Summary
Cancers often relapse after adoptive therapy, even though specific T cells kill cells from the same cancer efficiently in vitro. We found that tumor eradication by T cells required high affinities of the targeted peptides for MHC class I. Affinities of at least 10 nM were required for relapse-free regression. Only high-affinity peptide-MHC interactions led to efficient cross-presentation of antigen, thereby stimulating cognate T cells to secrete cytokines. These findings highlight the importance of targeting peptides with high affinity for MHC class I when designing T cell-based immunotherapy.
Introduction
Relapse of cancers is very common even following combinatorial therapy of surgery, chemotherapy, radiation, and/or immunotherapy. For maximal efficacy, drugs depend on reaching the necessary concentration in the tumor microenvironment (Skipper, 1986). This critical concentration concept also applies to cellular effectors such as neutrophils and T cells (Budhu et al., 2010; Li et al., 2002; Li et al., 2004). While cellular effectors or drugs at optimal concentrations can eradicate all sensitive cancer cells, relapse may still occur because of the outgrowth of variants. Cancer cells show extremely high genetic instability and cancers always contain variants that are resistant to destruction by a particular drug or T cell (Anders et al., 2011; Hanson et al., 2000), very similar to what is found for viruses (Hensley et al., 2009).
For complete eradication, it is important to eliminate every residual cancer cell including heritable variants (Singh et al., 1992; Spiotto et al., 2004; Zhang et al., 2007). However, factors responsible for T cell elimination of variants have not been determined. In experiments designed to explore the reason for failed T cell treatment, we took a reductionist approach, ultimately directing our focus to the target peptides, and in particular to their affinities for MHC class I. We selected several peptides that, when targeted, caused tumor eradication, and others that caused relapse. To reduce the influence of differences between cancers, we used two cancer cell lines that were both transduced to express the different peptides. To reduce differences due to expression levels, we used the same design of triple peptides fused to fluorescent proteins. Proteasomal cleavage of proteins may not generate (Chapiro et al., 2006; Popovic et al., 2011) or destroy immunogenic peptides (Schultz et al., 2002). To minimize differences in proteasomal cleavage of the fusion proteins, we designed peptide triplets separated by “Ala-Ala-Tyr” cleavage sites. We targeted antigens with no known oncogenic activity to reduce the possibility that the nature of a particular targeted antigen prevented the cancer from escaping. To exclude the influence of other T cells helping or regulating the relevant CD8+ T cells, TCR-transgenic T cells with a single specificity were adoptively transferred into hosts, which were TCR-transgenic for an irrelevant target. Finally, a single adoptive T cell transfer regimen was used, without providing any additional stimulation such as vaccination or administration of cytokine.
