Abstract
The vast majority of patients with primary dystonia are adults with focal or segmental distribution of involuntary movements. Although ∼10% of probands have at least one first- or second-degree relative to dystonia, large families suited for linkage analysis are exceptional. After excluding mutations in known primary dystonia genes (TOR1A, THAP1 and CIZ1), whole-exome sequencing identified a GNAL missense mutation (c.682G>T, p.V228F) in an African-American pedigree with clinical phenotypes that include cervical, laryngeal and hand-forearm dystonia. Screening of 760 subjects with familial and sporadic primary dystonia identified three Caucasian pedigrees with GNAL mutations [c.591dupA (p.R198Tfs*13); c.733C>T (p.R245*); and c.3G>A (p.M1?)]. These mutations show incomplete penetrance. Our findings corroborate those of a recent study which used whole-exome sequencing to identify missense and nonsense GNAL mutations in Caucasian pedigrees of mixed European ancestry with mainly adult-onset cervical and segmental dystonia. GNAL encodes guanine nucleotide-binding protein G(olf), subunit alpha [Gα(olf)]. Gα(olf) plays a role in olfaction, coupling D1 and A2a receptors to adenylyl cyclase, and histone H3 phosphorylation. African-American subjects harboring the p.V228F mutation exhibited microsmia. Lymphoblastoid cell lines from subjects with the p.V228F mutation showed upregulation of genes involved in cell cycle control and development. Consistent with known sites of network pathology in dystonia, immunohistochemical studies indicated that Gα(olf) is highly expressed in the striatum and cerebellar Purkinje cells, and co-localized with corticotropin-releasing hormone receptors in the latter.
INTRODUCTION
Dystonia, defined as a syndrome of involuntary, sustained muscle contractions affecting one or more sites of the body, frequently causing twisting and repetitive movements or abnormal postures, is a genetically and clinically heterogeneous movement disorder (1). Dystonias are categorized by etiology (primary, secondary, dystonia-plus, and heredodegenerative diseases with dystonia), age of onset [early (<20 years) or late (≥20 years)] and anatomical distribution (focal, segmental, multifocal, hemi-dystonia or generalized) (1,2). Most cases of primary dystonia begin in adults and primary adult-onset dystonia is more common in females (2,3). Cervical dystonia (CD) or spasmodic torticollis is the most common form of focal dystonia, characterized by involuntary contractions of the neck muscles producing abnormal posturing of the head upon the trunk (4). In the USA, primary dystonia may be less common among African-Americans than Caucasians (5,6). Genetic factors contribute to the pathogenesis of adult-onset primary dystonia since 10% of patients have one or more affected first- or second-degree relatives (2,7). Familial and sporadic dystonia appear to share the same genetic underpinnings (8).
To date, six genes (TOR1A, THAP1, CIZ1, ANO3, GNAL and TUBB4A) and additional genetic loci (DYT13 and DYT21) have been linked to primary dystonia (9–18). Mutations in TOR1A are typically associated with early-onset generalized dystonia, whereas mutations in THAP1 most commonly cause segmental craniocervical dystonia. Mutations in CIZ1 have only been reported in patients with adult-onset cervical dystonia (12). In aggregate, these genes account for <10% of adult-onset cases of primary dystonia. Although adult-onset primary dystonia has a considerable heritable component, penetrance is reduced and the identification of genetic etiologies had been hampered by the availability of large pedigrees that were sufficiently powered for linkage analysis. With the advent of whole-exome sequencing, smaller pedigrees have proven suitable for the identification of sequence variants (SVs) causally associated with dystonia.
While the contributions of TOR1A and THAP1 to primary dystonia are well established, the roles of CIZ1, ANO3, TUBB4A and GNAL have not been demonstrated in independent patient cohorts. In the present study, we confirm that familial adult-onset primary dystonia can result from mutations in GNAL, which encodes guanine nucleotide-binding protein, alpha activating activity polypeptide, olfactory type (Gα(olf), Golfalpha), a key player in signal transduction within the olfactory neuroepithelium and basal ganglia (19,20).
RESULTS
Linkage analysis and exome sequencing
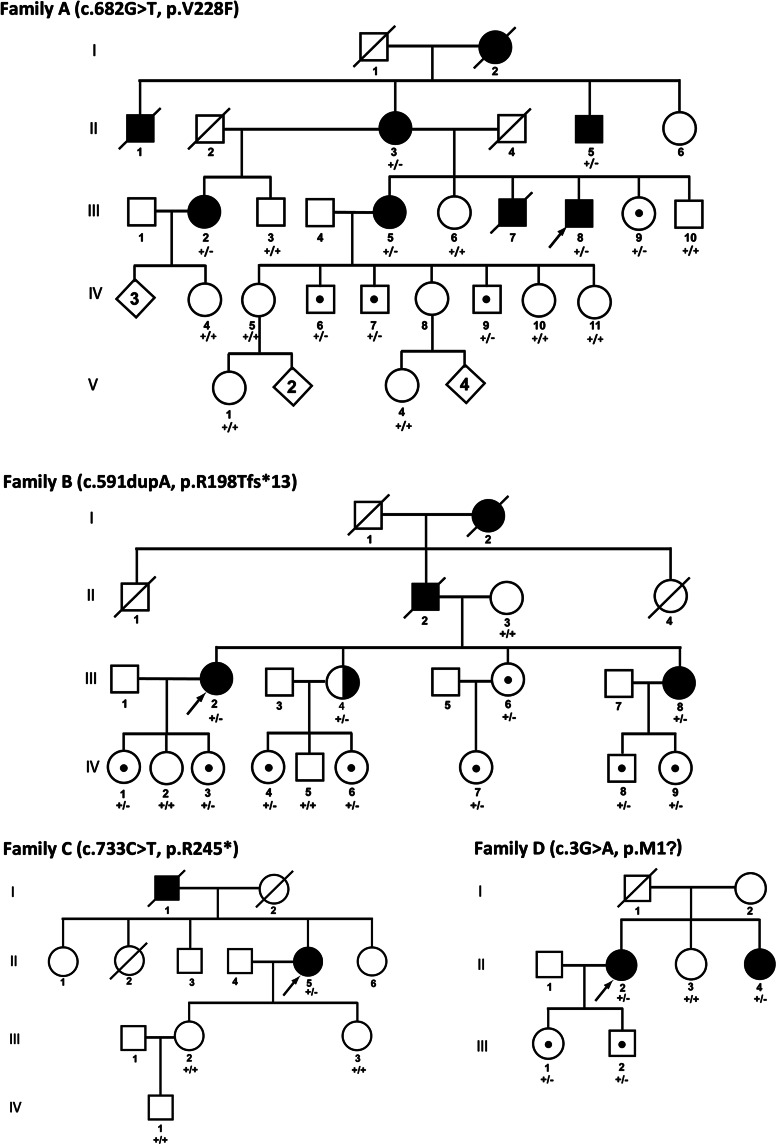
GNAL mutations were identified in the four independent pedigrees (Fig. 1). The largest family (A) was employed for linkage analysis and whole-exome sequencing. As previously described (6), the members of this pedigree reported ages of onset from 45 to 63 years (Table 1). All affected subjects in Families A, B, C and D had dystonia and varying degrees of objective microsmia with otherwise normal neurological examinations. In particular, no subject showed clinical evidence of ataxia, spasticity, oculomotor abnormalities, Parkinsonism or neuropathy.
