Abstract
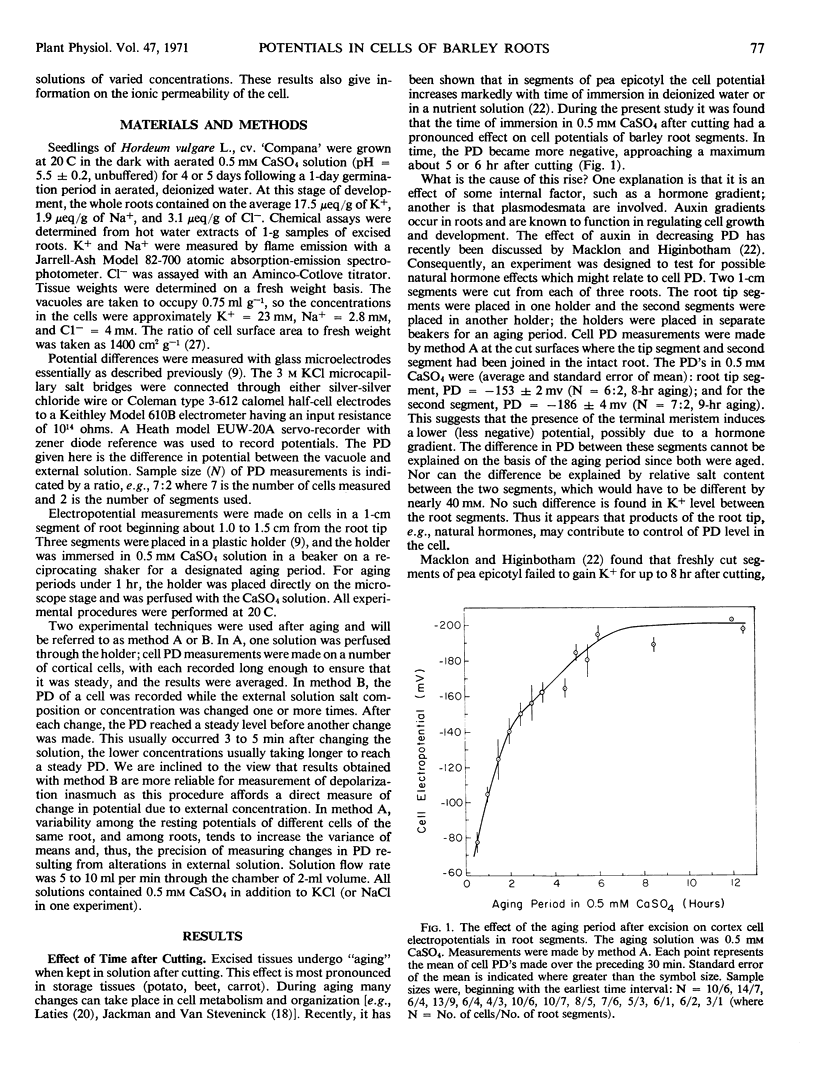
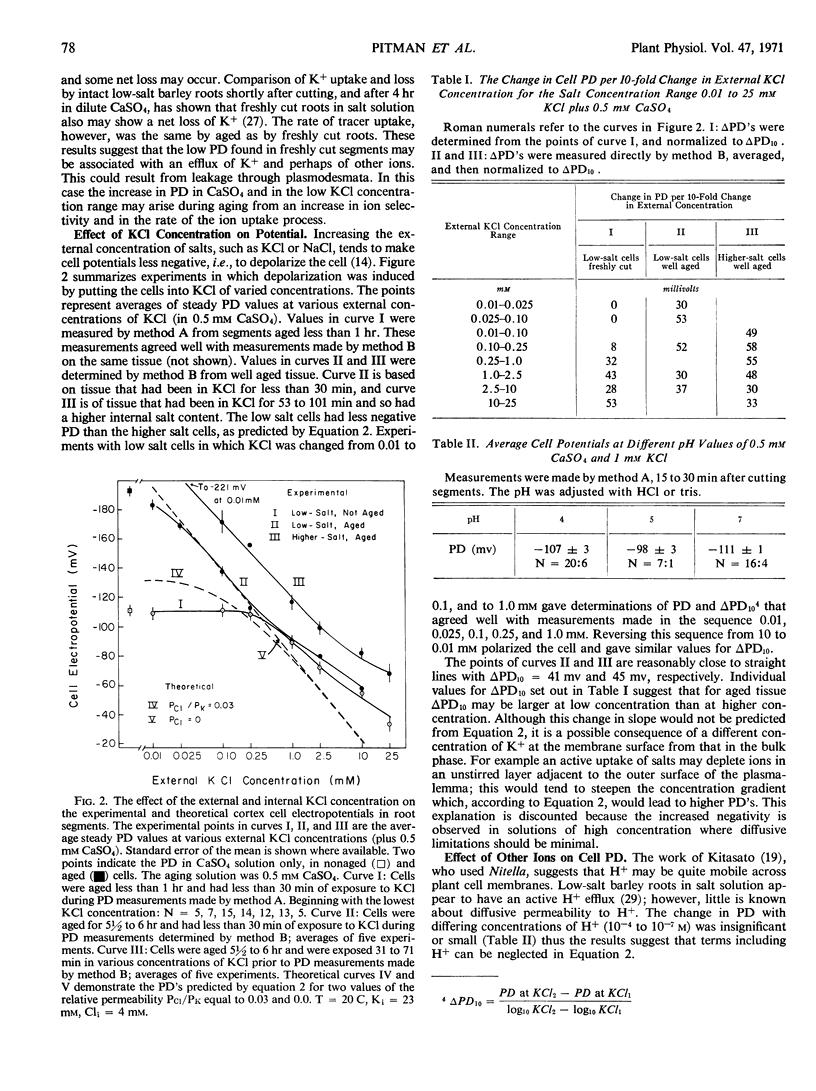
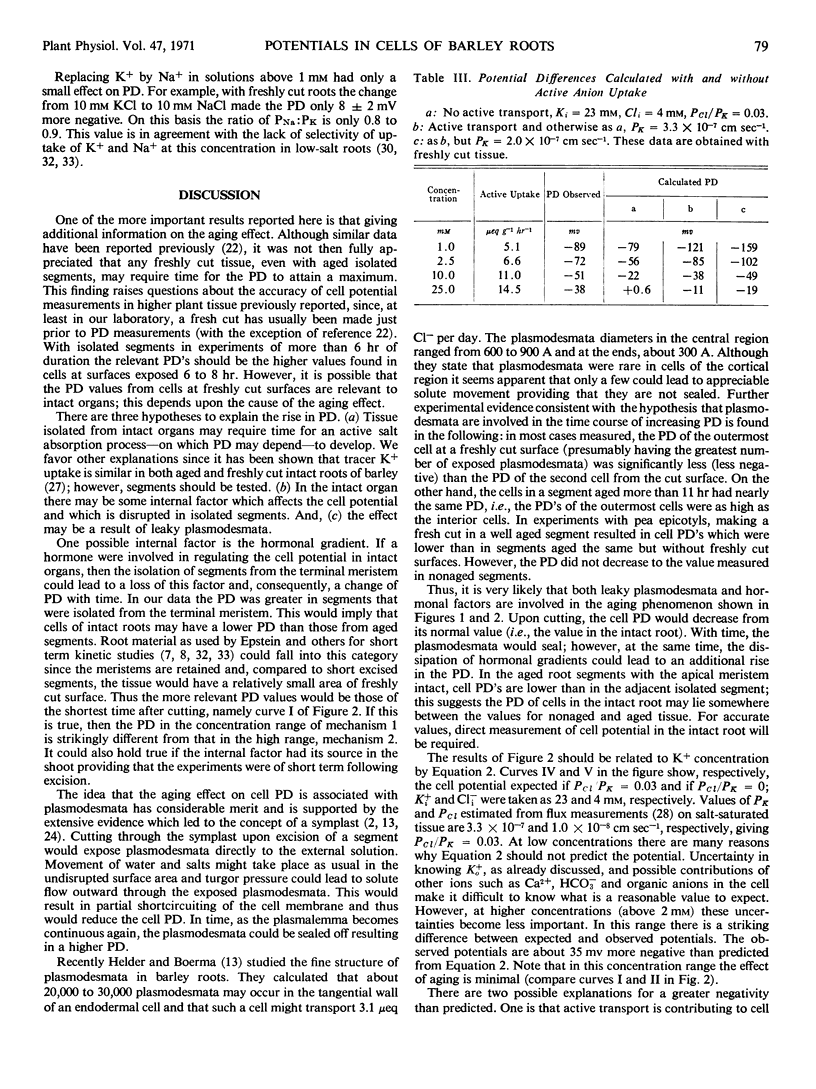
Single cell electropotentials of barley (Hordeum vulgare L., cv. `Compana') root cortex were measured at different external concentrations of KCl in the presence of Ca2+. The roots were low in salt from seedlings grown on 0.5 mm aerated CaSO4 solution. Thus, the conditions were equivalent to those used to define the dual mechanisms found with radioactive tracer-labeled ion uptake. In 0.5 mm CaSO4 alone, there is an increase with time of cell negativity from about -65 millivolts 15 minutes after cutting segments to about -185 millivolts in 6 to 8 hours. Two possible hypotheses, not mutually exclusive, are offered to explain this aging effect: that cutting exposes plasmodesmata which are leaky initially but which seal in time, and that some internal factors, e.g., hormones diffusing from the apex, have a regulatory effect on the cell potential, an influence which becomes dissipated in isolated segments and permits the development of a higher potential difference. In any case changes in selective ion transport must be involved. The cell potentials at KCl concentrations above 2.0 mm are more negative than would be expected for a passive diffusion potential. It is suggested that this discrepancy may be due to an electrogenic pump or to a higher K+ concentration in the cytoplasm than in the remainder of the cell, or perhaps to both. Whether there is a clear relationship between cell potential and mechanisms 1 and 2 of cation transport depends upon whether the cell potentials of freshly cut or of aged tissue represent the values relevant to intact roots.