Abstract
Background
Current post-operative thyroid replacement dosing is weight-based with adjustments made following TSH values. This method can lead to significant delays in achieving euthyroidism and often fails to accurately dose over and underweight patients. Our aim was to develop an accurate dosing method that utilizes patient BMI data.
Study Design
A retrospective review of a prospectively collected thyroid database was performed. We selected adult patients undergoing thyroidectomy with benign pathology who achieved euthyroidism on thyroid hormone supplementation. BMI and euthyroid dose were plotted and regression was used to fit curves to the data. Statistical analysis was performed using STATA 10.1 (StataCorp, College Station, TX).
Results
122 patients met inclusion criteria. At initial follow-up, only 39 patients were euthyroid (32%). 53% of patients with a BMI>30 were overdosed, while 46 % of patients with BMI<25 were under-dosed. The line of best fit demonstrated an overall quadratic relationship between BMI and euthyroid dose. A linear relationship best described the data up to a BMI of 50. Beyond that, the line approached 1.1 mcg/kg. A regression equation was derived for calculating initial levothyroxine dose (mcg/kg/day = −0.018*BMI +2.13 {F statistic =52.7, root mean squared error of 0.24}).
Conclusion
The current standard of weight based thyroid replacement fails to appropriately dose underweight and overweight patients. BMI can be used to more accurately dose thyroid hormone using a simple formula.
Introduction
Following total thyroidectomy, optimal replacement of thyroid hormone is imperative, yet often challenging to achieve. Suppressive doses of levothyroxine increase the risks of accelerated bone loss, fractures, arrhythmias, and decreased left ventricular function (1–4). Prolonged periods of under treatment are associated with the clinical features of hypothyroidism, weight gain, dyslipidemia and cardiovascular dysfunction (5–7). Both over-treatment and under-treatment are sources of dissatisfaction for patients and a potential source of increased healthcare costs due to the increased frequency of laboratory testing and physician visits (8).
The majority of recommendations on thyroid hormone therapy originates from literature on primary hypothyroidism and is applied to the surgically induced hypothyroid patients (5, 9–13). In primary hypothyroidism, residual thyroid tissue may produce endogenous thyroid hormone complicating the true requirement of exogenous thyroxine. Thus, studies examining the surgically induced hypothyroid patients are ideal to accurately assess thyroid hormone replacement in this subset. Common practice for initial dosing of levothyroxine (LT4) is weight based, with a recommended range of 1.6–1.7 mcg/kg/day with certain reports advocating up to 2.1 mcg/kg (9–13). Dose adjustments are subsequently made following serial thyroid stimulating hormone (TSH) concentrations and clinical evaluation. Goal TSH levels are dependent on pathology. In thyroid cancer, TSH suppression is preferred as adjuvant treatment to reduce tumor reoccurrence (14). Therefore, this study focuses on patients with benign thyroid disease whose goal is achieving a TSH value in the normal range.
According to the surgical literature, the time to achieve euthyroidism following thyroidectomy is highly variable ranging from 2 weeks to −2.5 years, with a median of 3.6 months (15). Post-operatively, many patients require multiple dose adjustments over time prior to achieving euthyroidism (15). The conventional method of thyroid replacement therapy involved an empiric dose of 100–150 micrograms per day. Following this regimen, between 21–37% of patients attained a euthyroid state at initial follow up (15,16). The generation of sensitive TSH immunoassays allowed serial titrations of levothyroxine contingent on TSH values, creating weight-based dosing (WBD) more feasible (17). Unfortunately, widely practiced WBD for initial thyroid hormone replacement has not improved predictability of actual euthyroid dose (16,18). Sukumar et al (19) compared the empiric dosing method to WBD and found the latter to require significantly more visits before reaching goal TSH levels.
Multiple variables effecting LT4 requirements have been evaluated including age, sex, body weight, lean body mass, ideal body weight, body surface area, menopausal state, hormonal status, and pathology (18, 20–24). Moreover, co-ingestion of calcium supplements, ferrous sulfate, proton pump inhibitors, bile acid sequestrants, and sucralfate can modify LT4 absorption can easily be modified by and further complicate post-operative dosing (25–28).