Figure 1.
Family pedigrees. Filled symbols, definitely affected. Half-filled symbols, probably affected. Symbols with central dots, unaffected carriers. Arrows, probands. GNAL genotypes: wild-type (+/+) and heterozygous mutant (+/−). The genotypes of three subjects from Family B have recently been reported (13).
Table 1.
GNAL mutant phenotypes
| Subject | Age | Gender | Age of onset | Anatomical distribution | GNAL mutation |
|---|---|---|---|---|---|
| A-II-03 | 80 | F | 50 | Segmental dystonia (cervicala, blepharospasm, oromandibular, spasmodic dysphonia), anosmia | c.682G>T (p.V228F) |
| A-II-05 | 78 | M | NA | Segmental dystonia (spasmodic dysphonia, hand-forearm) | c.682G>T (p.V228F) |
| A-III-02 | 67 | F | 63 | Generalized dystonia (cervicala, hand-forearm, lower limb) | c.682G>T (p.V228F) |
| A-III-05 | 55 | F | 50 | Segmental dystonia (cervicala, oromandibular, spasmodic dysphonia, hand-forearm), moderate microsmia | c.682G>T (p.V228F) |
| A-III-08 | 49 | M | 45 | cervical dystoniaa, severe microsmia | c.682G>T (p.V228F) |
| B-III-02 | 56 | F | 38 | cervical dystoniaa | c.591dupA (p.R198Tfs*13) |
| B-III-08 | 46 | F | 37 | cervical dystoniaa | c.591dupA (p.R198Tfs*13) |
| B-III-04 | 54 | F | NA | cervical dystonia (probable) | c.591dupA (p.R198Tfs*13) |
| C-II-05 | 55 | F | 45 | cervical dystonia, moderate microsmia | c.733C>T (p.R245*) |
| D-II-02 | 55 | F | 40 | cervical dystonia | c.3G>A (p.M1?) |
| D-II-04 | 44 | F | 41 | cervical dystoniaa | c.3G>A (p.M1?) |
aDystonic head tremor.
Eighteen subjects from Family A were genotyped with the Illumina HumanLinkage-24 Bead Chip. Call rates were over 99.6% for all 18 samples and reproducibility was 100% for 6 samples subjected to technical replication (Supplementary Material, Table S1). SNP genotypes were analyzed with Superlink-Online SNP version 1.0 (21). The highest multi-point LOD score was 1.10 (Supplementary Material, Table S2). LOD scores of 1 or less were obtained within the DYT7, DYT13 and DYT21 loci.
Whole-exome capture and massively parallel sequencing was performed on two definitely affected and one unaffected subject from Family A. Over 99.5% of exons were covered at ≥2× and over 96.1% of exons were covered at ≥20× (Supplementary Material, Table S3). After filtering and elimination of read errors with Sanger sequencing, three potentially pathological SVs were common to the two affected subjects and absent from the unaffected subject. However, only a single SV co-segregated with dystonia in Family A (GNAL; c.682G>T, p.V228F).
Linkage analysis of Family A using c.682G>T GNAL genotypes with penetrance values of 0.5 and 0.99 yielded LOD scores of 2.90 and 4.03, respectively, at rs879588 (Supplementary Material, Table S4; Fig. S1). This SNP is located near GNAL on Chr 18p11.2. Haplotype analysis of Chr 18p showed that SNPs near GNAL co-segregated with c.682G>T in subjects with dystonia (Supplementary Material, Fig. S2). In silico analyses with ClustalW2 (22), Polyphen-2 (23), SIFT (24) and MutationTaster (25) indicated that c.682G>T (p.V228F) altered a highly conserved amino acid and was disease causing (Supplementary Material, Table S5).
Mutation screening and in silico analysis
High-resolution melting (7,12) and Sanger sequencing were used for GNAL mutation screening in 760 subjects with mainly CD and 768 neurologically-normal controls (Supplementary Material, Tables S6–S7). Five additional novel SVs were identified and in silico analyses predicted three of these variants to be pathogenic: c.591dupA (p.R198Tfs*13), c.733C>T (p.R245*) and c.3G>A (p.M1?). Two (c.591dupA and c.733C>T) of these three mutations are predicted to cause premature stop codons and probably induce nonsense-mediated decay (NMD), while c.3G>A disrupts the start codon of Isoform 2.
Clinical characteristics of subjects with GNAL mutations
As seen in Table 1, 9 of the 11 affected subjects from Families A–D were female. The age of onset ranged from 37 to 63 years. The mean age of onset was 45 years. Among the 11 affected subjects, 7 had isolated focal dystonia manifest as CD. There was a single subject with generalized dystonia and three with segmental dystonia. Seven subjects had a dystonic head tremor.
GNAL mutations show incomplete penetrance. Unaffected carriers were present in Families A, B and D (Fig. 1; and Supplementary Material, Table S8). Of the 14 unaffected carriers included in this study, there were five males and nine females. The ages of unaffected carriers ranged from 9 to 51 years with a mean of 29 years.
Role of Gα(olf) in olfaction
When data from all four families were grouped together, the differences in mean University of Pennsylvania Smell Identification Test (UPSIT) scores ± standard error of the mean among manifesting carriers (n = 7, 30.8 ± 3.1), non-manifesting carriers (n = 8, 33.7 ± 2.8) and non-carrier neurologically normal family members (n = 14, 35.1 ± 3.7) were not significant (26). However, an independent one-tailed t-test restricted to data from Family A indicated that manifesting and non-manifesting mutation carriers (n = 6, 25.5 ± 2.9) had lower UPSIT scores than non-carrier neurologically normal family members (n = 5, 33.0 ± 1.1; P < 0.026). Microsmia was not self-reported in Families A–D, but only detected through objective UPSIT testing.