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ETHERTON B., HIGINBOTHAM N. Transmembrane potential measurements of cells of higher plants as related to salt uptake. Science. 1960 Feb 12;131(3398):409–410. doi: 10.1126/science.131.3398.409. [DOI] [PubMed] [Google Scholar]
- Epstein E., Rains D. W. CARRIER-MEDIATED CATION TRANSPORT IN BARLEY ROOTS: KINETIC EVIDENCE FOR A SPECTRUM OF ACTIVE SITES. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1320–1324. doi: 10.1073/pnas.53.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Rains D. W., Elzam O. E. RESOLUTION OF DUAL MECHANISMS OF POTASSIUM ABSORPTION BY BARLEY ROOTS. Proc Natl Acad Sci U S A. 1963 May;49(5):684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Mineral ion contents and cell transmembrane electropotentials of pea and oat seedling tissue. Plant Physiol. 1967 Jan;42(1):37–46. doi: 10.1104/pp.42.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitasato H. The influence of H+ on the membrane potential and ion fluxes of Nitella. J Gen Physiol. 1968 Jul;52(1):60–87. doi: 10.1085/jgp.52.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACROBBIE E. A., DAINTY J. Ion transport in Nitellopsis obtusa. J Gen Physiol. 1958 Nov 20;42(2):335–353. doi: 10.1085/jgp.42.