Results
Cancer cells expressing different peptides are killed by T cells with similar efficacy in vitro
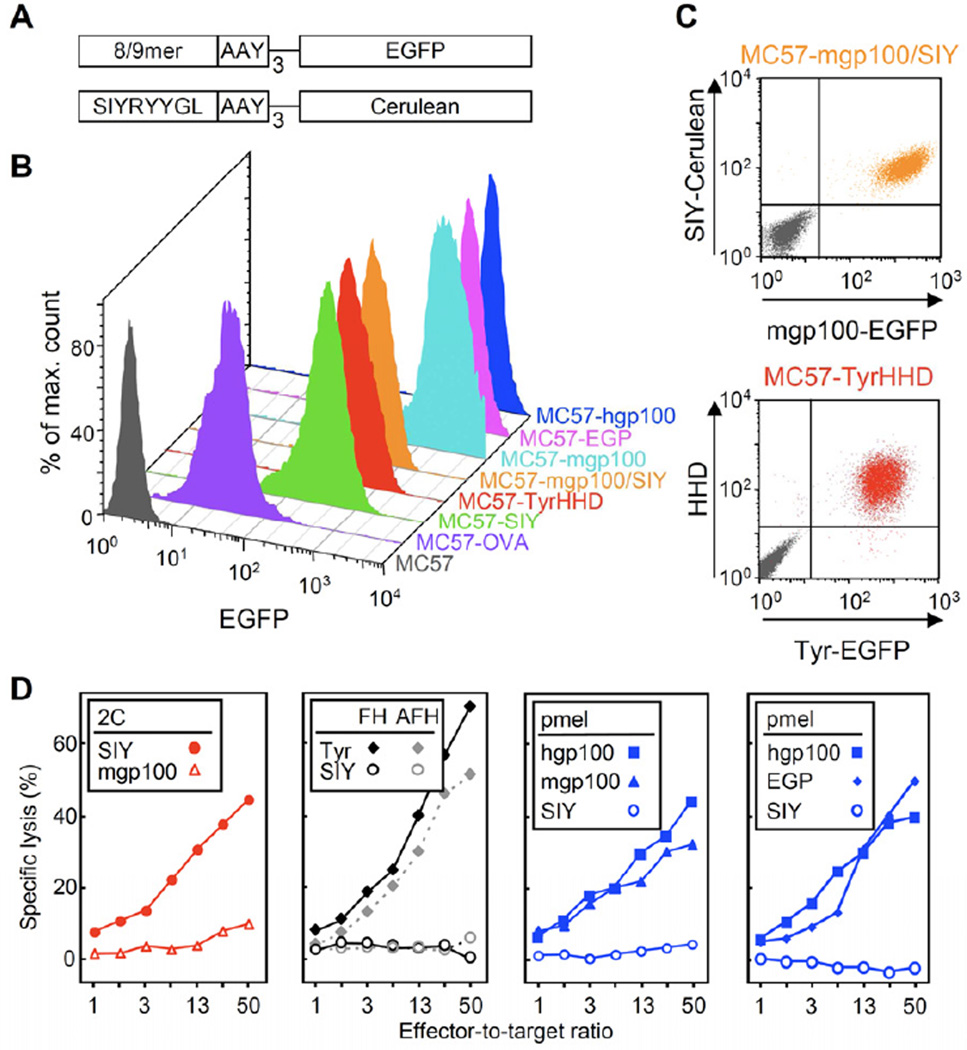
EGFP was fused to minigenes encoding the peptides OVA257, SIY, mouse Tyrosinase369–377 (Tyr369), mouse or human gp10025–33 (mgp10025 and hgp10025, respectively) and EGP; EGP differs from mgp10025 only in the third amino acid (EGPRNQDWL versus EGSRNQDWL), while it shares the proline at position 3 with hgp10025 (KVPRNQDWL). A Cerulean fusion gene was generated only for SIY (Figure 1A and C, top). The fibrosarcoma line MC57 of C57BL/6 origin was used to generate lines that expressed the fusion genes at high levels (Figure 1B). Furthermore, the HHD MHC class I molecule was co-transduced with the Tyr369-EGFP fusion protein to generate MC57-TyrHHD (Figure 1C, bottom).
Figure 1. Six transduced cancer cell lines that express antigens at high levels were effectively killed in vitro.
(A) Diagram of fusion proteins constructed to express antigen in MC57 cancer cells. Triple repeats of peptide and AAY proteasomal cleavage sites were fused to fluorescent proteins: OVA257, SIY, mouse tyrosinase369–377 (Tyr369), mouse or human gp10025–33 (mgp10025 and hgp10025, respectively) and EGP; a Cerulean fusion gene was generated only for SIY. (B) Flow cytometric analysis of peptide-EGFP fusion proteins expressed by the transduced MC57 fibrosarcoma lines. (C) MC57-mgp100/SIY expressed mgp10025 and SIY antigens as EGFP and Cerulean fusion proteins, respectively. The HLA-A2/Db chimeric protein HHD was co-transduced with the Tyr369-EGFP fusion protein to generate MC57-TyrHHD. Parental MC57 (gray) was analyzed for comparison. (D) Cytolysis of MC57 target cells overexpressing SIY, mgp10025, hgp10025, EGP, or Tyr369 and HHD (Tyr) by 2C, pmel, AFH (Tyr-negative) or FH (Tyr-positive) T cells in a 4.5 h 51Cr-release assay. Cancer cells expressing non-cognate peptide were used as negative controls. These data are compiled of three experiments and are representative for seven independent experiments. See Figure S1 for induction of vitiligo by FH and pmel T cells.
Assays in vitro demonstrated similar killing of the cancer lines by cognate peptide-activated T cells (Figure 1D). 2C T cells, whose TCR binds SIY, killed the MC57-SIY line, and pmel T cells killed MC57 cells expressing mouse gp10025, human gp10025 or EGP. Interestingly, Tyr369-specific T cells derived from the FH TCR-transgenic, tyrosinase (Tyr)-deficient albino mouse (AFH) or Tyr-positive black mouse (FH) killed MC57-TyrHHD target cells similarly well. Together, the results imply there is sufficient direct presentation of all processed peptides and sufficient avidity of the T cells for efficient killing in vitro.