A few studies suggest lean body mass (LBM) predicts LT4 requirement in both surgically induced and primary hypothyroid patients (23, 24). However more recent literature has shown no superior predictive value of LBM compared to actual body weight (15,19). From a practical standpoint, an accurate calculation of lean body mass (LBM) requires complicated techniques that are impractical in the clinical setting. Research using ideal body weight (IBW) has disclosed inconsistent outcomes (18, 20). Despite the variability of IBW and LBM, an inverse relationship between levothyroxine dose/kg and overall body weight has been consistently demonstrated (15,16,19). Lighter patients require a higher LT4 dose per kg compared to heaver patients (15, 16). Body surface area has also been strongly correlated with thyroxine requirement (19). Therefore, it is reasonable to hypothesize that BMI, given its inclusion of both height and weight, may be a superior predictive factor of initial thyroid hormone replacement following total thyroidectomy.
Literature on thyroid hormone replacement in surgically induced hypothyroidism remains scarce and lacks a single predictive factor with a strong correlation to accurately dose levothyroxine following surgery. The purpose of this study was to develop a simple algorithm incorporating BMI to improve predictability of initial LT4 dose.
Patients and Methods
A retrospective review of a prospectively collected thyroid database was performed, and data was abstracted between January 2008 and December 2010. Inclusion criteria were patients who underwent a total thyroidectomy for benign disease, followed-up at 6–8 weeks for postoperative TSH measurement, and reached normal thyroid function after surgery. Thyroid cancer patients were not included because of the higher dose required to achieve their goal of a suppressed TSH. Excluded were those <18 years old, patients pregnant within one year following surgery, those who received T3 supplementation or desiccated thyroid hormone preparations, and gastric bypass patients. A total of 146 patients met criteria and were included in this study. Twenty-four patients were then excluded for not achieving euthyroidism for reasons including irregular follow up visits, transfer back to home facility, and patient noncompliance with medications. Variables collected included age, autoimmune disease, BMI, and estrogen use. Height and weight were objectively measured at the pre-operative clinic visit prior to surgery and used to calculate BMI.
Prior to surgery, patients were given verbal instructions regarding proper administration of levothyroxine. Patients were instructed to take levothyroxine on an empty stomach, to wait 30 minutes prior to eating, and to separate their thyroid hormone from calcium, vitamins, or iron supplementation by 4 hours; and to take levothyroxine at the same time every day. Total thyroidectomy was performed by one of two endocrine surgeons at our institution. Thyroid hormone replacement was initiated on post-operative day one, with a recommended dose of 1.6 mcg/kg/day based on actual body weight. All patients were prescribed name brand thyroid hormone unless the cost was prohibitive due to lack of insurance coverage. All patients were maintained on the same LT4 preparation they were initiated on to avoid brand switches as a confounding factor.
Patients were seen at a 6–8 week post-operative visit where TSH values were obtained and dose adjustments made accordingly by the surgeon. The primary end point was achievement of euthyroidism; defined as a serum TSH of 0.45–4.50 uIU/mL. Either our endocrine surgery nurse practioner or an endocrinologist followed those patients over or under dosed after the initial visit, every 6–8 weeks with serial TSH measurements and dose titration. The time to achieve euthyroidism was defined as the time from their surgery to the first normal TSH value.
Statistical Analysis
Multivariate logistic regression was performed to identify the relative impact of age, autoimmune disease, BMI and estrogen use as predictors of failure to achieve euthyroid at initial 6–8 week visit. Gender was excluded due to lack of male patients to provide accurate predictive ability.
Binary comparisons were performed with the student’s t-test and Pearson Chi square test where appropriate. Comparisons of multiple groups were carried out by analysis of variance (ANOVA). Euthyroid dose and BMI were plotted and a quadratic equation was used to determine the limit on BMI. Next, a line of best fit was used to derive a formula from the linear portion of the curve. Patients with a BMI > 50 were excluded. Statistical analysis was performed using STATA 10.1 Results were expressed as the mean ± standard error of the mean. P<0.05 was considered significant.