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon A. E., Higinbotham N. Electropotential in excised pea epicotyls. Plant Physiol. 1968 Jun;43(6):888–892. doi: 10.1104/pp.43.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G. Adaptation of barley roots to low oxygen supply and its relation to potassium and sodium uptake. Plant Physiol. 1969 Sep;44(9):1233–1240. doi: 10.1104/pp.44.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G., Saddler H. D. Active sodium and potassium transport in cells of barley roots. Proc Natl Acad Sci U S A. 1967 Jan;57(1):44–49. doi: 10.1073/pnas.57.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G. Simulation of Cl Uptake by Low-salt Barley Roots as a Test of Models of Salt Uptake. Plant Physiol. 1969 Oct;44(10):1417–1427. doi: 10.1104/pp.44.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains D. W., Epstein E. Sodium absorption by barley roots: its mediation by mechanism 2 of alkali cation transport. Plant Physiol. 1967 Mar;42(3):319–323. doi: 10.1104/pp.42.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains D. W., Epstein E. Sodium absorption by barley roots: role of the dual mechanisms of alkali cation transport. Plant Physiol. 1967 Mar;42(3):314–318. doi: 10.1104/pp.42.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. M., Epstein E. The dual mechanisms of alkali cation absorption by plant cells: their parallel operation across the plasmalemma. Proc Natl Acad Sci U S A. 1968 Oct;61(2):447–453. doi: 10.1073/pnas.61.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]