Relative expression of GNAL
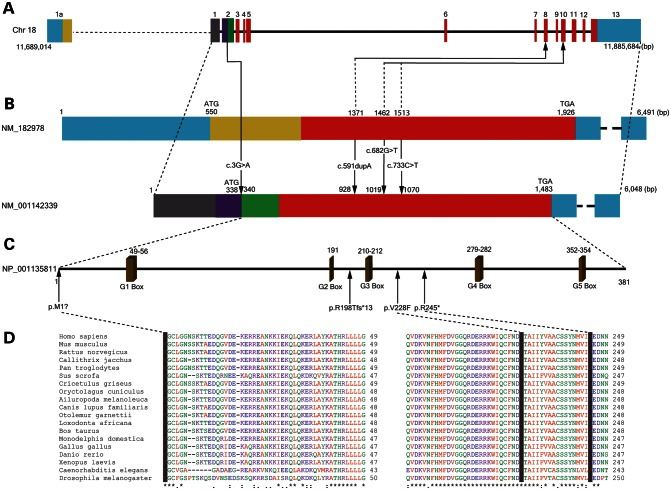
GNAL has three isoforms. Isoform 1 (NM_182978.3) is the longest, whereas Isoform 2 (NM_001142339.2) is the major isoform (Fig. 2). GenBank (http://www.ncbi.nlm.nih.gov/genbank/) cDNAs whose sequences support the existence of Isoform 3 have not been found in brain. Relative expression of Isoforms 1 and 2 was examined in human brain and leukocytes using quantitative RT–PCR (QRT–PCR; Supplementary Material, Table S9). Overall GNAL expression was highest in striatum and fetal whole brain, whereas relative expression of Isoform 2 to 1 was highest in striatum and cerebral cortex. Suggestive of NMD, overall leukocyte expression of GNAL was reduced in one subject from Family B (c.591dupA). We were unable to detect Isoform 1 in leukocytes. The c.3G>A mutation in Family D had no apparent effect on leukocyte expression of GNAL.
Figure 2.
Organization of GNAL gene, transcripts and full-length protein. (A) Structure of GNAL on Chr 18p presented in the 5′ to 3′ direction showing the location of four identified mutations in probands with dystonia. (B) The long and major isoforms of GNAL differ at Exon 1. (C) Missense mutations in highly conserved regions of Gα(olf) are shown in relationship to GTP binding domains (G1–G5). (D) The three Gα(olf) amino acids altered by missense mutations in GNAL show conservation in mammals (chimpanzees, marmosets, pigs, mice, rats, hamsters, rabbits, dogs, galagos, elephants, cows, and opossums) non-mammalian vertebrates (chickens, zebrafish, and frogs) and invertebrates (roundworms and fruit flies).
Immunohistochemistry of Gα(olf) in the rat central nervous system
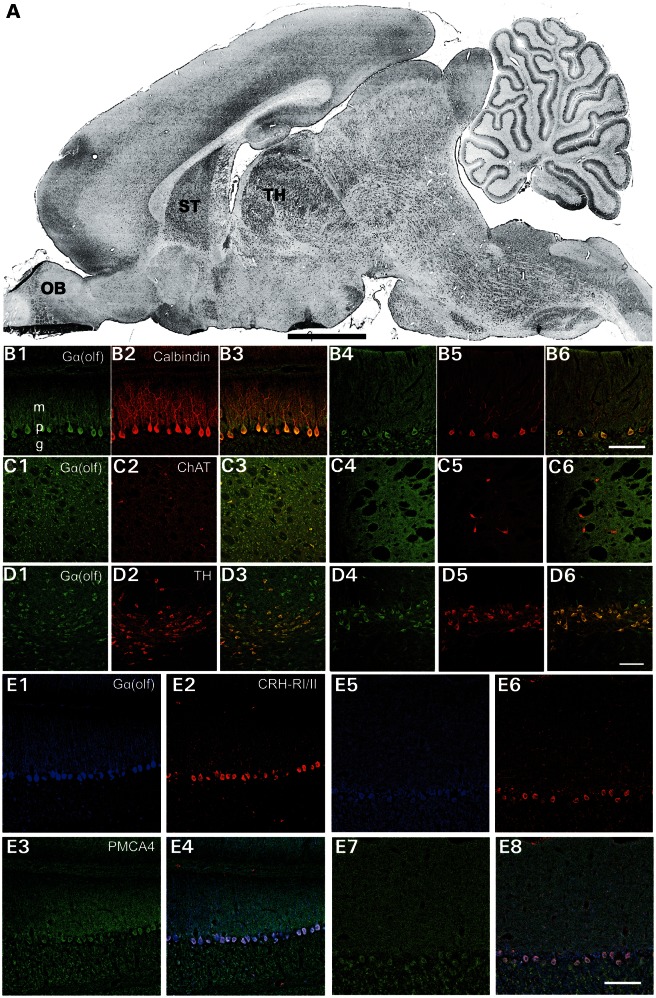
Since previous studies of Gα(olf) had focused on the olfactory bulb and striatum, we performed immunohistochemistry with a rabbit polyclonal anti-Gα(olf) antibody to obtain a more complete picture of Gα(olf)'s distribution in the rat central nervous system (Supplementary Material, Table S10). Gα(olf) immunoreactivity (IR) was present in olfactory bulb, striatum, thalamus, substantia nigra and cerebellum at P14 and in adult rat brains (Fig. 3). In cerebellum, Gα(olf)-IR was most prominent in Purkinje cells with weaker IR in granule cells. At P14, Gα(olf)-IR was seen throughput the dendritic arbor of Purkinje cells. However, in adult cerebellum, Gα(olf)-IR was largely restricted to the soma and proximal dendrites of Purkinje cells. In Purkinje cells, Gα(olf) co-localized with CRH-RI/II, but not PMCA4. At P14, Gα(olf)-IR was concentrated in the somas and proximal dendrites of medium spiny and cholinergic neurons within the striatum. In adult brain, more diffuse striatal Gα(olf)-IR was apparent. Gα(olf)-IR was localized to ChAT positive in striatal neurons at P14. However, in adult rat brain, Gα(olf)-IR in striatal cholinergic neurons was weak. At P14, Gα(olf)-IR was prominent in both dopaminergic and non-dopaminergic neurons of the substantial nigra. In adult rat brain, Gα(olf)-IR remained robust in TH-positive neurons of the substantia nigra but was less conspicuous in TH-negative neurons.