Results
Patient Characteristics
122 patients met inclusion criteria for benign thyroid disease. Pathology was comprised of benign, multi-nodular goiter, adenoma, Hashimoto’s thyroiditis, Graves’s disease, and hyperplasia (table I). Fifty patients had autoimmune disease (Graves’ disease or Hashimoto’s Thyroiditis). Study participants included 21 males and 101 females. Our study group ranged in age from 18–75.6 years with an average of 49.0 years. Before surgery, mean weight was 84.5 kg (range 44.9–200.9 kg) and mean height of 1.7 meters (range 1.49–1.88 meters). BMI followed a Gaussian distribution, with an average of 30.5 (range 15.1–71.5). The mean initial dose of LT4 following total thyroidectomy was 1.58 mcg/kg/day. The median follow up duration was 4 months, but this was highly variable as patients were followed until they were euthyroid (range of 2–29 months).
Table I.
Baseline Characteristics and Statistics of Study Participants (n=122)
| Characteristics | n | Percentile |
|---|---|---|
| Gender | ||
| Women | 101 | 82.8 |
| Men | 21 | 17.2 |
| Pathology | ||
| Benign | 10 | 8.2 |
| Multi-nodular goiter | 48 | 39.3 |
| Adenoma | 11 | 9.0 |
| Hashimoto’s Thyroiditis | 28 | 23.0 |
| Grave’s disease | 22 | 18.0 |
| Hyperplasia | 3 | 2.5 |
| Descriptive Statistics | Mean ± SEM |
|---|---|
| Age, years | 49.0 ± 1.2 |
| Height, meters | 1.7 ± .0.007 |
| BMI, kg/m2 | 30.5 ± 0.8 |
| Initial dose of LT4*, mcg/kg/day | 1.58 ± 0.02 |
Following weight-based dosing regimen of 1.6 mcg/kg/day
BMI=body mass index calculated as weight in kilograms divided by the square of the height in meters
LT4=levothyroxine
Status at 6–8 weeks post-operatively
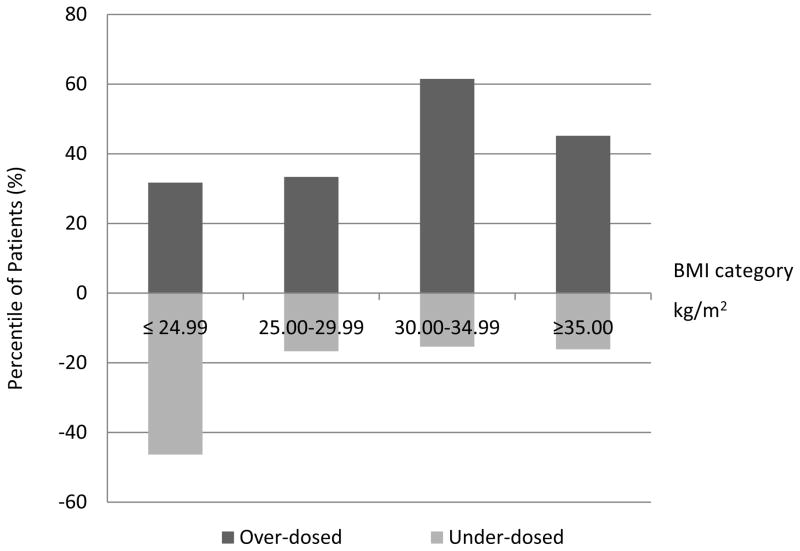
At initial 6–8 week post-operative visit, 39 patients were euthyroid (32%), 32 were under-dosed (26.2%) and 51 were over-dosed (41.8%, table II). In comparing patients who achieved euthyroidism at 6–8 weeks to those who did not, age and gender were not significantly different (p=0.93 and p=0.71, respectively). Additionally, there was no significant difference of initial dosing between those that achieved euthyroidism and those that did not (p=0.34) Patients initially over treated tended to be older than patients initially under treated, however this did not reach significance (p=0.06). The average BMI in those over-dosed was significantly higher compared to under-dosed patients (32.3 and 27.0, respectively, p=0. 018). In other words, patients with a lower BMI tended to be under dosed, while those of a higher BMI were more often over dosed (figure 1). Further, persons with a BMI <25.0 were significantly associated with under replacement of LT4 (figure I, p=0. 001). BMI was significantly different between those patients that achieved euthyroidism and those that did not (table II, p=.030). The incidence of autoimmune disease also did not differ between those with normal and abnormal TSH values (p=0.69).
Table II.
