject from the entire brain and for each region of interest (ROI).
ASL perfusion data
Imaging data processing and analyses were carried out with Statistical Parametric Mapping software (SPM8, Wellcome Department of Cognitive Neurology, UK, implemented in MatlabR2008b, Math Works, Natick, MA). A Matlab and SPM-based toolkit, ASLtbx (http://www.cfn.upenn.edu/~zewang/ASLtbx.php), was used to quantify CBF values and reconstruct CBF maps for perfusion analyses. For each participant, functional images were first realigned to correct for head motion and smoothed in space using a three-dimensional, 6mm full width at half maximum (FWHM) Gaussian kernel. The perfusion-weighted image series were then generated by pair-wise subtraction of the label and control images, followed by conversion to absolute CBF image series based on a single-compartment CASL perfusion model (12). Thus, the resulting CBF data sets contained 30 acquisitions with an effective TR of 8 s. One mean CBF image was generated and co-registered with an anatomical image for each individual participant.
Anatomical images
Anatomical data from the subjects were processed using the FMRIB Software Library (FSL) toolkit (http://www.fmrib.ox.ac.uk/fsl/) to correct for spatial inhomogeneity and to perform nonlinear noise reduction. Brain surfaces were reconstructed and inflated from the MPRAGE images using the FreeSurfer toolkit (http://surfer.nmr.mgh.-harvard.edu/) as described previously (13,14). Individual whole brain (right and left hemisphere) surface maps were then registered to a common FreeSurfer template surface pseudo-hemisphere (fsaverage_sym) using the FreeSurfer spherical registration system (15). As no hemispheric lateralizing differences were expected (nor found in preliminary analyses), the data from the two hemispheres for each subject were combined within a single, pseudo-hemisphere representation that is left-right transform symmetric. SPM8 was also used to calculate the volumetric transformation matrix for each subject to standard Montreal Neurological Institute (MNI) space.
ROIs
The boundary of V1 cortex was defined for each subject by anatomical features of the cortical surface and further constrained to the predicted location of the central 10° of visual field representation (16,17). These ROIs were then projected back to the volumetric space for each subject. A lateral geniculate nucleus (LGN) ROI was defined within standard volumetric (MNI) space using the Juelich Histological atlas (18). This region was then projected back to the volumetric space for each subject via an inverse of the transformation matrix calculated using SPM8. The average BOLD percentage signal change across voxels within each ROI for each subject and for each stimulus condition was then extracted.
Cortical surface analysis
The volumetric beta maps of percentage signal response to visual stimulation were projected from each subject to the cortical surface atlas. Each map underwent 10mm, two-dimensional Gaussian smoothing restricted to the cortical sheet. A map-wise, random-effects analysis across subjects modeled the effects of group (MWA, MWoA and controls) on cortical response, as well as nuisance covariates for the effects of age and gender. Permutation of group label assignment was used to calculate a map-wise false discovery rate (FDR) threshold (q=0.05) separately for each comparison between groups.
Behavioral data on visual sensory sensitivity
Participants completed a previously published and validated survey designed to measure interictal visual discomfort (9). This survey consists of 23 questions using a four-point scale to assess somatic, perceptual, and performance difficulties experienced in visual tasks, with increasing scores indicating increased visual discomfort. A total visual discomfort score (VDS) for each subject was calculated by adding the individual point responses for all questions. The VDS survey was added to the study procedures after study enrollment had begun, thus VDS data were not available for all subjects.
Statistical analysis
Groups of subjects were compared using t test or Wilcoxon rank sum tests as indicated. Means and standard deviations or medians and intraquartile ranges (IQR) are presented as appropriate. Correlations between variables were tested using Pearson correlation coefficient. An association was considered significant if p<0.05. All tests were two sided.
Results
A total of 75 participants were included: 25 MWA (four males/21 females), 25 MwoA (four males/21 females), and 25 controls (four males/21 females). The age distribution was matched between controls (mean age, 32±6 years), MWA (mean age, 32±6 years) and MwoA (mean age, 32±6 years) groups. The mean age of onset of migraines was 14±6 years for the MWA group and 19±8 years for the MwoA group. Median headache frequency in an average month was two (IQR one to 10) per month for the MWA group and two (IQR range one to 15) per month for the MwoA group. Mean headache frequency in an average month was three±three per month for the MWA group and four±four per month for the MwoA group. Median headache frequency in the month immediately preceding scanning was two (IQR one to 12) per month for the MWA group and two (IQR one to 15) per month for the MwoA group. All MWA subjects had visual aura, with eight subjects also having additional aura symptoms (vertigo, three; unilateral numbness/paresthesias, three; aphasia, two).