Figure 3.
Immunohistochemical localization of Gα(olf) in P14 and adult rat brain. (A) Para-sagittal rat brain section. OB, olfactory bulb, ST, striatum. TH, thalamus. (B1–6), Double-label fluorescence immunohistochemistry for simultaneous detection of Purkinje cell marker calbindin (red), TH or ChAT (red) with Gα(olf) (green) in P14 (B1-3) and adult (B4-6) rat brain. Gα(olf) -IR was present in ChAT-positive cholinergic neurons in striatum (C1–6) and TH-positive dopaminergic neurons in substantia nigra (D1–6). (E1–8), Triple-label fluorescent immunohistochemistry for simultaneous detection of PMCA4 (green), CRH-RI/II (red) and Gα(olf) (blue) in P14 (E1–4) and adult (E5–8) rat brains. Scale bar, 2 mm for A and 100 μm for the remaining images.
Gene expression analysis
Given the diverse role of G-proteins in regulating myriad cellular processes, gene expression studies were performed to identify pathways possibly dysregulated by mutant Gα(olf) [p.V228F]. These experiments employed RNA derived from lymphoblastoid cell lines established for four affected carriers (three females and one male) and four non-carriers (three females and one male) from Family A. In comparison to endogenous control and other dystonia-associated genes, GNAL was expressed at relatively low levels in lymphoblastoid cell lines (Supplementary Material, Table S11). However, the p.V228F mutation elicited highly reproducible effects on the transcriptome (Supplementary Material, Table S12).
In total, 82 genes were upregulated and 29 were downregulated (Supplementary Material, Tables S13 and S14; Fig. S3). Our gene set enrichment identified 15 significant KEGG pathways (Supplementary Material, Table S15). Upregulated pathways included Wnt signaling (LRP5, PLCB2 and FZD3), cytokine–cytokine interactions (IL17RB, TNFRSF14 and CXCL10) and arrhythmogenic right ventricular cardiomyopathy (ACTN1, DMD and LMNA). Top canonical pathways upregulated, as indicated by IPA, included vitamin D receptor/retinoic acid X receptor (VDR/RXR) and G-protein receptor-coupled signaling (EMR2, FZD3, OXTR, PDE6G, PLCB2 and PRKCE) (Supplementary Material, Tables S16 and S17). The top dysregulated networks were involved in cell cycle control, development, cell death and cellular proliferation (Table 2).
Table 2.
Ingenuity pathway analysis—top dysregulated networks
| Network | Genes | Scorea | Focus genesb | Top functions |
|---|---|---|---|---|
| 1 | CSTB, DMD, F13A1, FHL3, FLOT2, FZD3, MIR155HG, MSX1, NFIC, NT5C3, PABPC3, PDE6G, SIK3, TSC22D1 | 24 | 14 | Cell cycle, cellular development, connective tissue development and function |
| 2 | EMR2, EXOC6, FXYD2, HES1, IFITM3, IKZF1, KRTAP17-1, LIN7A, RAB9A, SFMBT2, SP140, SYT11 | 19 | 12 | Cellular development, cellular growth and proliferation, hematological system development and function |
| 3 | AKAP7, CERS6, CKLF, CLINT1, HLA-DRB4, IL17RB, SLC2A8, TNFRSF14, TPST1, TRAF3IP3 | 15 | 10 | RNA damage and repair, cell death and survival, gene expression |
aNetwork score is the negative log of the P-value for the likelihood that network molecules would be found together by chance. Higher scores indicate a greater statistical likelihood that molecules depicted in the network are interconnected.
bNumber of significantly dysregulated genes associated with that pathway in subjects from Family A with the p.V228F mutation.
DISCUSSION
Previous work from several groups had suggested that a dystonia-associated gene resides on Chr 18p (16,27–33). In 1996, Leube et al. reported a large German pedigree with CD and linkage to the region telomeric to D18S1153 on Chr 18p with a maximal LOD score of 3.17 (34). Follow-up studies narrowed this dystonia locus to a 30 cM region near D18S1098, but also presented evidence of locus heterogeneity in adult-onset CD (16,35). More recent work has shed doubts on the DYT7 locus for CD on Chr 18p (36). Although CD and other forms of adult-onset dystonia are genetically heterogeneous, dystonia is a relatively common manifestation of 18p deletion syndrome (MIM 146390) (27,29–33). Dystonia in 18p deletion syndrome can be focal, segmental or generalized. Some subjects with the 18p deletion syndrome also exhibit myoclonus and white matter abnormalities on magnetic resonance imaging of the brain. Nasir et al. (31) identified a Chr 18p 15 Mb deletion that included GNAL in a mother and her son both affected with dystonia. Moreover, linkage to Chr 18p was reported in three brothers with late-onset hand-forearm dystonia (28). When integrated with recent work describing several missense and nonsense GNAL mutations in familial dystonia (13), the data reported herein indicate that (1) GNAL is one dystonia-associated gene on Chr 18p, and (2) Gα(olf) deficiency or dysfunction mainly causes adult-onset CD.
Gα(olf) belongs to a class of GTP-binding proteins (G proteins) which couple G-protein receptors to adenylyl cyclase. Heterotrimeric G protein complexes are composed of three subunits (α, β and γ). G proteins are categorized into four subfamilies according to their α-subunits (Gαs, Gαi/o, Gαq and Gα12). Gα(olf) which has 88% amino acid homology to Gαs is considered to be a member of the Gαs family. Although Gα(olf) was originally discovered in the olfactory neuroepithelium and striatum, it has been identified in pancreatic β-cells, vestibular end organs, testis, spleen, lung and heart (37,38). Our immunohistochemical work indicates that Gα(olf) is widely expressed in brain, especially in motor regions that have previously been associated with dystonia.
At the network level, dystonia has been considered as a disorder of the (1) basal ganglia, (2) olivocerebellar pathways or (3) their interaction (39–41). In dystonic rats, dystonia has been associated with abnormal neurotransmission at the climbing fiber–Purkinje cell synapse (42). Climbing fiber activity leads to the release of CRH which facilitates induction of long-term depression at both parallel fiber and climbing fiber synapses (43). In the striatum, Gα(olf) is upregulated after lesioning the nigrostriatal dopaminergic pathway which contributes to levodopa-induced dyskinesias in models of Parkinson's disease (19). Mutant Gα(olf) and Gα(olf) deficiency may precipitate dystonia by limiting activation of adenylate cyclase in dopamine D1 receptors in striatal medium spiny neurons of the direct pathway (13).