Status at 6–8 week follow up post operatively
| TSH (uIU/mL) | |||||
|---|---|---|---|---|---|
| Variables | All | Over-dosed (<0.45) | Euthyroid (0.45–4.5) | Under-dosed (>4.5) | p value |
| n (%) | 122 | 51 (41.80%) | 39 (31.96%) | 32 (26.23%) | |
| Age, years | 49.0 ± 1.2 | 51.3 ± 1.8 | 48.8 ± 2.2 | 45.5 ± 2.6 | p=0.19 |
| BMI, kg/m2 | 30.5 ± 0.8 | 32.3 ± 1.5 | 31.0 ± 1.2 | 27.0 ± 1.5 | p=0.030 |
| Initial dose of LT4, mcg/kg/day | 1.58 ± 0.02 | 1.61 ± 0.03 | 1.58 ± 0.05 | 1.53 ± 0.03 | p=0.34 |
| Eventual Euthyroid dose of LT4, mcg/kg/day | 1.61 ± 0.03 | 1.46 ± 0.03 | 1.58 ± 0.05 | 1.89 ± 0.07 | p<.0001 |
TSH= Thyroid Stimulating Hormone
BMI= body mass index calculated as weight in kilograms divided by the square of the height in meters
LT4= levothyroxine
Data are represented as the mean +/− SEM
Differences among groups analyzed with ANOVA
Fig. I.
Distributions of patients not euthyroid at initial 6–8 week follow up by BMI category
BMI=body mass index calculated as weight in kilograms divided by the square of the height in meters
Achievement of euthyroidism
Of the 83 patients who did not achieve euthyroidism at initial postoperative visit, the average time to achieve normal thyroid function was 7.5 months. At one year, 107 (87.8%) patients had achieved a euthyroid state. The average euthyroid dose was 1.61±0.03 mcg/kg/day, however this euthyroid dose was significantly different between those that were over dosed, under dosed, and euthyroid at initial follow up (table II, p<.0001). Those with a BMI <30 required a significantly higher dose/kg to achieve euthyroidism compared to patients with a BMI >30 (table III, p<0.001). Table III demonstrates a trend acutely observed in this study that as BMI increases, the dose/kg required to achieve euthyroidism decreases.
Table III.
Eventual Euthyroid dose of Levothyroxine by BMI Category
| BMI, kg/m2 | Number of patients | Mean euthyroid dose (mcg/kg/day) |
|---|---|---|
| ≤ 24.99 | 41 | 1.84±0.07* |
| 25.00–29.99 | 24 | 1.63±0.05 |
| 30.00–34.99 | 26 | 1.50±0.05 |
| ≥ 35.00 | 31 | 1.39±0.05 |
|
| ||
| p= <0.001 | ||
BMI= body mass index calculated as weight in kilograms divided by the square of the height in meters
Data are represented as the mean +/− SEM
Differences among groups analyzed with ANOVA
Gender did not influence the dose of LT4 needed to achieve euthyroidism (p=0.41). In patients with either Hashimoto’s thyroiditis or Grave’s disease, a statistically greater dose was required compared to those without autoimmune disease (p=0.03).
Multivariate analysis
On multivariate analysis, BMI was the only significant factor predicting achievement of euthyroid dose at initial post-operative visit. Analysis indicated patient age, autoimmune disease, and estrogen use did not independently influence achievement of normal TSH values (table IV). For those patients over the age of 50, with a BMI over 25, more than half were initially overdosed and required adjustments, and only one third achieved euthyroidism on initial dose. Those in the group over 50 years of age with a BMI less than 25 were predominately under dosed, supporting a relationship between BMI and euthyroid dose independent of age.
Table IV.