Comparison of BOLD amplitude across groups in V1 and LGN
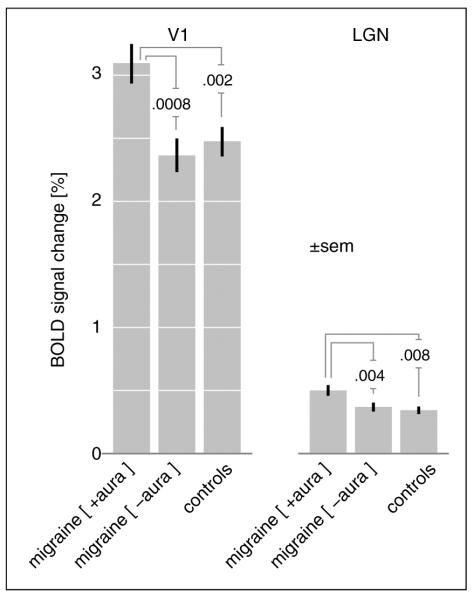
There was a significant difference in the amplitude change of BOLD signal in V1 in response to photic stimulation in the MWA compared to MwoA group (MWA 3.09±0.77% vs. MwoA 2.36±0.66%, p=0.0008) as well as between the MWA and control group (MWA 3.09±0.77% vs. control 2.47±0.57%; p=0.002) (Figure 1). There was no significant difference between the MwoA and control groups (p=0.5). Thus, V1 cortex hyperresponsiveness to light stimulation was restricted to the MWA population. We then examined whether V1 hyperresponsiveness was related to aura burden by dividing the MWA subjects into those whose migraine headaches were associated with aura more than half the time (n=11) compared to those who had aura with less than half of their headaches (n=14). While V1 hyperresponsiveness was greater in those with higher aura burden, this was not statistically significant (MWA>half 3.33±0.93% vs. MWA <half 2.90±0.59%, p=0.17).
Figure 1.
Change in BOLD fMRI signal in V1 and lateral geniculate nucleus (LGN) in response to visual stimuli across groups. BOLD fMRI: blood oxygen level-dependent functional magnetic resonance imaging.
We next asked if this difference in responsiveness is present earlier in the visual pathway. The LGN was defined using a volumetric atlas and the BOLD fMRI response compared across groups. Again, there was a larger amplitude of BOLD response in the MWA compared to MwoA group (MWA 0.53±0.24% vs. MwoA 0.38±0.16%, p=0.01) as well as between the MWA and control group (MWA 0.53±0.24% vs. control 0.36±0.15%; p=0.004). There was no significant difference between the MwoA and control groups (p=0.65).
Given prior electrophysiological data showing habituation in migraine subjects occurring over a time scale of minutes (19), we then examined the average striate time-series of BOLD response between the groups (Figure 2), recognizing that our stimulus was discontinuous and therefore not comparable to that typically used to demonstrate habituation. We found that the enhanced response in the MWA group persisted throughout the 8-minute study. We obtained the difference in amplitude between the MwA and control groups for each of the 20 blocks of stimulation in the left and right hemisphere, and examined the correlation of this measure with time over the 8-minute scan, and found no significant effect (r=−0.23, p=0.33). The short duration of our stimulus blocks with respect to the hemodynamic response function precluded an examination of differences in habituation of the amplitude of response over the shorter, within-block time-scale.
Figure 2.
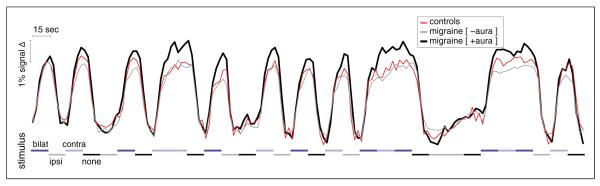
Population average BOLD time-series from V1. The average time-series data (expressed as percentage signal change) within the left V1 cortex for each subject was obtained for the 8-minute scan. The time-series from each subject was adjusted to remove a linear drift term and effects of no-interest (“spike” transients) and then averaged within groups. The three plots were set to have the same initial value at time zero. Stimulus timing is indicated by shades of gray and blue along the x-axis. The time-series from the right hemisphere shows qualitatively the same amplitude effect and persistence (data not shown).