Although mutations in GNAL had not previously been associated with olfactory dysfunction in humans, two Iranian families with isolated congenital anosmia showed linkage to 18p11.23-q12.2 (44). More importantly, mice deficient in Gα(olf) are anosmic (20). In our study, microsmia was most prominent in those members of African-American Family A also affected with dystonia. However, in the Caucasian families, microsmia was absent or variable in mutation carriers. These data suggest that, like dystonia, the penetrance of olfactory dysfunction may be reduced and dependent on genetic background. In addition, alternate olfactory signaling pathways may compensate for Gα(olf) deficiency given that, in contrast to the Caucasian families, Family A harbored a missense mutation in GNAL.
At the cellular level, some forms of primary dystonia have been characterized as neurodevelopmental disorders and several dystonia associated proteins (THAP1, TAF1 and CIZ1) play important roles at the G1–S checkpoint of the cell cycle (2,10,12,45). Similarly, our gene expression studies identified dysregulated cellular networks involved in cell cycle, development and gene expression. Although lymphoblastoid cells do not faithfully model many aspects of neuronal function, most cellular processes are shared and possible links among dystonia-associated proteins should not be ignored. In this regard, administration of the dopamine receptor blocker haloperidol induces prolonged increases in the levels of Ser10 phosphorylated histone H3 in dopamine D2 receptor-expressing neurons of the dorsomedial and the dorsolateral striatum and this effect is mediated through adenosine A2A receptor-mediated activation of Gα(olf) (46,47). Histone H3 phosphorylation at Ser10 inhibits checkpoint kinase 1, a key component of the ATM/ATR arm of the G1–S checkpoint pathway (48). Therefore, it is conceivable that loss of Gα(olf) function could disturb G1–S cell cycle control.
MATERIALS AND METHODS
Human subjects
Human studies were conducted in accordance with the Declaration of Helsinki, with formal approval from the institutional review boards at each participating study site. All genetic and phenotypic analyses and publication of the results were approved by the University of Tennessee Health Science Center Institutional Review Board (#01-07346-XP). Enrollment of patients with primary dystonia and neurologically normal controls is described in previous publications (7,12). Genomic DNA was extracted from peripheral whole blood or saliva (Oragene DNA Self-Collection kit, DNA Genotek®, Kanata, Ontario, Canada). Total leukocyte RNA was extracted with the LeukoLOCK™ Total RNA Isolation System (Ambion, Austin, TX, USA) as previously described (7).
Given the established role of Gα(olf) in olfaction and to investigate the potential utility of olfactory testing as a preclinical diagnostic tool in primary dystonia, Families A–D were examined with the 40-item University of Pennsylvania Smell Identification Test (26) (UPSIT; Sensonics, Inc., Haddon Heights, NJ, USA). UPSIT data were analyzed with SAS 9.3 (SAS Institute, Inc., Cary, NC, USA).
Linkage analysis
Eighteen subjects from Family A were genotyped with the HumanLinkage-24 Bead Chip (Illumina, San Diego, CA, USA). SNP genotypes were analyzed with Superlink-Online SNP version 1.0 (http://cbl-hap.cs.technion.ac.il/superlink-snp/) (21) using a dominant model with a mutant allele frequency of 0.0001 and penetrance of 0.5 (Supplementary Material, Table S2).
Exome sequencing and variant analysis
In-solution whole-exome capture and massively parallel sequencing was performed on two definitely affected and one unaffected subject from Family A using the Agilent SureSelectXT All Exon Kit 51 Mb (Santa Clara, CA, USA). Three micrograms of genomic DNA from two affected and one control subject was sheared to yield 100–450 bp fragments. Sheared DNA was then subjected to Illumina paired-end library preparation followed by enrichment for target sequences (Agilent SureSelectXT Automated Target Enrichment for Illumina Paired-End Multiplexed Sequencing). Enriched DNA fragments were sequenced on Illumina's HiSeq 2000 platform as paired-end 100 base reads (Otogenetics Co., Norcross, GA, USA).
Percentage of exome coverage was based on exons targeted by the 51 Mb All Exon v4 kit which incorporates Consensus Coding Sequence (CCDS), NCBI Reference Sequence (RefSeq) and GENCODE annotations. Sequence reads (FASTQ) were mapped to the human reference genome (NCBI build 37.1) with NextGENe® (SoftGenetics, State College, PA, USA). The average read length for all three samples was 97 nt. To maximize the probability of detecting the causal SV, all base changes occurring in two or more reads in any individual sample were classified as variants for downstream analyses. A variant comparison was done between the two affected and one unaffected subjects to filter down the number of SVs in order to identify the putative causal variant. With NextGENe® software, all filtering parameters were implemented simultaneously: (i) homozygous SVs, (ii) intergenic SVs, (iii) deep intronic SVs (≥12 nt from splice sites), (iv) SVs reported in dbSNP Build 135, (v) synonymous SVs and (vi) non-pathogenic non-synonymous SVs (12,49). In order to filter benign missense variants, the pathogenicity of non-synonymous single amino acid substitutions was interrogated with five in silico tools: PolyPhen-2, MutationTaster, SIFTnew, LRTnew (Likelihood Ratio Test) and PhyloPnew (50). Conservative scores were employed for filtering: PolyPhen-2 (≥0.5), MutationTaster (≥0.5), SIFTnew (>0.5), LRTnew (≥0.5) and PhyloPnew (≥0.8). We retained those SVs that met at least four of these five criteria. Remaining SVs were filtered if identified in two or more subjects included in the 1000 Genomes Project (http://www.1000genomes.org) or Exome Sequencing Project (http://evs.gs.washington.edu/EVS/). Candidate genes were also eliminated if not expressed in brain [Allen Brain Atlas (http://www.brain-map.org/) and BioGPS (http://biogps.org)].
QRT-PCR
For QRT–PCR, leukocyte RNA was reverse transcribed to cDNA with Ambion's RETROscript™ kit using 500 ng of total RNA as a template. The reaction mixture was incubated at 44°C for 1 h and then at 92°C for 10 min. QRT–PCR was performed using the Roche Applied Science (Penzberg, Germany) LightCycler® 480 system with GNAL specific primers and Roche Universal ProbeLibrary probes under the following conditions: 95°C for 10 min; 45 cycles at 94°C for 10 s, 60°C for 10 s and 72°C for 20 s. GNAL-specific primers, which were designed using the Roche Universal ProbeLibrary Assay Design Center to span at least one intron, showed efficiencies of 92.5–95.9% and R2 values >0.99 (Supplementary Material, Table S9). The GNAL primer pairs for all isoforms, Isoform 1 and Isoform 2, generated single amplicons that produced single well-defined bands on agarose gel electrophoresis. Furthermore, Sanger sequencing confirmed the identity of these bands as GNAL cDNA. Cyclophilin D was chosen from a panel of six endogenous controls (β-actin, β-tubulin, TATA-binding protein, hypoxanthine-guanine phosphoribosyltransferase, cyclophilin D and S19), since it showed the highest efficiency (97.8%), smallest sample-to-sample variance and generated an R2 of 0.996. All samples were run in triplicate and median values were employed for statistical analyses.