Multivariate Analysis of factors effecting euthyroid failure of initial LT4 dose post-operatively
| Parameter | p value |
|---|---|
| Age | 0.215 |
| Autoimmune disease | 0.110 |
| BMI | 0.008 |
| Estrogen use | 0.309 |
BMI= body mass index calculated as weight in kilograms divided by the square of the height in meters
Development of BMI-based Formula for Postoperative T4 Dosing
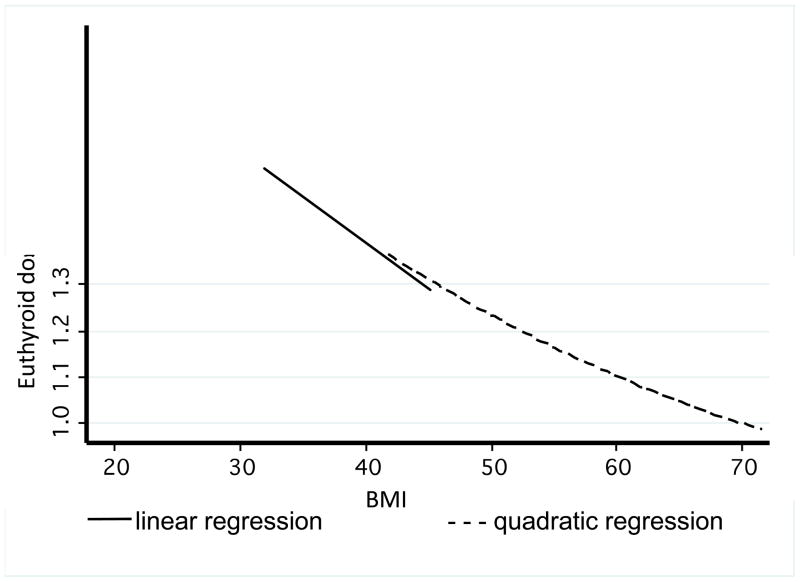
Following the multivariate analysis results, euthyroid dose and BMI were plotted. Regression analysis demonstrated an overall quadratic relationship between euthyroid dose and BMI, but the curve appeared linear up to a BMI of 50, and beyond that, approached 1.1 mcg/kg (figure II). As BMI increased, euthyroid dose/kg decreased. There were 6 patients with a BMI over 50. A dosing algorithm for patients with a BMI <50 was computed using linear regression for the linear portion of the curve (figure III).
Fig. II.
Regression Analysis
Fig. III.
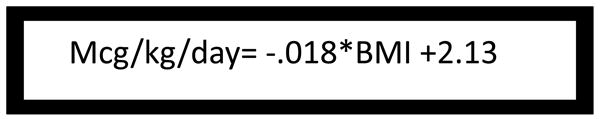
Dosing algorithm for Levothyroxine post-operatively in those patients with a BMI < 50
F=52.7, root mean squared error of .24
BMI=body mass index calculated as weight in kilograms divided by the square of the height in meters
Discussion
Our study reinforces current literature that a purely WBD method fails to accurately and efficiently predict thyroid hormone replacement in the surgically induced hypothyroid population. In our study, only 32% of patient’s achieved euthyroidism upon initial follow up. A similar study by Jonklaas (18) evaluated the effectiveness of WBD following total thyroidectomy for benign thyroid disease and thyroid cancer. At 6–8 week follow up, 32% of patients were at goal and by 1 year, 90% were adequately replaced (18). In our study, 87.8% of patients were euthyroid at one year.
Mistry et al (16) compared three different dosing methods in patients undergoing total thyroidectomy. Following the WBD method 25% of patients were correctly replaced on their initial dose (16). The empiric dosing method was inferior with 21% of patients’ euthyroid at follow up. Their proposed dosing method, a derived regression equation including weight and age, correctly dosed only 28% of patients (16).
The relationship observed between BMI and euthyroid dose predictability is the most noteworthy finding of our study. Our results substantiate tendencies to overdose patients who are overweight while under dosing patients of normal BMI following a WBD regimen. This result is physiologically intuitive, as fat cells function primarily as energy storage as opposed to expenditure (29). It would therefore be expected that overweight individuals require less dose/kg of thyroid replacement and may be at risk of over supplementation following WBD. In the present study, the average dose per kg that adequately replaced thyroxine decreased with increasing BMI.
Further, this phenomenon has significant implications when compared to studies on anorexia nervosa, obesity, and thyroid function. Anorexia nervosa is associated with both low TSH and lower levels of T3, understood to be an adaptive process for preserving resting energy potential (30,31). The low T3 levels are contributed to an impaired ability to convert T4 to T3 in the periphery (30,32,33). The opposite is demonstrated in the obese population. Compared to patients of normal weight, obese patients have slightly increased TSH and free T3 levels and produce a greater ratio of free T3 to reverse T3 (30, 34–36). Thus, when obese patients receive LT4 post-operatively, their ability to convert more thyroxine to T3 may explain the lower ideal dose/kg of LT4 observed in our study. Although our patients are not in a starving state, the higher thyroxine dose/kg in lower BMI patients may be due an impairment of T4 conversion (30,32,33).