T cells targeting SIY, OVA257 or Tyr369 eradicate large tumors
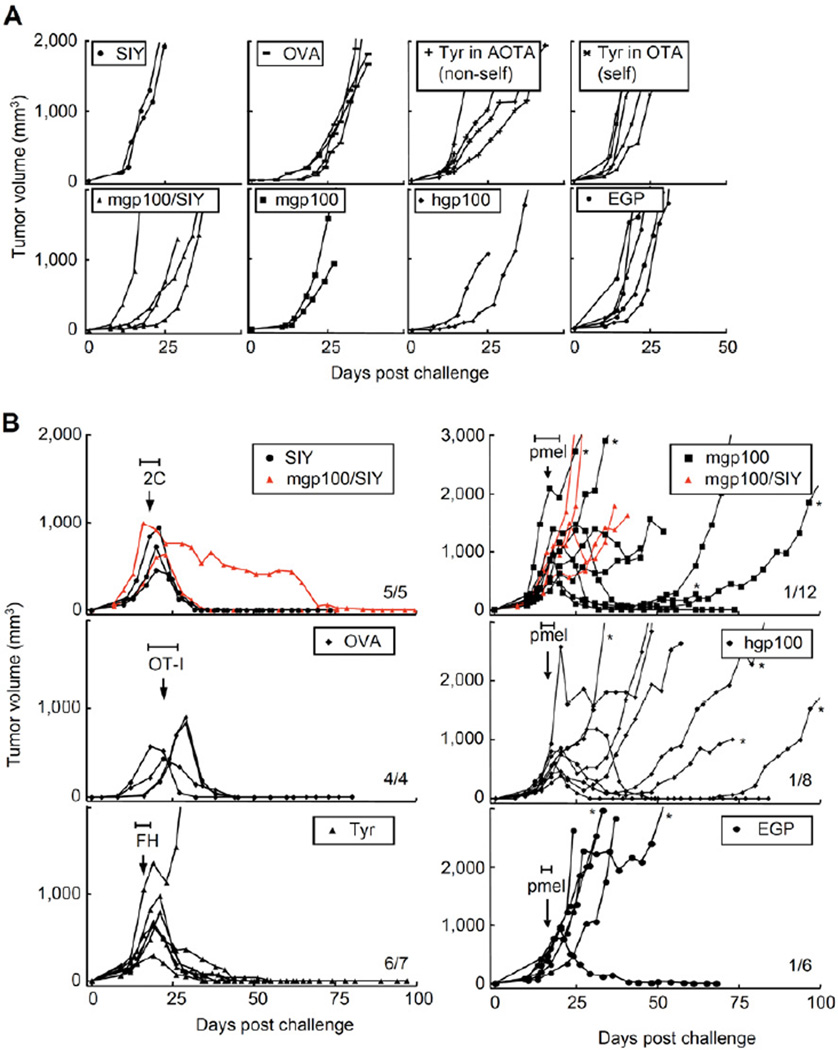
SIY-expressing MC57-SIY cells were injected in TCR-transgenic mice of irrelevant specificity (OT-I). OVA257-transfected cancer cells were injected in 2C TCR-transgenic mice; MC57-TyrHHD cancer cells were grown in OT-I TCR- and AAD-transgenic mice, which did (OTA) or did not express tyrosinase (albino, AOTA). In all cases, cancer cells produced progressively growing tumors within one week (Figure 2A). At least 2 weeks after cancer cell injection, when tumors reached about 500 mm3, mice were treated with T cells. As published by our laboratory, tumors expressing SIY and treated with 2C T cells were eradicated (Figure 2B upper left, Table 1 and Table S1) (Spiotto et al., 2004). Here we also show that OVA257-expressing tumors treated with OT-I T cells were rejected (Figure 2B middle left) and FH T cells eradicated Tyr-positive tumors (Figure 2B lower left). In this last experiment, FH T cells derived from a Tyr-positive donor were transferred into a Tyr-positive host and eradicated a Tyr-expressing tumor. Together, this and other experiments using FH TCR-transgenic T cells from Tyr-negative donors (AFH) and/or Tyr-negative hosts (AOTA) showed that tumors could be rejected (i) whether the targeted peptide was self or non-self for the tumor-bearing host and (ii) whether the targeted peptide was self or non-self for the donor T cells (Figure S2A). This may be unique to our model, since a different model showed that low ubiquitous expression of a transgene prevented the rejection of antigen-expressing tumors through the induction of tolerance (Buschow et al., 2010). Levels of antigen expression in the host and/or tumor, type of cells that express the self-antigen and the source of T cells may likely influence the outcome. Taken together, targeting any of the three peptides SIY, OVA257 or Tyr369 caused eradication of established, large solid tumors.
Figure 2. Targeting SIY, OVA257 or Tyr369 eradicated large tumors while targeting mgp10025, hgp10025 or EGP caused initial tumor regression but was followed by relapse.