BOLD: blood oxygen level-dependent.
Whole brain analysis comparing BOLD activation across groups
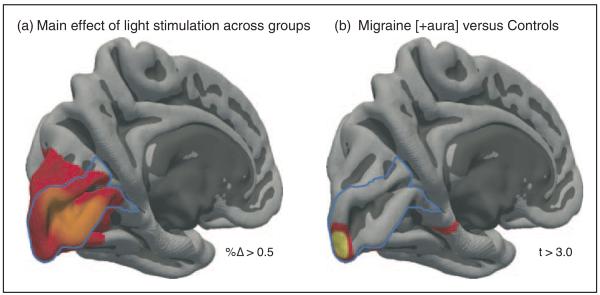
In both the V1 and lateral geniculate ROIs, larger responses were seen for the MWA group. To determine whether these enhanced responses extend beyond the early visual pathway, we conducted a random-effects analysis across the entire cortical surface after combining the data from each hemisphere within subjects. Figure 3(a) presents the main effect of visual stimulation across the three groups. As would be expected, BOLD fMRI responses are seen both within striate cortex (outlined in blue), as well in adjacent extra-striate cortex (V2 and V3). We then compared the evoked response between groups. No differences at a map-wise threshold were seen between the MwoA and control groups. For the MWA group, however, there was an isolated increased response at the occipital pole, corresponding to the foveal representation (Figure 3(b)).
Figure 3.
Evoked cortical responses to visual stimulation. The evoked response for each subject was obtained for each hemisphere and then averaged within a symmetric pseudo-hemisphere. (a) The main effect of visual stimulation across the three groups, thresholded to show cortical locations with a greater than 0.5% BOLD signal change to stimulation. (b) Regions of significantly greater (t > 3.0, q = 0.05) evoked response in the migraine with aura group compared to controls.
BOLD: blood oxygen level-dependent.
We then considered whether this apparent focality of the increased response within the foveal representation of V1 could be an effect of thresholding. Assuming this were the case, a more extensive region of V1 cortex actually has an increased response in MWA, but thresholding reveals only the tip of this area of effect. In Figure 3(b), the occipital pole region of increased response in MWA extended for 12.5mm along the calcarine sulcus, corresponding to approximately 1.5 degrees of visual field representation. We examined the difference map in Figure 3(b) at a much reduced statistical threshold, corresponding to a vertex-wise p=0.05 (uncontrolled). Even at this much lower threshold, the area of increased response in MWA remained confined to the occipital pole, extending 16.0mm along the calcarine sulcus corresponding to approximately two degrees of visual field representation. Based on this, we conclude that the hyperresponsive effect is relatively specific to the foveal representation of area V1.
Comparison of resting perfusion between groups
To evaluate the possibility that differences in the baseline resting metabolic state might be related to subsequent differences in activation, we analyzed quantified CBF data from each subject acquired using ASL perfusion MRI and tested for differences between and within groups. We reasoned that a decreased neural resting state would be accompanied by decreased resting CBF within area V1, as has been seen in other studies of altered neural physiology (20).
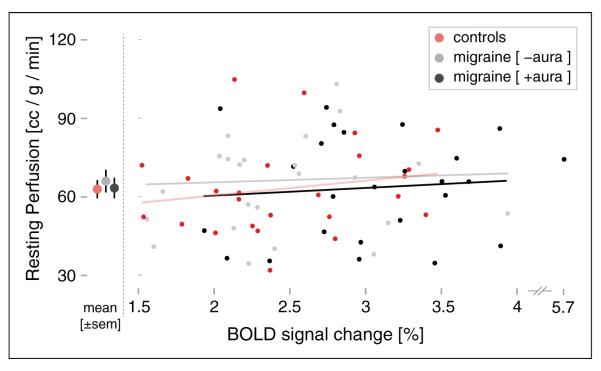
There were no significant differences in the resting state mean CBF values in V1 between the MWA, MwoA, and control groups (MWA 63.5±20.0 ml/100 g/min; MwoA 66.2±23.6 ml/100 g/min; control 63.1±17.3 ml/100 g/min; MwA vs. controls, p=0.93, MwoA vs. controls, p=0.58). We then asked if, within a group, the degree of evoked BOLD response was related to the V1 resting CBF prior to visual stimulation (Figure 4). No systematic relationship between these measures was seen for any group (MWA: r=0.046, p=0.82; MwoA: r=−0.014, p=0.95; controls: r=0.214, p=0.30).