While the increased prevalence of thyroid disease among older women is well known, age as a predictor for LT4 dosing is debated (16, 18, 20, 201, 37). Studies on primary hypothyroidism conclude that LT4 requirements decrease with age due to a slowing rate of thyroxine degradation (3, 13, 38). A recent retrospective review compared male patients to pre- and post-menopausal women and found age-based differences could be accredited instead to changes in body weight, body composition, or hormonal status in women (21). Likewise, our multivariate analysis did not find age to be a predictive factor for initial euthyroid dose.
Certain medications consumed concurrently with LT4 can affect the therapeutic requirement. Patients on both LT4 and estrogen show an estrogen-induced increase in thyroxine binding concentration, slightly lowering the amount of free thyroxine available (22). Clinically, close follow-up of TSH levels is recommended in these patients to make appropriate dose adjustments as needed (22). Our multivariate analysis did not find estrogen use to be an independent factor of failure to achieve euthyroidism.
The pathology of hypothyroidism can also influence the required dose of thyroid hormone. In Hashimoto’s Thyroiditis and Graves Disease, if thyroid tissue remains, persistent endogenous production of thyroxine can effect LT4 requirements (10). In the present study, we included only patients who underwent total thyroidectomy to control for this factor. Autoimmune diseases were included in our analysis to assess if the pathology itself, regardless of residual tissue, affected the patients ability to become euthyroid following surgery. Surprisingly, we found a significantly greater dose of LT4 required to achieve euthyroidism in patients with autoimmune disease suggesting a persistent role of immune activity influencing thyroxine replacement, despite complete removal of thyroid tissue. The etiology of this is unclear and understudied, but may be due to circulating antibodies or altered metabolism.
Several factors limited this analysis. There was some variation in starting dose as not all of our patients were initiated on exactly 1.6 mcg/kg/dose (table II). Due to insurance coverage, not all of our patients received the same preparation of LT4 that may have influenced efficacy and achievement of euthyroidism. Second, the retrospective nature of our study did not allow us to account whether patients were taking medications that affect levothyroxine dosing, or whether patients had mal-absorptive issues or cardiac disease, all of which can result in sub-optimal dosing. After a thyroidectomy at our institution, patients are placed on calcium, which raises concern regarding interference with levothyroxine absorption. All patients were treated with standard protocol of calcium supplementation after thyroidectomy, and given instructions on proper administration of calcium so this should limit the extent of this bias. In addition, calcium supplementation was stopped in almost all patients at 2-week follow-up.
The importance of strict adherence to daily thyroxine supplementation was communicated, however patient compliance may also have affected the delay in achieving euthyroidism. Patients were given thorough instructions on how to properly take thyroid hormone and warned of the effect of food and supplements on the absorption rate.
Although the overall fit for the proposed algorithm is not perfect, the results of this study clearly emphasize the role of BMI in determining a postoperative dosing regimen. A follow up study at our institution is currently underway to investigate prospectively the use of the proposed algorithm and its efficacy.
Despite these limitations, our study reaffirms that WBD regimen insufficiently predicts thyroxine requirements postoperatively. We propose BMI as a predictive factor and suggest its use in a clinically simple equation may better predict LT4 dosing, and shorten the time to achieving euthyroidism.
Selected Abbreviations
- BMI
body mass index
- LT4
levothyroxine
- TSH
thyroid stimulating hormone
- WBD
weight-based dosing
- LBM
lean body mass
- IBW
ideal body weight
- ANOVA
analysis of variance
Footnotes
Presented as a poster at the 2012 ACS Annual Clinical Congress Conference in Chicago, IL.