(A) Cancer cell lines formed progressively growing tumors within one week. TCR-transgenic mice with irrelevant specificity were challenged s.c. with 2×106 cancer cells. MC57-SIY, -mgp100, -mgp100/SIY, -hgp100 and -EGP were injected into OT-I mice; MC57-OVA grew in 2C mice and MC57-TyrHHD grew in AOTA (Tyr-deficient, non-self) and OTA (Tyr-positive, self). Graphs represent single mice in 11 experiments, listed as non-treated controls in Tables 1, S1 and S3. (B) At least 2 weeks after cancer cell injection, when tumors reached about 500 mm3, each mouse was treated once with cognate T cells (treatment between days 13 and 26, depending on tumor size, as indicated by the horizontal bars (H)). Average size of tumors at day of treatment: 2C: 720 mm3, ranging from 448 to 995 mm3; OT-I: 608 mm3, ranging from 440 to 715 mm3; FH: 517 mm3, ranging from 250 to 848 mm3; pmel, targeting mgp10025: 601 mm3, ranging from 325 to 980 mm3; targeting hgp10025: 470 mm3, ranging from 264 to 936 mm3; targeting EGP: 337 mm3, ranging from 180 to 600 mm3. MC57-TyrHHD was grown in OTA (self) and treated with FH T cells (self). The number of rejected tumors per number of tumors treated is indicated. Data are derived from 15 independent experiments, compiled in Tables 1 and S1. See also Figure S2.
* Tumors were isolated and analyzed for antigen expression (see Figure 3).
Table 1.
Abbreviations, conditions and summary of results for key experiments.A
| Target peptide on cancer cells |
Hosts |
T cells |
Tumor rejection |
|||||
|---|---|---|---|---|---|---|---|---|
| Desig- nation |
Sequence | MHC | Affinity of peptide for MHC (IC50 [nM])B |
Desig- nation |
Relationship of antigen to recipient |
Desig- nation |
Relationship of antigen to donor |
|
| SIY | SIYRYYGL | Kb | 1.1 | OT-I | non-self | 2C | non-self | 5/5C,D,E,F |
| none | 0/6C | |||||||
| OVA257–264 | SIINFEKL | 0.9 | 2C | non-self | OT-I | non-self | 4/4G | |
| none | 0/4G | |||||||
| Tyr369–377 | FMDGTMSQV | A2 | 4.2H | OTA | self | FH | self | 6/7I |
| none | 0/5I | |||||||
| hgp10025–33 | KVPRNQDWLJ | Db | 186 | OT-I | non-self | pmel | non-self | 1/8D |
| none | 0/2 | |||||||
| EGP | EGPRNQDWL | 454 | OT-I | non-self | pmel | non-self | 1/6E | |
| none | 0/5 | |||||||
| mgp10025–33 | EGSRNQDWL | 22,975 | OT-I | self | pmel | self | 1/12F | |
| none | 0/6 | |||||||
See Table S1 for details.
IC50 values represent the geometric mean of 5 or more experiments.
p = 0.002;
p < 0.005;
p = 0.015;
p < 0.001;
p < 0.029;
A higher IC50 value of 65 nM was published for this peptide earlier (Colella et al., 2000). The differences in affinity measurements likely arose as a result of small differences in reagents, methodology, and procedures.
p = 0.015
only the underlined amino acids differ between the three gp100 peptide variants
Large mgp10025, hgp10025, or EGP-expressing tumors relapse after initial regression caused by transferred T cells
In contrast, T cells targeting the self-peptide mgp10025 and the non-self heteroclitic peptides hgp10025 or EGP, did not result in tumor eradication. MC57 lines overexpressing mgp10025, hgp10025 or EGP were injected into OT-I TCR-transgenic mice and produced progressively growing tumors within one week (Figure 2A). At least 2 weeks after cancer cell injection, when the tumors reached about 500 mm3, the mice were treated with pmel T cells. The tumors regressed initially, but eventually almost all tumors relapsed (Figure 2B right panels, Table 1 and Table S1).
To exclude any non-antigenic differences in the cancer lines (caused by transduction and sorting), we used a cell line that expressed both SIY and mgp10025 antigens (MC57-mgp100/SIY, Figure 1C). When mice bearing these tumors were treated with 2C or pmel T cells, the outcome was the same as when tumors from single antigen lines were treated (Figure 2B upper panels). In conclusion, neither human nor mouse gp10025 expressed by the cancer cells supported rejection by pmel T cells.
These findings were not limited to the MCA-induced cancer line MC57 but were confirmed using the UV-induced cancer line 8101 (Figure S2A and D). The line was transduced to overexpress SIY, human or mouse gp10025. Again, we observed eradication of established tumors by adoptive T cell transfer only when SIY was targeted. Interestingly, in this model, targeting hgp10025 was more effective than targeting mgp10025; tumors expressing hgp10025 regressed after pmel transfer, while tumors expressing mgp10025 continued to grow uninhibitedly.