Figure 4.
Relationship between resting cerebral perfusion and evoked BOLD response to visual stimulation in V1. Note that there is a discontinuity in right of the x-axis to accommodate a subject with a larger BOLD response.
BOLD: blood oxygen level-dependent.
To test whether a difference in CBF might be seen using a larger relevant region of interest, we also examined resting mean CBF in the entire posterior cerebral artery distribution. This also showed no difference between groups (MWA 46.2 ml/100 g/min vs. MwoA 47.6 ml/100 g/min vs. controls 47.6 ml/100 g/min; all comparisons NS). A further examination testing CBF in V1 scaled by global mean CBF similarly showed no difference between groups. Neither of these two alternate measures of resting regional CBF was significantly related to evoked BOLD response.
VDS
We obtained VDSs to assess behavioral differences between groups and to test for a relationship between cortical response and symptom severity. Scores were available for 25/25 MWA, 17/25 MWoA and 19/25 control subjects. The median VDS was 9 (IQR 4–16) in the MWA group and 8 (IQR 5–16) in the MwoA group compared to 4 (IQR 1–7) in the control subjects (MWA vs. controls, p=0.008; MwoA vs. controls, p=0.005). There was no difference in VDSs between the MWA and MwoA groups (p=0.93). We then tested if the VDS was associated with the degree of evoked BOLD response. No significant relationship was seen between BOLD response and VDSs in any of the three groups (MWA, r=0.243, p=0.21; MwoA, r=0.229, p=0.37; controls, r=0.023, p=0.91).
Discussion
Our results demonstrate a robust difference in BOLD fMRI activation along the visual pathway in MWA compared to MwoA and control subjects. While this finding is generally consistent with prior electro-physiological data suggesting interictal cortical hyperresponsiveness in migraine, it differs in that hyperresponsiveness was seen only in the MWA group (5). An important strength of the current study was the inclusion of a relatively large cohort of MWA and MwoA subjects, allowing separate analysis of the cortical response in the two groups. The cortical response in the MwoA group was nearly identical to the control population in our study, suggesting that the finding of cortical hyperresponsiveness, at least with the visual stimulus paradigm we used, is confined to patients with MWA. An interesting parallel to our observations is a prior MR spectroscopy study that examined brain metabolism interictally in a similar cohort of MWA, MwoA, and control subjects, and demonstrated metabolic alterations after visual stimulation suggestive of hyperresponsive neuronal activity in the MWA but not MwoA group compared to controls (21). Whether MWA and MwoA should be treated as a single disease process with a spectrum of manifestations or two separate diseases entirely is a long-standing question (22). A number of clinical differences between the two headache types have been emphasized, and a large population-based twin study found an inheritance pattern strongly suggesting the two are distinct disorders (23,24). On the other hand, many MWA subjects also experience typical MwoA, and medications that suppress cortical spreading depression are effective prophylactic medications for MWA and MwoA (25).
The finding of cortical hyperresponsiveness only in the MWA group is also notable in that the MWA and MwoA groups in our study had equivalent levels of interictal visual discomfort, with both differing markedly from controls in this respect. The visual discomfort score used in our study encompasses multiple aspects of visual sensitivity (9). These results suggest that visual cortex hyperresponsiveness may not be the main correlate for the behavioral perception of interictal visual sensitivity, and is perhaps consistent with recent data suggesting that the light sensitivity perceived by migraineurs may be mediated by the non-image forming melanopsin-mediated visual pathway (26). On a related note, we found that the enhanced response to visual stimulus within striate cortex was confined to the occipital pole, which is the region of foveal representation. Given that the large majority of MWA patients describe the aura as starting centrally and spreading peripherally, this lends further support to the idea that cortical hyperresponsiveness may be directly connected to aura itself as opposed to being a more general phenomenon related to sensory processing (27).
