Abstract
Spermine (SPM) and spermidine (SPD), endogenous polyamines (PA) with the ability to modulate various ion channels and receptors in the brain, exert neuroprotective, antidepressant, antioxidant and other effects in vivo such as increasing longevity. These PA are preferably accumulated in astrocytes, and we hypothesized that SPM increases glial intercellular communication by interacting with glial gap junctions. Results obtained in situ, using Lucifer yellow propagation in the astrocytic syncitium of 21–25 day old rat CA1 hippocampal slices, showed reduced coupling when astrocytes were dialyzed with standard intracellular solutions (ICS) without SPM. However, there was a robust increase in the spreading of Lucifer yellow via gap junctions to neighboring astrocytes when the cells were patched with ICS containing 1 mM SPM; a physiological concentration in glia. Lucifer yellow propagation was inhibited by gap junction blockers. Our findings show that the glial syncitium propagates SPM via gap junctions and further suggest a new role of polyamines in the regulation of the astroglial network in both normal and pathological conditions.
Keywords: polyamines, spermine, dye propagation, astrocyte coupling, gap junction channels
Introduction
Astrocytes act as important players in the biochemical control of the brain microenvironment via ion and molecular signaling. Prominent features of astrocytes are their highly negative membrane potential and their ability to signal to each other via gap junctions [1]. In addition, astrocytes release glio-transmitters into the extracellular space via unapposed connexin hemichannels, and strongly affect neurons and blood vessels [1,2]. Gap junction channels are formed of connexin subunits, which belong to the large family of connexin proteins, with twenty-one connexin molecules identified in vertebrates to-date [1]. Functional gap junctions have a central aqueous pore of ~1.0–1.5 nm diameter that permits the passage of ions (electrical coupling) and molecules of less than ~1.1 kDa (metabolic coupling) [2,3]. Exchange of information in the glial syncitium through gap junctions is tightly regulated by a variety of extra- and intracellular factors, including PKC, calcium and ATP [3].
Expression of connexin subunits is regulated throughout development and typically different cell types express more than one connexin isoform. In the adult rodent brain, Cx43 is considered to be the dominant subunit in astrocytes, accompanied by lower expression levels of Cx30 and Cx26 subunits [1]. Cx43 mRNA and protein levels can change dramatically in neurodegenerative diseases [4,5], epilepsy [6] and excitotoxic injury [7]. The consequences of wide-spread gap junctional communication in astrocytes are still largely unknown, although suggested functions involve potassium buffering [8], glutamate and GABA uptake [1], large-scale distribution of energetic substrates and spreading of intercellular signaling molecules [1]. Furthermore, molecular signaling between astrocytes is only stopped when both Cx43 and Cx30 are genetically knocked out [9,10].
Methodologically, the extent of gap junctional intercellular coupling is usually examined by monitoring propagation of fluorescent signals in situ after intracellular injection of low-molecular weight dyes in the glial syncitium. Results of such studies in brain slices revealed dependence of dye-spreading on the area of the brain, charge, shape and molecular weight of the dye and the developmental status of the animal [11–14].
The endogenous polyamine spermine (SPM) is accumulated almost exclusively in glial cells, not in neurons [15,16], and could potentially modify astrocytic gap junctional coupling. However, in standard whole-cell voltage-clamp conditions, endogenous polyamines will be washed out within two minutes of attainment of whole-cell configuration in glial cells [17]. These polyamine molecules are potent modulators of receptors and channels in neurons and glia such as glutamate (NMDA, AMPA and kainate) receptors, Kir channels [17,18], TRP channels and others [19] with SPM being more potent than lower molecular weight polyamines, such as spermidine [18]. In addition, SPM causes an unusual biphasic block of glial inward rectifier channels [17]. Finally, SPM has also been shown to block Cx40 gap junctions [20]; this connexin, however, is not found in astrocytes.
Given that SPM is accumulated in glia we asked to what extent does SPM affect gap junction channels in these cells, and how does SPM influence either electrical communication or drug permeation via gap junctional coupling in situ? In this study, we first aimed to characterize the effects of intracellular SPM on Lucifer yellow-propagation from one astrocyte to another by testing SPM-dependent biochemical coupling in stratum radiatum area of CA1 hippocampus, using acute brain slices of adult Sprague-Dawley rats. This model was used previously and shows a very low rate of astrocytic Lucifer yellow-coupling [13,14] and should allow us to easily visualize gap junctional coupling in response to SPM. We show that SPM is a key and potent modulator of gap junction channels in the astrocytic syncitium.