Treatment of tumors expressing human gp10025 but not murine gp10025 or EGP results in outgrowth of antigen-loss variants
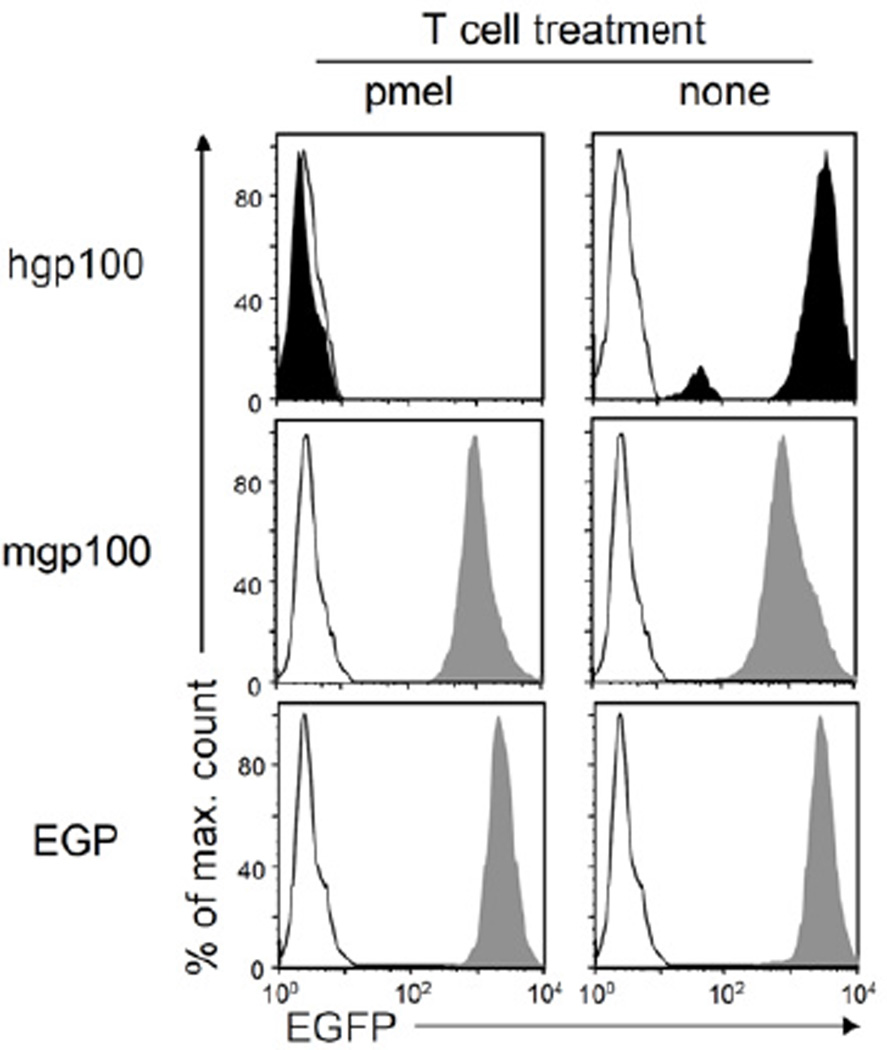
We isolated cancer cells from tumors expressing mgp10025, hgp10025 or EGP that had relapsed following treatment with pmel T cells (Figure 2B) and analyzed these for antigen-loss variants (ALV). All MC57-hgp100 tumors had lost EGFP expression, which indicated loss of hgp10025, as both were expressed as a single fusion protein (one representative tumor shown in Figure 3). Importantly, the tumor isolated from a non-treated mouse retained EGFP expression. MC57-mgp100 and MC57-EGP tumors treated with pmel had also not lost EGFP expression. All lines expressed mgp100-EGFP or EGP-EGFP at levels similar to the isolate from a non-treated mouse (Figure 3). These data suggest that pmel T cells were capable of killing all hgp10025-expressing MC57 cancer cells but were not capable of killing all mgp10025- or EGP-expressing cancer cells in the respective tumors. These findings seem to be influenced also by the targeted cancer cell, as relapsed tumors formed by 8101-hgp100 cancer cells all retained expression of the antigen (data not shown).
Figure 3. Outgrowth of antigen-loss variants after pmel T cell treatment of cancer cells expressing hgp10025 but not of cancers expressing mgp10025 or EGP.