References
- 1.Uzzan B, Campos J, Cucherat M, et al. Effects on Bone mass of long-term treatment with thyroid hormones: a meta-analysis. J Clin Endocrinol Metab. 1996;81:4278–4289. doi: 10.1210/jcem.81.12.8954028. [DOI] [PubMed] [Google Scholar]
- 2.Biondi B, Fazio S, Carella C, et al. Cardiac effects of long term thyrotropin-suppressive therapy with levothyroxine. J Clin Endocrinol Metab. 1993;77:334–338. doi: 10.1210/jcem.77.2.8345037. [DOI] [PubMed] [Google Scholar]
- 3.Sawin CT, Geller A, Wolf PA, et al. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N Engl J Med. 1994;331:1249–1252. doi: 10.1056/NEJM199411103311901. [DOI] [PubMed] [Google Scholar]
- 4.Bauer DC, Ettinger B, Nevitt MC, Stone KL Study of osteoporotic fractures research group. Risk for fracture in women with low serum levels of thyroid stimulating hormone. Ann Intern Med. 2001;134:561–568. doi: 10.7326/0003-4819-134-7-200104030-00009. [DOI] [PubMed] [Google Scholar]
- 5.Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174–180. doi: 10.1056/NEJM199407213310307. [DOI] [PubMed] [Google Scholar]
- 6.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287–293. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 7.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 8.Palestini N, Grivon M, Durando R, et al. Thyroidectomy for Graves’ hyperthyroidism Retrospective study of patients’ appreciation. Ann Ital Chir. 2007;78(5):405–412. [PubMed] [Google Scholar]
- 9.Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764–770. doi: 10.1056/NEJM198703263161302. [DOI] [PubMed] [Google Scholar]
- 10.Gordon MB, Gordon MS. Variations in adequate levothyroxine replacement therapy in patients with different causes of hypothyroidism. Endocr Pract. 1999;5:233–238. doi: 10.4158/EP.5.5.233. [DOI] [PubMed] [Google Scholar]
- 11.Verhaert N, Vander Poorten V, Delaere P, et al. Levothyroxine replacement therapy after thyroid surgery. B-ENT. 2006;2:129–133. [PubMed] [Google Scholar]
- 12.Palit TK, Miller CC, 3rd, Miltenburg DM. The efficacy of thyroidectomy for Graves; disease: a meta-analysis. J Surg Res. 2000;90:161–165. doi: 10.1006/jsre.2000.5875. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum RL, Barzel US. Levothyroxine replacement dose for primary hypothyroidism decreases with age. Ann Intern Med. 1982;96:53–55. doi: 10.7326/0003-4819-96-1-53. [DOI] [PubMed] [Google Scholar]
- 14.Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid. 2010;20(2):135–146. doi: 10.1089/thy.2009.0311. [DOI] [PubMed] [Google Scholar]
- 15.Olubowale O, Chadwick DR. Optimization of thyroxine replacement therapy after total or near total thyroidectomy for benign thyroid disease. British J of Surg. 2006;93:57–60. doi: 10.1002/bjs.5157. [DOI] [PubMed] [Google Scholar]
- 16.Mistry D, Atkin S, Atkinson H, Gunasekaran S, et al. Predicting Thyroxine requirements following total thyroidectomy. Clin Endo. 2011;74:384–387. doi: 10.1111/j.1365-2265.2010.03940.x. [DOI] [PubMed] [Google Scholar]
- 17.Garces J, Barsano CP. Immunoradiometric assay for basal thyroid-stimulating hormone levels: strategy for the management of thyroxine replacement. South Med J. 1988;81(9):1127–1131. doi: 10.1097/00007611-198809000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Jonklaas J. Sex and Age differences in levothyroxine dosage requirement. Endocr Pract. 2010;16:71–79. doi: 10.4158/EP09257.OR. [DOI] [PubMed] [Google Scholar]
- 19.Sukumar R, Agarwal A, Gupta S, et al. Prediction of LT4 replacement dose to achieve euthyroidism in subjects undergoing total thyroidectomy for benign thyroid disorders. World J Surg. 2010;34:527–531. doi: 10.1007/s00268-009-0345-3. [DOI] [PubMed] [Google Scholar]
- 20.Baehr KM, Lyden E, Treude K, et al. Levothyroxine dosing following thyroidectomy is affected by more than just body weight. The Laryngoscope. 2012;122:834–848. doi: 10.1002/lary.23186. [DOI] [PubMed] [Google Scholar]