Methods
Animals
All procedures were performed in accordance with the National Institutes of Health guidelines for the humane treatment of laboratory animals and the Institutional Animal Care and Use Committee.
Electrophysiology and morphology
Transverse, 350 µm thick hippocampal slices were prepared from the brains of Sprague-Dawley female rats age P21-P25, dissected in ice-cold ACSF saturated with 5%CO2/95%O2. Slices were cut on a vibratome (VT1000S Leica, Germany) and incubated for recovery in a standard ACSF solution containing (in mM): 127 NaCl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 10 glucose, 26 NaHCO3, gassed with 5%CO2/95%O2, pH 7.4 at 35°C for 20 minutes and then at room temperature (osmolarity 305 mosm/l). After 30 minutes of total incubation, slices were placed in a recording chamber and continuously superfused with ACSF at room temperature (23–24°C). Cells were visualized using an Olympus infrared microscope equipped with DIC (BX51WI Olympus, Japan). DP30BW digital camera and DP Controller software (Olympus, Japan) were used to visualize and record the spread of Lucifer yellow fluorescence through the glial cell syncitium. Counting of coupled cells was done in a single X-Y plane, not in 3D dimensions (non-confocal) and was equally applied for all slices and procedures tested.
Two piezoelectric micromanipulators (MX7500 with MC- 1000 drive, Siskiyou, Inc., Grants Pass, OR) were used for voltage-clamp and current-clamp recording and for positioning micropipettes. Astrocytes were clamped using patch pipettes made from borosilicate glass tubing (O.D. 1.5 mm, I.D. 1.0 mm; World Precision Instr., Sarasota, FL) pulled in four steps using a P-97 puller (Sutter Instr. Co., Novato, CA) and filled with intracellular solutions (ICS) containing (in mM) for K-gluconate solution A (similar to [14,16]): 117 K-gluconate, 13 KCl, 2 MgCl2, 10 HEPES, pH adjusted to 7.2 with KOH (osmolarity 275 mosm/l). Solution B was as A with addition of 1 K2-ATP, 10 Phosphocreatine, 0.3 Na2-GTP and EGTA 0.1 (osmolarity ~301 mosm/l, pH=7.2). Solution C was as A with substitution of potassium salts by cesium salts (in mM): CsF 125, CsCl 20 with addition of EGTA 2.5 mM (osmolarity ~308 mosm/l, pH=7.2). Lucifer yellow potassium salt CH (Sigma-Aldrich, St. Louis, MO) at a final concentration of 2 mM in the above ICSs was used throughout the dye-propagation experiments. Carbenoxolone (CBX) (3-hydroxy-11-oxoolean-12-en-30-oic acid chloride salt) and 18β-Glycyrrhetinic acid dissolved in ACSF to 200 µM were obtained from Sigma-Aldrich (St.Louis, MO) and were used as blockers of gap junctions in the brain slice preparations. SPM (chloride salt, Sigma-Aldrich, MO) was used at a concentration close to that found in glial cells, 1 mM [17]. After filling with ICS, the final micropipette resistance was near 8 MΩ which was optimized for astrocyte recordings to achieve gigaseals on cell membranes > 3GΩ. Voltage clamping and current recording in whole-cell patch-clamp mode were performed using a MultiClamp 700A patch clamp amplifier with a DigiData 1322A interface (Molecular Devices, Inc., Sunnyvale, CA). The pClamp 10 software package (Molecular Devices, Inc., CA) was used for data acquisition and analysis. Astrocyte recordings were accepted only if membrane potential was negative to ~–70 mV and if cells had linear current-voltage relation (passive astrocytes) and low input resistance (less than 20MΩ). Note: brain slices older than 1 hour were not used.
Data Analysis
Data were analyzed using Origin 8 software (OriginLab, Northampton, MA) and are reported as mean ± standard error of the mean. Significant differences between groups of data were evaluated using Student`s paired t-test.