Cancer cells of relapsed tumors expressing mgp10025, EGP (both gray) or hgp10025 (black) were isolated after pmel T cell treatment, adapted to culture, and analyzed for peptide-EGFP fusion gene expression (left panels). MC57 cells (white histogram) cultured in vitro and MC57-mgp100, MC57-EGP (both gray) or MC57-hgp100 (black) cells isolated from non-treated mice (right panels) were analyzed as controls. Isolated lines from mgp100- or hgp100-expressing tumors are representative for four lines each, the isolate from the EGP-expressing tumor is representative for two lines; all lines were isolated after relapse (respective tumors were marked with * in Figure 2B). The repeatability in independent experiments strongly suggests that loss of antigen expression from the hgp10025 cancer cells was not an artifact caused by adaptation or post-isolation culturing. See also Figure S3.
While we did not observe significant differences when targeting either human or mouse gp10025 in treatments of established tumors, we did see differences in protection against cancer cell inoculations. Pmel T cells prevented the outgrowth of MC57-hgp100 but not of MC57-mgp100 tumors (Figure S3A and C). MC57-mgp100 cells formed tumors in which a large fraction of cells still expressed the antigen (Figure S3B). Taken together, pmel T cells showed a stronger effect when targeting hgp10025 compared to mgp10025 and EGP.
Tumor eradication correlates with high affinity of targeted peptides for MHC
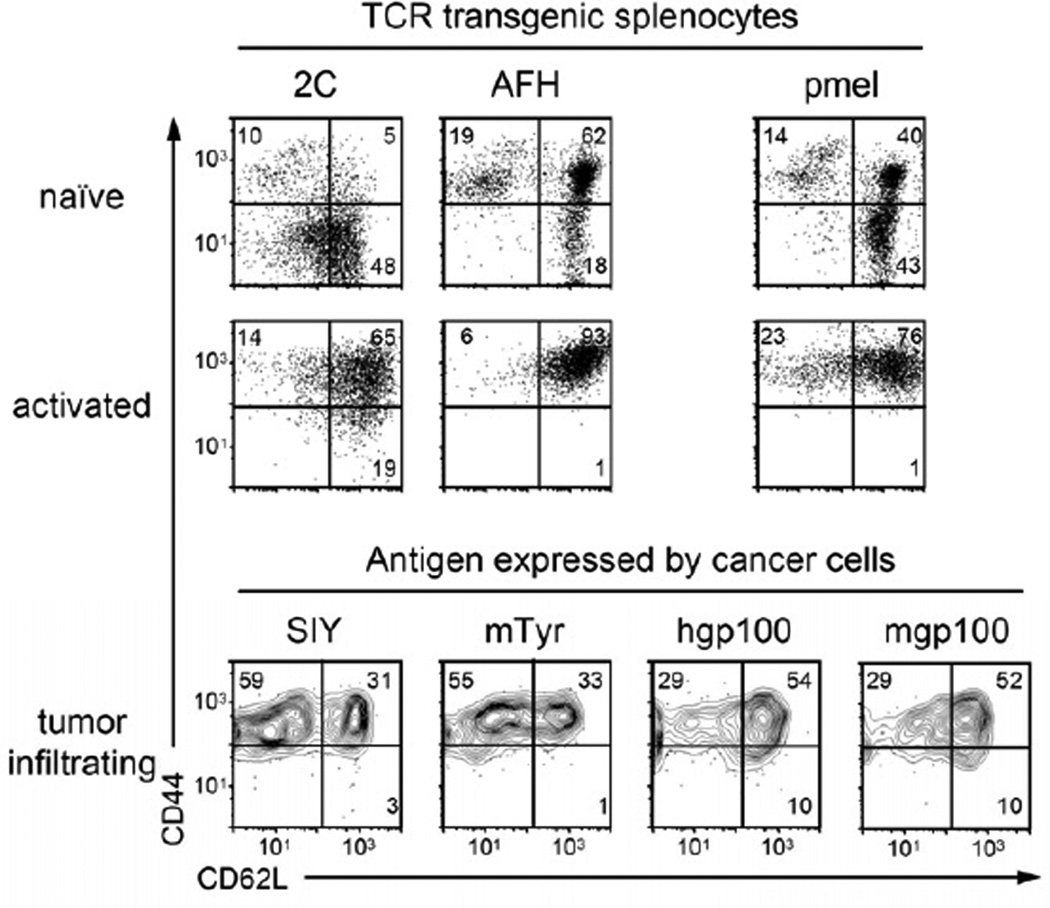
In an effort to understand why targeting some peptides led to eradication while targeting others resulted in relapse, we first analyzed the activation status of the T cells transferred to treat the different tumors (Figure 4). Upon transfer, after peptide stimulation in vitro, all T cells showed the same CD44hi and CD62Lhi phenotype of activated T cells (Figure 4) and demonstrated very similar killing capabilities in vitro (Figure 1). It is worth mentioning that splenocytes from self-reactive TCR-transgenic mice (pmel and AFH) showed an antigen-experienced phenotype (CD44hi), while T cells from the non-self reactive TCR-transgenic 2C mice showed a truly naïve phenotype. However, this difference was overcome after peptide stimulation in vitro. Interestingly, when isolated from tumors four days after adoptive transfer, the T cells that led to eradication of tumors (2C and AFH) showed a more effector-like phenotype compared (CD62Llo, CD44hi) to the more central memory-like phenotype (CD62Lhi, CD44hi) found for pmel T cells. Together, these data suggest that the differences in tumor rejection were not due to differing activation statuses of the T cells at the time of transfer.