Adult human whole brain total RNA [FirstChoice® Human Brain Reference RNA (1 mg/ml)] was obtained from Ambion. Fetal human whole brain and adult human cerebral cortex, cerebellum, and substantia nigra total RNA were purchased from Clontech (Mountain View, CA, USA). Striatum total RNA was acquired from Agilent.
Immunohistochemistry
Perfusion-fixed [normal saline-4% paraformaldehyde in 0.1 m phosphate-buffered saline (PBS)] postnatal day 14 (P14) and adult Sprague–Dawley rat brains were sectioned in the mid-sagittal or coronal planes and processed for detection of Gα(olf) with Purkinje cell marker (calbindin D-28K), tyrosine hydroxylase (TH), choline acetyltransferase (ChAT), a parallel fiber marker (PMCA4) and corticotropin releasing hormone receptors I and II (CRH-RI/II) (Supplementary Material, Table S10). After endogenous peroxidases were quenched, slides were blocked and exposed to a primary rabbit polyclonal anti-Gα(olf) antibody (Novus Biologicals, Littleton, CO, USA; 1:100) overnight. After rinsing with PBS, slides were exposed to biotinylated goat anti-rabbit antibody (1:500) for 4 h, followed by rinsing and exposing to streptavidin for 1 h. Labeling was visualized with nickel-intensified 3, 3′-diaminobenzidine solution (Vector, Burlingame, CA, USA). For double-label fluorescent immunohistochemistry, mouse anti-calbindin monoclonal, mouse anti-TH polyclonal or a goat anti-choline acetyltransferase (ChAT) polyclonal antibodies were used in combination with rabbit anti-Gα(olf). The secondary antibodies were Cy2-tagged donkey anti-rabbit, and rhodamine red-X (RRX)-tagged donkey anti-mouse or anti-goat. Sections were incubated with secondary antibodies for 4 h and then rinsed, dehydrated, cleared and coverslipped with 1,3-diethyl-8-phenylxanthine mounting compound (DPX; Sigma-Aldrich, St. Louis, MO, USA). For triple-label fluorescent immunohistochemistry, goat anti-CRH-RI/II polyclonal, mouse anti-PMCA4 monoclonal antibodies were used in combination with rabbit anti-Gα(olf). The secondary antibodies were Cy2-tagged donkey anti-mouse, and rhodamine red-X (RRX)-tagged donkey anti-goat and Cy5-tagged donkey anti-rabbit. Sections were visualized with both epifluorescence (Leica DM 6000B; Wetzlar, Germany) and confocal laser-scanning (Zeiss LSM 710; Thornwood, NY, USA) microscopes.
Lymphoblastoid transformation and RNA extraction
Lymphocytes from four affected carriers and four non-carriers from Family A were transformed to lymphoblastoid cell lines for downstream analysis. To establish lymphoblastoid cell lines, peripheral blood mononuclear cells were separated by centrifugation on a sodium diatrizoate polysucrose gradient and transformed with Epstein-Barr virus (Coriell, Camden, NJ, USA). The lymphoblastoid cell lines were propagated in RPMI-1640 medium (Sigma-Aldrich) supplemented with 20% fetal bovine serum (Sigma-Aldrich) and 2 mm l-glutamine at 37°C in 5% CO2. Confluent cells (1×106 cells/ml) were harvested for RNA isolation. Ambion's TRI Reagent® was used to isolate RNA. The quality of total RNA derived from leukocytes and lymphoblastoid cell lines was accessed with a NanoDrop® ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), and Agilent 2100 Bioanalyzer using the Agilent RNA 6000 Nano Chip kit.
Gene expression studies
Total RNA from lymphoblastoid cell lines of eight subjects were processed on Illumina® HumanHT-12 v.4 expression microarray platform (Illumina, San Diego, CA, USA), flexible for high-throughput processing of 12 samples per beadchip, to assess the expression levels in each individual specimen. These arrays investigate whole-genome expression, providing coverage for more than 47 000 transcripts and known splice variants across the human transcriptome. Total RNA (200 ng) was processed using the Illumina® TotalPrep™ RNA Amplification kit (Applied Biosystems, Carlsbad, CA, USA) according to the company's protocol. The raw data were processed for errors and quality checks using Illumina's proprietary GenomeStudio® software. Data were normalized and summarized further with GeneSpring GX® 12.0 software (Agilent® Technologies). Unpaired t-tests were used to filter significant probes. Probes that were significant at P ≤ 0.05 with a mean fold change of ≥1.5 were retained for downstream analyses.
Enrichment analysis of differentially expressed genes was performed using WebGestalt (http://bioinfo.vanderbilt.edu/webgestalt/) (49). The differentially expressed gene set was compared with the human genome using the hypergeometric test followed by correction for multiple testing using the Benjamini & Hochberg (BH) method at a significance level of 0.05 (FDR < 0.05) (51). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were accessed from WebGestalt (52,53). Dysregulated cellular networks were examined with Ingenuity Pathway Analysis (IPA; Ingenuity, Redwood City, CA, USA).