Results
SPM significantly increases spreading of Lucifer yellow through carbenoxolone (CBX) sensitive gap junctions between astrocytes located in the stratum radiatum of rat CA1 hippocampus
A first set of the experiments was performed to answer the question: does SPM affect Lucifer yellow propagation from one astrocyte to another in the astrocyte syncitium via CBX-sensitive connexin gap junctions? Experiments were performed using hippocampal brain slices under constant slice perfusion (1 mL/min) with ACSF. Identified astrocytes (by their size, shape and location) in the stratum radiatum layer of CA1 hippocampus were current-clamped in whole-cell mode. The cells were dialyzed for 10 min with potassium-based or cesium-based, Lucifer yellow-containing ICS with either zero or 1 mM SPM. After 10 min of dialysis without any additional electrical current injection, the number of coupled cells was counted, then the pipette was carefully withdrawn from the cell and fluorescent micrographs of the slice were obtained (Fig. 1). To avoid using astrocytes that were artificially decoupled during slice preparation (traumatic zone), we recorded only from astrocytes located at least 100 µm below the surface of the slice [14]. There was no obvious difference in astrocyte coupling when using either simple ICS-A, or ICS-B with supplements such as ATP, GTP, phosphocreatine, or when using cesium based ICS-C (data not shown). Also, 18β-Glycyrrhetinic acid (200 µM) produced similar results to CBX (not shown). Therefore, we used ICS-A and CBX for data collection and statistics in our Lucifer yellow propagation studies. Lucifer yellow was used to study coupling as previously described [10,13,14]. Both control and SPM-treated recordings were done in each slice, in non-overlapping areas within 30 min of each. In some experiments, controls were performed first, while in others SPM-treatment occurred first. Microscopic images revealed marked increases in dye-propagation through astrocytic gap junctions using SPM-enriched ICS (Fig.1B) as compared to SPM-free control ICS (Fig.1A). To examine the mode of propagation, we examined the effects of CBX (200 µM), a gap junction-uncoupler. Slices were perfused with CBX prior to penetration with a pipette containing ICS with or without SPM. This manipulation effectively blocked the effect of SPM in perfused slices (Fig. 1C), reducing the number of coupled cells to 1.9±0.2 (N=8) when compared to 11.0±1.4 in SPM-dialyzed, non-CBX treated cells (N=15; p<0.0001).
Figure 1. Microscopic images of Lucifer yellow propagation in the astrocytic syncytium of adult rat.

A. Two cells were coupled in the control experiment using 117 mM K+ gluconate-based intracellular solution containing no spermine, while in B. injection of the cell with the same intracellular solution enriched by 1 mM spermine revealed a robust increase in cell-to-cell coupling (18 cells stained total). C. The SPM-induced increase in coupling was abolished in slices pretreated with 200 µM carbenoxolone. Images were taken 10 min after loading the fluorescent dye through the patch pipette. Note the different scale bar in B.
Lack of correlation between Lucifer yellow coupling and astrocytic membrane potential
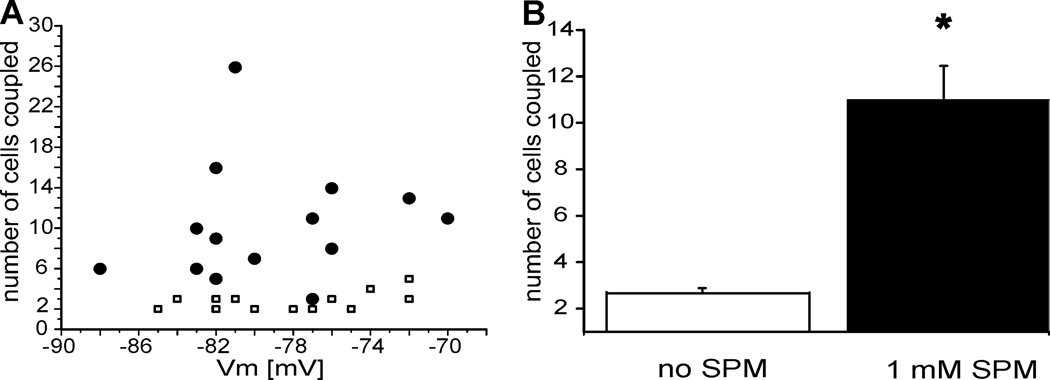
A second set of experiments was performed to test membrane potential-dependence of Lucifer yellow propagation in CBX-free ACSF. Astrocytic membrane potentials ranged from −70 to −90 mV, when measured 1 minute after entering current-clamped whole-cell mode, and we counted the number of coupled cells 10 min after patch rupture. We found no significant relationship between the number of dye-coupled cells and the resting membrane potential in either control (Fig 2A, white squares, N=13) or SPM-treated astrocytes (Fig. 2A, black filled circles, N=14). The data averaged from different rats are presented in Figure 2B. These further illustrate the significant (p<0.01) increase in cell coupling after inclusion of SPM in the patch solution (Fig 2B). The maximal number of coupled astrocytes was 26 when using ICS with SPM (Fig. 2A, filled circles), while two to no more than 5 astrocytes were coupled in SPM-free ICS (Fig. 2A, open squares).