Figure 4. T cells transferred to treat the tumors expressing the different peptides showed the same phenotype of activated T cells.
T cells of 2C AFH and pmel TCR-transgenic mice were tested for their activation status in “naïve”, untreated mice (splenocytes), at day 3 after peptide activation in vitro, and on day 4 after adoptive transfer (tumor infiltrating cells). Cells were analyzed by flow cytometry for expression of CD44 and CD62L, and gated on CD8+ T cells expressing the cognate Vβ-chain: Vβ8 for 2C, Vβ11 for AFH and Vβ13 for pmel. Data are representative for two or three independent experiments for the data in vivo and in vitro data, respectively.
As another variable that could influence the efficacy of tumor rejection, we analyzed the affinities of the peptides for the presenting MHC molecules. In a cell-free competition binding assay, the concentration of inhibitor peptides needed to displace half of the probe peptide (IC50 in nanomolar (nM)) was determined. IC50 values are reasonable approximations of real KD values (see Methods). A wide range in binding affinities was measured (Table 1). There was a strong correlation between affinity of the peptide for MHC and tumor eradication. The three target peptides supporting tumor eradication, OVA257, SIY, and Tyr369, displayed strong binding to their cognate MHC (0.9, 1.1 and 4.2 nM, respectively). These high affinities stood in stark contrast to the affinities we measured for mgp10025, EGP and hgp10025 (22,975, 454 and 186 nM, respectively). These three peptides bound the MHC poorly and when targeted resulted in relapse rather than tumor eradication.
Stromal cells isolated from tumors formed by cancer cells expressing peptides with high affinity to MHC stimulate cognate T cells effectively
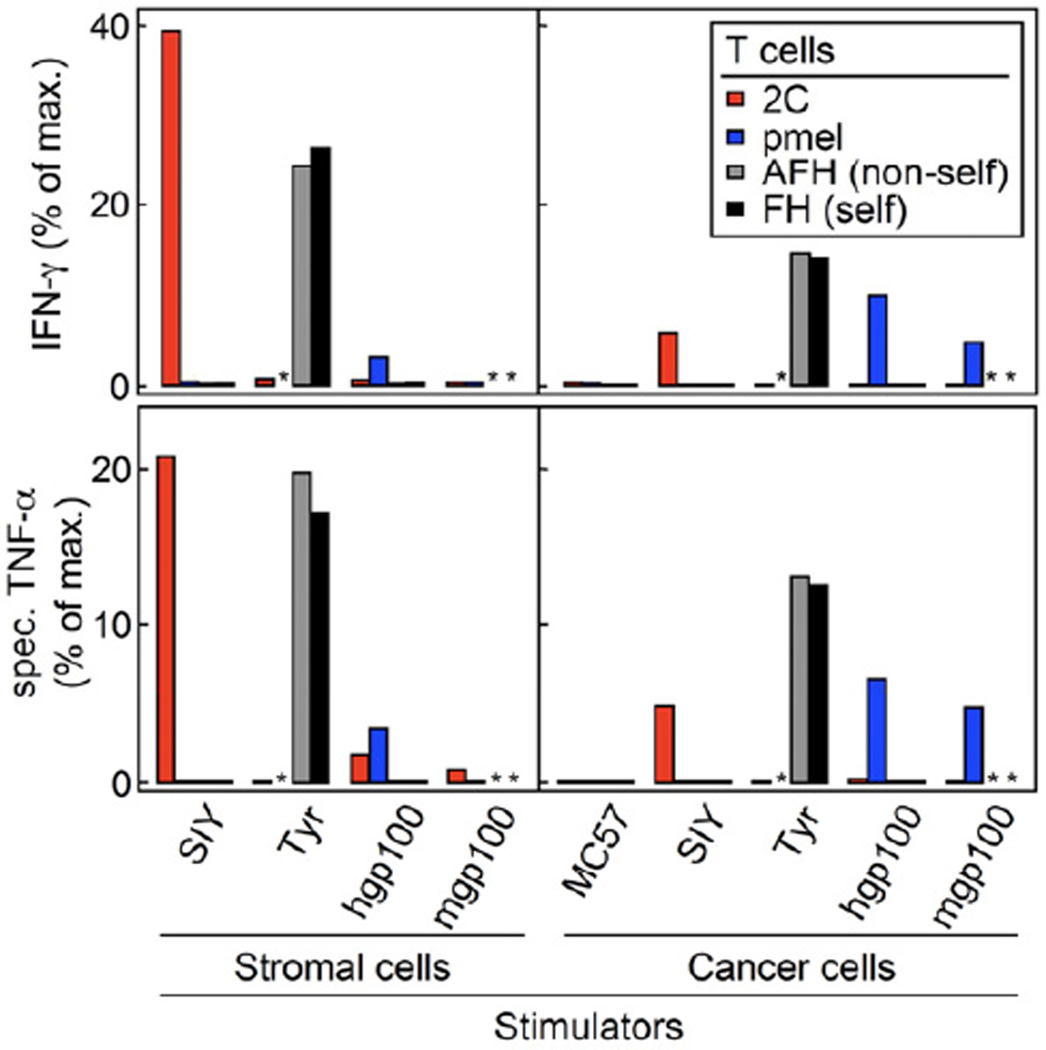
To analyze why high peptide-MHC affinities were required for tumor eradication, we performed assays to evaluate the level of cross-presentation in growing tumors. MC57 and 8101 lines expressing SIY, mgp10025 or hgp10025 were grown in OT-I TCR-transgenic or Rag1−/− mice, respectively; MC57-TyrHHD was grown in AOTA (non-self) mice. SIY was used as a representative peptide for the two highest binding peptides OVA257 and SIY, and only the relatively best and worst binding gp100 peptides (human and mouse gp100) were analyzed in comparison. Enriched populations of CD11b+ stromal cells were obtained from at least 2-week-old untreated tumors and were compared in their ability to stimulate T cells in vitro to analyze the level of cross-presentation of the different peptides expressed by the tumors. For comparison, we used the transduced MC57 and 8101 cancer