SUPPLEMENTARY MATERIAL
FUNDING
M.S.L. was supported by the Bachmann-Strauss Dystonia & Parkinson Foundation, Dystonia Medical Research Foundation and NIH/NINDS R01 NS069936. A.P. was supported by governmental funding of clinical research within the Swedish National Health Services (ALF-YF) and by the Swedish Parkinson Foundation (Parkinsonfonden). Z.K.W is partially supported by the NIH/NINDS P50 NS072187, Mayo Clinic Florida (MCF) Research Committee and Dystonia Medical Research Foundation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank J. Searcy and A. Strongosky for their assistance with collecting clinical data.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Fahn S. Concept and classification of dystonia. Adv. Neurol. 1988;50:1–8. [PubMed] [Google Scholar]
- 2.LeDoux M.S. The genetics of dystonias. Adv. Genet. 2012;79:35–85. doi: 10.1016/B978-0-12-394395-8.00002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeDoux M.S. Dystonia: phenomenology. Parkinsonism Relat. Disord. 2012;18(Suppl 1):S162–164. doi: 10.1016/S1353-8020(11)70050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J., Brin M.F., Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov. Disord. 1991;6:119–126. doi: 10.1002/mds.870060206. [DOI] [PubMed] [Google Scholar]
- 5.Marras C., Van den Eeden S.K., Fross R.D., Benedict-Albers K.S., Klingman J., Leimpeter A.D., Nelson L.M., Risch N., Karter A.J., Bernstein A.L., et al. Minimum incidence of primary cervical dystonia in a multiethnic health care population. Neurology. 2007;69:676–680. doi: 10.1212/01.wnl.0000267425.51598.c9. [DOI] [PubMed] [Google Scholar]
- 6.Puschmann A., Xiao J., Bastian R.W., Searcy J.A., LeDoux M.S., Wszolek Z.K. An African-American family with dystonia. Parkinsonism Relat. Disord. 2011;17:547–550. doi: 10.1016/j.parkreldis.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao J., Zhao Y., Bastian R.W., Perlmutter J.S., Racette B.A., Tabbal S.D., Karimi M., Paniello R.C., Wszolek Z.K., Uitti R.J., et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 2010;74:229–238. doi: 10.1212/WNL.0b013e3181ca00ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defazio G., Abbruzzese G., Girlanda P., Liguori R., Santoro L., Tinazzi M., Berardelli A. Phenotypic overlap in familial and sporadic primary adult-onset extracranial dystonia. J. Neurol. 2012;259:2414–2418. doi: 10.1007/s00415-012-6514-6. [DOI] [PubMed] [Google Scholar]
- 9.Ozelius L.J., Hewett J.W., Page C.E., Bressman S.B., Kramer P.L., Shalish C., de Leon D., Brin M.F., Raymond D., Corey D.P., et al. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat. Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs T., Gavarini S., Saunders-Pullman R., Raymond D., Ehrlich M.E., Bressman S.B., Ozelius L.J. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat. Genet. 2009;41:286–288. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- 11.Charlesworth G., Plagnol V., Holmstrom K.M., Bras J., Sheerin U.M., Preza E., Rubio-Agusti I., Ryten M., Schneider S.A., Stamelou M., et al. Mutations in ANO3 cause dominant craniocervical dystonia: ion channel implicated in pathogenesis. Am. J. Hum. Genet. 2012;91:1041–1050. doi: 10.1016/j.ajhg.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao J., Uitti R.J., Zhao Y., Vemula S.R., Perlmutter J.S., Wszolek Z.K., Maraganore D.M., Auburger G., Leube B., Lehnhoff K., et al. Mutations in CIZ1 cause adult-onset primary cervical dystonia. Ann. Neurol. 2012;71:458–469. doi: 10.1002/ana.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuchs T., Saunders-Pullman R., Masuho I., Luciano M.S., Raymond D., Factor S., Lang A.E., Liang T.W., Trosch R.M., White S., et al. Mutations in GNAL cause primary torsion dystonia. Nat. Genet. 2012;45:88–92. doi: 10.1038/ng.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hersheson J., Mencacci N., Davis M., MacDonald H.N., Trabzuni D., Ryten M., Pittman A., Paudel R., Kara E., Fawcett K., et al. Mutations in the autoregulatory domain of β-tubulin 4a cause hereditary dystonia. Ann. Neurol. 2013 doi: 10.1002/ana.23832. DOI:10.1002/ana.23832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad F., Davis M.B., Waddy H.M., Oley C.A., Marsden C.D., Harding A.E. Evidence for locus heterogeneity in autosomal dominant torsion dystonia. Genomics. 1993;15:9–12. doi: 10.1006/geno.1993.1003. [DOI] [PubMed] [Google Scholar]
- 16.Leube B., Hendgen T., Kessler K.R., Knapp M., Benecke R., Auburger G. Sporadic focal dystonia in Northwest Germany: molecular basis on chromosome 18p. Ann. Neurol. 1997;42:111–114. doi: 10.1002/ana.410420117. [DOI] [PubMed] [Google Scholar]
- 17.Norgren N., Mattson E., Forsgren L., Holmberg M. A high-penetrance form of late-onset torsion dystonia maps to a novel locus (DYT21) on chromosome 2q14.3-q21.3. Neurogenetics. 2011;12:137–143. doi: 10.1007/s10048-011-0274-9. [DOI] [PubMed] [Google Scholar]
- 18.Valente E.M., Bentivoglio A.R., Cassetta E., Dixon P.H., Davis M.B., Ferraris A., Ialongo T., Frontali M., Wood N.W., Albanese A. DYT13, a novel primary torsion dystonia locus, maps to chromosome 1p36.13–-36.32 in an Italian family with cranial-cervical or upper limb onset. Ann. Neurol. 2001;49:362–366. [PubMed] [Google Scholar]
- 19.Alcacer C., Santini E., Valjent E., Gaven F., Girault J.A., Herve D. Galpha(olf) mutation allows parsing the role of cAMP-dependent and extracellular signal-regulated kinase-dependent signaling in L-3,4-dihydroxyphenylalanine-induced dyskinesia. J. Neurosci. 2012;32:5900–5910. doi: 10.1523/JNEUROSCI.0837-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belluscio L., Gold G.H., Nemes A., Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- 21.Silberstein M., Tzemach A., Dovgolevsky N., Fishelson M., Schuster A., Geiger D. Online system for faster multipoint linkage analysis via parallel execution on thousands of personal computers. Am. J. Hum. Genet. 2006;78:922–935. doi: 10.1086/504158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 23.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., Sunyaev S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng P.C., Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz J.M., Rodelsperger C., Schuelke M., Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 26.Doty R.L., Shaman P., Dann M. Development of the University of Pennsylvania Smell Identification test: a standardized microencapsulated test of olfactory function. Physiol. Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 27.Awaad Y., Munoz S., Nigro M. Progressive dystonia in a child with chromosome 18p deletion, treated with intrathecal baclofen. J. Child Neurol. 1999;14:75–77. doi: 10.1177/088307389901400202. [DOI] [PubMed] [Google Scholar]