Figure 2. Intracellular spermine increases Lucifer yellow coupling between astrocytes located in the stratum radiatum of CA1 hippocampus.
A. Cell-to-cell coupling is not dependent on the membrane potential either in the control group (white-filled squares) or in the spermine-treated group (black-filled circles) B. Intracellular loading of fluorescent dye (2 mM Lucifer yellow) revealed significant difference between the number of cells coupled in the control group (2.7±0.2) and in the spermine-treated group (11±1.4). The asterisk indicates a significant difference between control and SPM-treated groups (p<0.01, N=15 in each group).
Discussion
This study describes a previously unrecognized role of the ubiquitously present polyamine, SPM, in the regulation of the astroglial network in situ. SPM is preferentially accumulated in hippocampal and cortical astrocytes, Bergman glia in cerebellum [15] and in retinal Müller glial cells [16]. SPM has marked effects on glial potassium channel activity [16,17] and may regulate other glial receptors and channels. Our results show that SPM, when included intracellularly at a concentration shown to be physiologically present in vivo in glial cells [17], dramatically increases Lucifer yellow propagation through the rat hippocampal glial syncitium. Cx43-based gap junctions are a likely route for inter-glial chemical propagation, and our results further show that blockade of gap junctions by CBX blocks the SPM enhancement of coupling, suggesting that SPM acts via gap junction activation.
In agreement with previous studies using the same experimental model (Sprague Dawley rats), we also show relatively low levels of Lucifer yellow propagation in the astrocytes of stratum radiatum of CA1 hippocampus [13,14] in ICS without SPM. However, when SPM was present in the cytoplasm (i.e., introduced through the patch pipette), Lucifer yellow diffusion to other astrocytes was considerably enhanced, and this enhancement was sensitive to CBXi. These data indicate that intracellular SPM at physiological concentrations facilitates gap junctional communication between astrocytes. Furthermore, these data imply that SPM itself propagates through gap junctions because the dye spreads further than just the adjacent astrocyte and can cause coupling of up to 26 cells in the syncitium.
Previously, it was shown that Cx40 gap junction channels are blocked by SPM in a voltage- and concentration-dependent manner [20], although Cx40 is not expressed in astrocytes [1–3] and SPM actions on glial gap junctions are not known. Glutamate residues at position 9 and 13 of Cx40 are responsible for SPM block. If these are replaced with the lysine residues found at these positions in Cx43 block by SPM is eliminated [21].
Possible cytosolic actions of SPM could involve direct interaction with connexin subunits expressed in astrocytes (Cx43, Cx30 and Cx26), as was previously shown in the case of non-glial expressed Cx40-containing homotypic gap junctions [18,21]. We cannot also rule out the possibility that the effect of SPM is indirect, through an action on intrinsic metabolic pathways of astrocytes. Gene activation is unlikely given the 10 minute time of our experiments. On the other hand, second messengers involving G-proteins and PKC with tyrosine kinase (which regulates connexin gap junctions and hemichannels) could be targets. To determine the exact mechanism of SPM action will require further study and is not considered for this short report.
Conclusion
Our data show that SPM significantly increases gap junction coupling in the astrocytic syncitium and suggest a novel role of polyamines in regulation of the astroglial network. Future experiments examining the astrocytic syncitium and its regulation should consider maintaining intracellular levels of SPM close to normal levels (which in most cells are typically at total concentrations in the order of 1 mM with estimated free concentrations from ~8 (in liver cells [22]), ~80 (in neurons [18,23]) to ~800 µM (in retinal glia [17]), thereby maintaining cell coupling (Figs. 1 and 2), K channel rectification [16], and other SPM-dependent regulatory features.
Acknowledgement
The authors thank Paola López Pieraldi and Natalia Skachkova for their superior technical assistance.