In addition to an enhanced light response in striate cortex, we found a greater BOLD fMRI response in the LGN, also specific to MWA. The replication of the pattern of group differences at an anatomically distant but functionally linked site buttresses the findings in striate cortex. Prior work has suggested alterations in thalamocortical connectivity (specifically gamma band oscillations) in MWA (28). Because of the complexity of reciprocal geniculostriatal connections, and the low temporal resolution of fMRI, we are unable to determine which one of these two regions is primary in driving the observed increased activation response. In contrast to a prior study showing differential activation in the middle temporal complex in migraine, we did not see group differences in evoked response at extra-striate cortical sites (29). However, our stimulus was not optimized for the recruitment of higher-order cortical areas.
The precise nature and mechanism of hyperresponsive cortical function seen in migraine remains a matter of controversy. Electrophysiological studies, primarily scalp recordings of visual-evoked potentials (VEPs), have consistently shown a lack of habituation to sustained stimuli as a primary abnormality in cortical reactivity in migraine (4,7). Given the temporal filtering of the hemodynamic response and the duration of stimulation used in our study, we were unable to test for differences in habituation at a time scale of seconds (11). We did test for differences in habituation on the scale of minutes across the scan session. While no evidence for differential habituation between groups was seen, our stimulus was discontinuous (Figure 2) and as such our results are not comparable to electrophysiological studies that have shown altered habituation in migraine over minutes using a continuous stimulus (19).
We used ASL perfusion MRI to assess resting cerebral perfusion within striate cortex for each subject. ASL is a non-invasive MRI modality that utilizes magnetically labeled arterial blood water as an endogenous tracer for noninvasive quantification of CBF (8). CBF is well correlated with metabolic activity (e.g. glucose consumption) in healthy participants as well as those with altered neural function, and thus represents a measure of resting cerebral metabolic state (30,31). We found no group differences in either resting blood flow in striate cortex or the interaction between resting CBF and BOLD response. This result was unchanged when we further examined regional CBF scaled to global brain blood flow and when we examined CBF using a larger cortical ROI (the posterior cerebral artery territory). A number of studies using MR spectroscopy have suggested disturbances of resting energy metabolism in migraine, possibly on the basis of mitochondrial dysfunction (32). Whether this is coupled to alterations in CBF or BOLD response remains an open question and an area for future study.
There have been relatively few prior investigations using functional neuroimaging to study the interictal cortical response in patients with migraine, and those that have been performed have generally studied small populations (e.g. three to 10 subjects in a group) and have examined the effect of manipulations of the parameters of visual stimulation, such as luminance level or spatial frequency, on cortical responsiveness. In six MWA subjects and controls, Huang and colleagues (33) studied the BOLD fMRI response in voxels adjacent to the calcarine sulcus with a set of vertical gratings that varied from 0.3 to 9.0 cycles per degree (cpd) with logarithmic spacing. Larger amplitude responses in the MwA cohort compared to controls were observed across all spatial frequencies, with the maximal difference observed at 1.2 cpd. In a subsequent study of a mixed migraine group (10 migraine, 10 control subjects), larger responses in V1 were seen compared to controls for a grating of 2.5 cycles per degree, but not for higher (7.9 cpd) or lower (0.3 cpd) spatial frequencies (34). Larger responses in the migraine group were found in extra-striate cortex for both 2.5 and 7.9 cpd stimulation. Two studies of stimulus luminance on migraine cortical responsiveness have been reported. In both studies, a measure of volume of cortical activation was used. This dependent measure is difficult to interpret, as it is confounded with amplitude by spatial smoothing and depends on the map-wise threshold selected. One used positron-emission tomography (PET) to examine the interaction of luminance and a simultaneous trigeminal pain stimulus in seven migraineurs compared to matched controls (35). A larger volume of occipital cortical response in migraineurs to light at two different levels and with or without a simultaneous pain stimulus was reported. Martin and colleagues used BOLD fMRI to study a mixed migraine group (seven MWA, 12 MwoA) over a range of stimulus luminance (36). At a “low” and “moderate” stimulus intensity, there was a greater extent (but not amplitude) of response in the occipital lobe in the migraineurs compared to controls. At two higher stimulus levels, close to maximal tolerability of brightness, no difference between the groups was seen. The properties of our stimulus roughly correspond to the lower spatial frequency of Huang et al. (34), and the “moderate” luminance of Martin et al. (36).