- 28.Bhidayasiri R., Jen J.C., Baloh R.W. Three brothers with a very-late-onset writer's cramp. Mov. Disord. 2005;20:1375–1377. doi: 10.1002/mds.20568. [DOI] [PubMed] [Google Scholar]
- 29.Graziadio C., Rosa R.F., Zen P.R., Pinto L.L., Barea L.M., Paskulin G.A. Dystonia, autoimmune disease and cerebral white matter abnormalities in a patient with 18p deletion. Arq. Neuropsiquiatr. 2009;67:689–691. doi: 10.1590/s0004-282x2009000400021. [DOI] [PubMed] [Google Scholar]
- 30.Kowarik M.C., Langer S., Keri C., Hemmer B., Oexle K., Winkelmann J. Myoclonus-dystonia in 18p deletion syndrome. Mov. Disord. 2011;26:560–561. doi: 10.1002/mds.23446. [DOI] [PubMed] [Google Scholar]
- 31.Nasir J., Frima N., Pickard B., Malloy M.P., Zhan L.P., Grunewald R. Unbalanced whole arm translocation resulting in loss of 18p in dystonia. Mov. Disord. 2006;21:859–863. doi: 10.1002/mds.20846. [DOI] [PubMed] [Google Scholar]
- 32.Postma A.G., Verschuuren-Bemelmans C.C., Kok K., van Laar T. Characteristics of dystonia in the 18p deletion syndrome, including a new case. Clin. Neurol. Neurosurg. 2009;111:880–882. doi: 10.1016/j.clineuro.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Tezzon F., Zanoni T., Passarin M.G., Ferrari G. Dystonia in a patient with deletion of 18p. Ital. J. Neurol. Sci. 1998;19:90–93. doi: 10.1007/BF02427563. [DOI] [PubMed] [Google Scholar]
- 34.Leube B., Rudnicki D., Ratzlaff T., Kessler K.R., Benecke R., Auburger G. Idiopathic torsion dystonia: Assignment of a gene to chromosome 18p in a German family with adult onset, autosomal dominant inheritance and purely focal distribution. Hum. Mol. Genet. 1996;5:1673–1677. doi: 10.1093/hmg/5.10.1673. [DOI] [PubMed] [Google Scholar]
- 35.Klein C., Ozelius L.J., Hagenah J., Breakefield X.O., Risch N.J., Vieregge P. Search for a founder mutation in idiopathic focal dystonia from northern Germany. Am. J. Hum. Genet. 1998;63:1777–1782. doi: 10.1086/302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winter P., Kamm C., Biskup S., Kohler A., Leube B., Auburger G., Gasser T., Benecke R., Muller U. DYT7 gene locus for cervical dystonia on chromosome 18p is questionable. Mov. Disord. 2012;27:1820–1822. doi: 10.1002/mds.25219. [DOI] [PubMed] [Google Scholar]
- 37.Regnauld K.L., Leteurtre E., Gutkind S.J., Gespach C.P., Emami S. Activation of adenylyl cyclases, regulation of insulin status, and cell survival by G alpha olf in pancreatic beta-cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R870–R880. doi: 10.1152/ajpregu.00374.2001. [DOI] [PubMed] [Google Scholar]
- 38.Wackym P.A., Cioffi J.A., Erbe C.B., Popper P. G-protein Golfalpha (GNAL) is expressed in the vestibular end organs and primary afferent neurons of Rattus norvegicus. J. Vestib. Res. 2005;15:11–15. [PMC free article] [PubMed] [Google Scholar]
- 39.Neychev V.K., Fan X., Mitev V.I., Hess E.J., Jinnah H.A. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Argyelan M., Carbon M., Niethammer M., Ulug A.M., Voss H.U., Bressman S.B., Dhawan V., Eidelberg D. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J. Neurosci. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeDoux M.S. Animal models of dystonia: lessons from a mutant rat. Neurobiol. Dis. 2011;42:152–161. doi: 10.1016/j.nbd.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LeDoux M.S., Lorden J.F. Abnormal spontaneous and harmaline-stimulated Purkinje cell activity in the awake genetically dystonic rat. Exp. Brain Res. 2002;145:457–467. doi: 10.1007/s00221-002-1127-4. [DOI] [PubMed] [Google Scholar]
- 43.Ohtsuki G., Piochon C., Hansel C. Climbing fiber signaling and cerebellar gain control. Front Neurosci. 2009;3:4. doi: 10.3389/neuro.03.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghadami M., Majidzadeh A.K., Morovvati S., Damavandi E., Nishimura G., Komatsu K., Kinoshita A., Najafi M.T., Niikawa N., Yoshiura K. Isolated congenital anosmia with morphologically normal olfactory bulb in two Iranian families: a new clinical entity? Am. J. Med. Genet. A. 2004;127A:307–309. doi: 10.1002/ajmg.a.30025. [DOI] [PubMed] [Google Scholar]
- 45.Makino S., Kaji R., Ando S., Tomizawa M., Yasuno K., Goto S., Matsumoto S., Tabuena M.D., Maranon E., Dantes M., et al. Reduced neuron-specific expression of the TAF1 gene is associated with X-linked dystonia-parkinsonism. Am. J. Hum. Genet. 2007;80:393–406. doi: 10.1086/512129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertran-Gonzalez J., Hakansson K., Borgkvist A., Irinopoulou T., Brami-Cherrier K., Usiello A., Greengard P., Herve D., Girault J.A., Valjent E., et al. Histone H3 phosphorylation is under the opposite tonic control of dopamine D2 and adenosine A2A receptors in striatopallidal neurons. Neuropsychopharmacology. 2009;34:1710–1720. doi: 10.1038/npp.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez-Collazo P., Snyder S.K., Chiffer R.C., Zlatanova J., Leuba S.H., Smith C.L. cAMP signaling induces rapid loss of histone H3 phosphorylation in mammary adenocarcinoma-derived cell lines. Exp. Cell Res. 2008;314:1–10. doi: 10.1016/j.yexcr.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liokatis S., Stützer A., Elsässer S.J., Theillet F.X., Klingberg R., van Rossum B., Schwarzer D., Allis C.D., Fischle W., Selenko P. Phosphorylation of histone H3 Ser10 establishes a hierarchy for subsequent intramolecular modification events. Nat. Struct. Mol. Biol. 2012;19:819–823. doi: 10.1038/nsmb.2310. [DOI] [PubMed] [Google Scholar]
- 49.Zhang B., Kirov S., Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–748. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X., Jian X., Boerwinkle E. dbNSFP: a lightweight database of human nonsynonymous SNPs and their functional predictions. Hum. Mutat. 2011;32:894–899. doi: 10.1002/humu.21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benjamini Y., Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. Ser. B. Met. 1995;57:289–300. [Google Scholar]
- 52.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanehisa M., Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.