Source of funding: This work was supported by NIH grants R01-NS065201-03 (to S.N.S) and 8G12-MD007583-27 (for core facilities at UCC) from NINDS and NIMHD. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Footnotes
Statement of conflicts of interest: none declared
References
- 1.Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MV, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 2003;26:610–617. doi: 10.1016/j.tins.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theis M, Sohl G, Eiberger J, Willecke K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 2005;28:188–195. doi: 10.1016/j.tins.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Zeng LH, Wong M. Impaired astrocytic gap junction coupling and potassium buffering in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2009;34:291–299. doi: 10.1016/j.nbd.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand-Schieber E, Werner P, Iacobas DA, Iacobas S, Beelitz M, Lowery SL, et al. Connexin43, the major gap junction protein of astrocytes, is down-regulated in inflamed white matter in an animal model of multiple sclerosis. J Neurosci Res. 2005;80:798–808. doi: 10.1002/jnr.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garbelli R, Frassoni C, Condorelli DF, Trovato Salinaro A, Musso N, Medici V, et al. Expression of connexin 43 in the human epileptic and drug-resistant cerebral cortex. Neurology. 2011;76:895–902. doi: 10.1212/WNL.0b013e31820f2da6. [DOI] [PubMed] [Google Scholar]
- 7.Vukelic JI, Yamamoto T, Hertzberg EL, Nagy JI. Depletion of connexin43-immunoreactivity in astrocytes after kainic acid-induced lesions in rat brain. Neurosci Lett. 1991;130:120–124. doi: 10.1016/0304-3940(91)90242-l. [DOI] [PubMed] [Google Scholar]
- 8.Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience. 2004;129:1045–1056. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallraff A, Kohling R, Heinemann U, Theis M, Willecke K, Steinhauser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 2006;26:5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 11.Schools GP, Zhou M, Kimelberg HK. Development of gap junctions in hippocampal astrocytes: evidence that whole cell electrophysiological phenotype is an intrinsic property of the individual cell. J Neurophysiol. 2006;96:1383–1392. doi: 10.1152/jn.00449.2006. [DOI] [PubMed] [Google Scholar]
- 12.D'Ambrosio R, Wenzel J, Schwartzkroin PA, McKhann GM, 2nd, Janigro D. Functional specialization and topographic segregation of hippocampal astrocytes. J Neurosci. 1998;18:4425–4438. doi: 10.1523/JNEUROSCI.18-12-04425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bordey A, Sontheimer H. Postnatal development of ionic currents in rat hippocampal astrocytes in situ. J Neurophysiol. 1997;78:461–477. doi: 10.1152/jn.1997.78.1.461. [DOI] [PubMed] [Google Scholar]
- 14.Cotrina ML, Kang J, Lin JH, Bueno E, Hansen TW, He L, et al. Astrocytic gap junctions remain open during ischemic conditions. J Neurosci. 1998;18:2520–2537. doi: 10.1523/JNEUROSCI.18-07-02520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laube G, Veh RW. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia. 1997;19:171–179. doi: 10.1002/(sici)1098-1136(199702)19:2<171::aid-glia8>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Skatchkov SN, Eaton MJ, Krusek J, Veh RW, Biedermann B, Bringmann A, et al. Spatial distribution of spermine/spermidine content and K(+)-current rectification in frog retinal glial (Muller) cells. Glia. 2000;31:84–90. doi: 10.1002/(sici)1098-1136(200007)31:1<84::aid-glia80>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Kucheryavykh YV, Shuba YM, Antonov SM, Inyushin MY, Cubano L, Pearson WL, et al. Complex rectification of Muller cell Kir currents. Glia. 2008;56:775–790. doi: 10.1002/glia.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams K. Interactions of polyamines with ion channels. Biochem J. 1997;325(Pt 2):289–297. doi: 10.1042/bj3250289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahern GP, Wang X, Miyares RL. Polyamines are potent ligands for the capsaicin receptor TRPV1. J Biol Chem. 2006;281:8991–8995. doi: 10.1074/jbc.M513429200. [DOI] [PubMed] [Google Scholar]
- 20.Musa H, Veenstra RD. Voltage-dependent blockade of connexin40 gap junctions by spermine. Biophys J. 2003;84:205–219. doi: 10.1016/S0006-3495(03)74843-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musa H, Fenn E, Crye M, Gemel J, Beyer EC, Veenstra RD. Amino terminal glutamate residues confer spermine sensitivity and affect voltage gating and channel conductance of rat connexin40 gap junctions. J Physiol. 2004;557:863–878. doi: 10.1113/jphysiol.2003.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe S, Kusama-Eguchi K, Kobayashi H, Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J Biol Chem. 1991;266:20803–20809. [PubMed] [Google Scholar]
- 23.Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]