- 21.Devdhar M, Drooger R, Pehlivanova M, et al. Levothyroxine replacement doses are affected by gender and weight, but not age. Thyroid. 2011;21:821–827. doi: 10.1089/thy.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arafah BM. Increased need for thyroxine in women with hypothyroidism during estrogen therapy. N Engl J Med. 2001;344:1743–1749. doi: 10.1056/NEJM200106073442302. [DOI] [PubMed] [Google Scholar]
- 23.Santini F, Pinchera A, Marsili A, et al. Lean Body Mass is a Major determinant of Levothyroxine dosage in the Treatment of Thyroid disease. J of Clin Endo. 2005;90(1):124–127. doi: 10.1210/jc.2004-1306. [DOI] [PubMed] [Google Scholar]
- 24.Sartorio A, Ferrero S, Trecate L, Bedogni G. Thyroid Function is more strongly associated with body impedance than anthropometry in healthy subjects. J Endo Invest. 2002;25:620–623. doi: 10.1007/BF03345086. [DOI] [PubMed] [Google Scholar]
- 25.Zamfirescu I, Carlson HE. Absorption of levothyroxine when co-administered with various calcium formulations. Thyroid. 2011;21(5):483–486. doi: 10.1089/thy.2010.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17(8):763–765. doi: 10.1089/thy.2007.0060. [DOI] [PubMed] [Google Scholar]
- 27.Flaux E, Kadri K, Levasseur C, et al. Hypothyroidism as a result of drug interaction between ferrous sulfate and levothyroxine. Rev Med Interne. 2010;31(10):e4–5. doi: 10.1016/j.revmed.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 28.Liwanpo L, Hershman JM. Conditions and Drugs interfering with thyroxine absorption. Best Practice & Research Clin Endo & Met. 2009;23:781–792. doi: 10.1016/j.beem.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Severi S, Malavolti M, Battistini N, Bedogni G. Some applications of indirect calorimetry to sports medicine. Acta Diabetol. 2001;38:23–26. doi: 10.1007/s005920170024. [DOI] [PubMed] [Google Scholar]
- 30.Reinehr T, Isa A, De Sousa G, et al. Thyroid hormones and their relationship to weight status. Horm Res. 2008;70:51–57. doi: 10.1159/000129678. [DOI] [PubMed] [Google Scholar]
- 31.Van Wymelbeke V, Brondel L, Brun J, Rigaud D. Factors associated with the increase in resting energy expenditure during re-feeding in malnourished anorexia nervosa patients. Am J Clin Nutr. 2004;80:1469–1477. doi: 10.1093/ajcn/80.6.1469. [DOI] [PubMed] [Google Scholar]
- 32.Nedvidkova J, Papezova H, Haluzik M, Schreiber V. Interaction between serum leptin levels and hypothalamo-hypophyseal-thyroid axis in patients with anorexia nervosa. Endocrine Research. 2000;26(2):219–230. doi: 10.3109/07435800009066163. [DOI] [PubMed] [Google Scholar]
- 33.Moshang T, Jr, Parks JS, Baker L, Vaidya V, Utiger RD, Bongiovanni AM, Snyder PJ. Low serum triiodothyronine in patients with anorexia nervosa. J Clin Endocrinol Metab. 1975 Mar;40(3):470–3. doi: 10.1210/jcem-40-3-470. [DOI] [PubMed] [Google Scholar]
- 34.Reinehr T. Obesity and thyroid function. Molecular And Cellular Endo. 2010;316:165–171. doi: 10.1016/j.mce.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90:4019–4024. doi: 10.1210/jc.2004-2225. [DOI] [PubMed] [Google Scholar]
- 36.Rotandi M, Leporati P, La Manna A, et al. Raised Serum TSH levels in patients with morbid obesity; is it enough to diagnose subclinical hypothyroidism? Eur J Endocrinol. 2009;160 (3):403–408. doi: 10.1530/EJE-08-0734. [DOI] [PubMed] [Google Scholar]
- 37.Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: The Whickham Survey. Clin Endocrinol. 1977;7(6):481–493. doi: 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 38.Davis FB, LaMantia RS, Spaulding SW, et al. Estimation of physiologic replacement dose of levothyroxine in elderly patients with hypothyroidism. Arch Intern Med. 1984;144:1752–1754. [PubMed] [Google Scholar]