The stimulus parameters we used were designed to maximize V1 responses. The results of Huang et al. suggest, however, that we might have found a slightly greater group difference if we had used a stimulus with a higher spatial frequency (33).
Our results clearly differ from some prior electrophysiological studies showing hyperresponsiveness in MWA and MwoA subjects. It is possible that differences in stimulus sequence, duration, and type between studies may account for these discrepant findings. Differences in patient characteristics, such as headache frequency and severity, across studies might also contribute to this variability. Our study population had above average headache frequency compared to that reported in epidemiologic studies of general migraine patients (37), but perhaps less frequent headaches then might be seen in specialized headache clinics with severely affected patients, from which many studies might recruit research subjects. We are able to reject several alternative explanations for the greater visual cortex response seen in MWA within our study. Our groups were well matched in the basic demographic features of age and gender, and both migraine groups within our study had similar headache frequency. As mentioned earlier, we also obtained VDSs for our subjects and replicated prior studies showing enhanced visual sensitivity in migraine (1–3). VDSs did not differ between MWA and MwoA, supporting the contention that it is aura itself that is associated with altered interictal cortical responses, as opposed to interictal symptoms of visual sensitivity. A small number of control and MwoA subjects were missing VDS data because collection of the VDSs was added to the study procedures after enrollment had begun. While this would be unlikely to introduce a systematic bias in terms of subject responses, it could have reduced the precision of our estimates of visual discomfort and limited statistical power to show an association between VDS and BOLD response overall.
In conclusion, we found enhanced geniculo-striate responses specific to subjects with MwA and not modulated by alterations in resting perfusion. Despite equivalent descriptions of headache frequency and interictal symptoms, it is the presence of aura that predicts altered cortical physiology. Furthermore, this altered cortical physiology appears to correlate neuroanatomically with the typical localization of visual aura symptoms (i.e. starting from the central visual field). These observations imply a direct connection between cortical hyperresponsiveness and aura itself, and suggest that future studies of cortical alteration in migraine should carefully segregate patients with and without aura.
Clinical implications
Patients with migraine, both with and without aura, have enhanced visual discomfort in between headache attacks.
Only patients with migraine with aura show evidence of enhanced visual cortex activation when presented with a visual stimulus, suggesting that migraine with aura should be viewed as relatively distinct from migraine without aura on a physiological basis.
Acknowledgments
Funding This study was funded by grants from the National Institute of Neurological Disorders and Stroke (NS061572 to B.C; NS058386, NS045839, RR002305 to J.D.).
Footnotes
Conflict of interest None declared.
References
- 1.Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache. 1997;37:492–495. doi: 10.1046/j.1526-4610.1997.3708492.x. [DOI] [PubMed] [Google Scholar]
- 2.Vanagaite J, Pareja JA, Storen O, et al. Light-induced discomfort and pain in migraine. Cephalalgia. 1997;17:733–741. doi: 10.1046/j.1468-2982.1997.1707733.x. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd AJ. Visual contrast processing in migraine. Cephalalgia. 2000;20:865–880. doi: 10.1046/j.1468-2982.2000.00119.x. [DOI] [PubMed] [Google Scholar]
- 4.Coppola G, Pierelli F, Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia. 2007;27:1427–1439. doi: 10.1111/j.1468-2982.2007.01500.x. [DOI] [PubMed] [Google Scholar]
- 5.Ambrosini A, Schoenen J. Electrophysiological response patterns of primary sensory cortices in migraine. J Headache Pain. 2006;7:377–388. doi: 10.1007/s10194-006-0343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98:4687–4692. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambrosini A, de Noordhout AM, Sándor PS, et al. Electrophysiological studies in migraine: A comprehensive review of their interest and limitations. Cephalalgia. 2003;23:13–31. doi: 10.1046/j.1468-2982.2003.00571.x. [DOI] [PubMed] [Google Scholar]
- 8.Detre JA, Wang J, Wang Z, et al. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. 2009;22:348–355. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- 9.Conlon EG, Lovegrove WJ, Chekaluk E, et al. Measuring visual discomfort. Vis cogn. 1999;6:637–663. [Google Scholar]
- 10.Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 11.Aguirre GK, Zarahn E, D'Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Aguirre GK, Rao H, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 15.Fischl B, Sereno MI, Tootell RB, et al. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinds OP, Rajendran N, Polimeni JR, et al. Accurate prediction of V1 location from cortical folds in a surface coordinate system. Neuroimage. 2008;39:1585–1599. doi: 10.1016/j.neuroimage.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benson NC, Butt O, Datta R, et al. Surface structure and retinotopic function are tightly linked within human striate cortex. Curr Biol. 2012 in press. [Google Scholar]
- 18.Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Afra J, Cecchini AP, De Pasqua V, et al. Visual evoked potentials during long periods of pattern-reversal stimulation in migraine. Brain. 1998;121(Pt 2):233–241. doi: 10.1093/brain/121.2.233. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Parrish TB. Caffeine dose effect on activation-induced BOLD and CBF responses. Neuroimage. 2009;46:577–583. doi: 10.1016/j.neuroimage.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarchielli P, Tarducci R, Presciutti O, et al. Functional 1H-MRS findings in migraine patients with and without aura assessed interictally. Neuroimage. 2005;24:1025–1031. doi: 10.1016/j.neuroimage.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson M, Blau JN. Are classical and common migraine different entities? Headache. 1985;25:211–213. doi: 10.1111/j.1526-4610.1985.hed2504211.x. [DOI] [PubMed] [Google Scholar]
- 23.Russell MB, Rasmussen BK, Fenger K, et al. Migraine without aura and migraine with aura are distinct clinical entities: A study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia. 1996;16:239–245. doi: 10.1046/j.1468-2982.1996.1604239.x. [DOI] [PubMed] [Google Scholar]
- 24.Russell MB, Ulrich V, Gervil M, et al. Migraine without aura and migraine with aura are distinct disorders. A population-based twin survey. Headache. 2002;42:332–336. doi: 10.1046/j.1526-4610.2002.02102.x. [DOI] [PubMed] [Google Scholar]
- 25.Ayata C, Jin H, Kudo C, et al. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59:652–661. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- 26.Noseda R, Kainz V, Jakubowski M, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain. 1996;119(Pt 2):355–361. doi: 10.1093/brain/119.2.355. [DOI] [PubMed] [Google Scholar]
- 28.Coppola G, Ambrosini A, Di Clemente L, et al. Interictal abnormalities of gamma band activity in visual evoked responses in migraine: An indication of thalamocortical dysrhythmia? Cephalalgia. 2007;27:1360–1367. doi: 10.1111/j.1468-2982.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 29.Antal A, Polania R, Saller K, et al. Differential activation of the middle-temporal complex to visual stimulation in migraineurs. Cephalalgia. 2011;31:338–345. doi: 10.1177/0333102410379889. [DOI] [PubMed] [Google Scholar]
- 30.Raichle ME. Behind the scenes of functional brain imaging: A historical and physiological perspective. Proc Natl Acad Sci U S A. 1998;95:765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jueptner M, Weiller C. Review: Does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage. 1995;2:148–156. doi: 10.1006/nimg.1995.1017. [DOI] [PubMed] [Google Scholar]
- 32.Reyngoudt H, Achten E, Paemeleire K. Magnetic resonance spectroscopy in migraine: What have we learned so far? Cephalalgia. 2012;32:845–859. doi: 10.1177/0333102412452048. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Cooper TG, Satana B, et al. Visual distortion provoked by a stimulus in migraine associated with hyperneuronal activity. Headache. 2003;43:664–671. doi: 10.1046/j.1526-4610.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Zong X, Wilkins A, et al. fMRI evidence that precision ophthalmic tints reduce cortical hyperactivation in migraine. Cephalalgia. 2011;31:925–936. doi: 10.1177/0333102411409076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulloche N, Denuelle M, Payoux P, et al. Photophobia in migraine: An interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry. 2011;81:978–984. doi: 10.1136/jnnp.2009.190223. [DOI] [PubMed] [Google Scholar]
- 36.Martin H, del Rio MS, de Silanes CL, et al. Photoreactivity of the occipital cortex measured by functional magnetic resonance imaging-blood oxygenation level dependent in migraine patients and healthy volunteers: Pathophysiological implications. Headache. 2011;51:1520–1528. doi: 10.1111/j.1526-4610.2011.02013.x. [DOI] [PubMed] [Google Scholar]
- 37.Lipton RB, Stewart WF, Diamond S, et al. Prevalence and burden of migraine in the United States: Data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]