lines grown in vitro. As seen for the similar killing in vitro of MC57 cells presenting the different peptides in Figure 1D, direct presentation also led to comparable amounts of IFN-γ and TNF-α secretion by cognate T cells (Figure 5). However, in the 8101 model more IFN-γ was found when targeting SIY versus hgp10025 and mgp10025 (Figure S2C). Even bigger differences occurred in both cancer models, when T cells were stimulated with stromal cells. CD11b+ stromal cells cross-presenting SIY and Tyr369 stimulated cognate T cells even more strongly than directly presenting cancer cells (Figure 5). In contrast, both gp10025 peptides were very poorly cross-presented. While stromal cells from hgp10025-expressing tumors stimulated T cells to secrete low levels of both cytokines, stromal cells from mgp10025 tumors did not stimulate T cells at all (Figure 5 and Figure S2C). The heteroclitic peptide EGP behaved similarly to hgp10025 when cross-presented; it stimulated pmel T cells to secrete low amounts of IFN-γ (Figure S4). Thus, the peptides with high affinities for MHC (SIY and Tyr369) were well cross-presented, while peptides with low affinities (all three gp10025 peptides) were so poorly cross-presented that the respective stromal cells could not efficiently stimulate cognate T cells ex vivo.
Figure 5. Only SIY and Tyr369 are cross-presented, as detected by cytokine secretion by T cells stimulated by stromal cells isolated from untreated tumors.
CD11b+ stromal cells were isolated from established untreated tumors and were co-cultured with peptide-activated T cells. Enriched stromal cells from tumors grown from MC57-SIY, -hgp100 and -mgp100 cells (all grown in OT-I mice) and -TyrHHD (grown in AOTA mice (non-self)) were co-cultured with 2C pmel AFH (non-self) or FH (self) TCR-transgenic T cells. Stromal cells from the various tumors were compared to cultured cancer cells expressing the same antigen. Supernatants were harvested after 24 h of co-culture and amounts of IFN-γ and TNF-α measured by ELISA. Data are shown as percent of maximal cytokine secretion (anti-CD3 and -CD28 antibody stimulation, defined as 100 %). For TNF-α, cytokine secretion by stromal cells without T cells was subtracted to obtain specific TNF-α secretion by T cells (443 to 975 pg/ml by 1×105 cells cultured in 200 µl for 24 h, depending on experiment). Unstimulated T cells served as negative control (below 0.7 % for all responders, not shown). Data shown are combined from two experiments and are representative for four independent experiments. See also Figure S4.
* not done
Destruction of tumor stroma is stronger when targeted peptides have high affinity for MHC
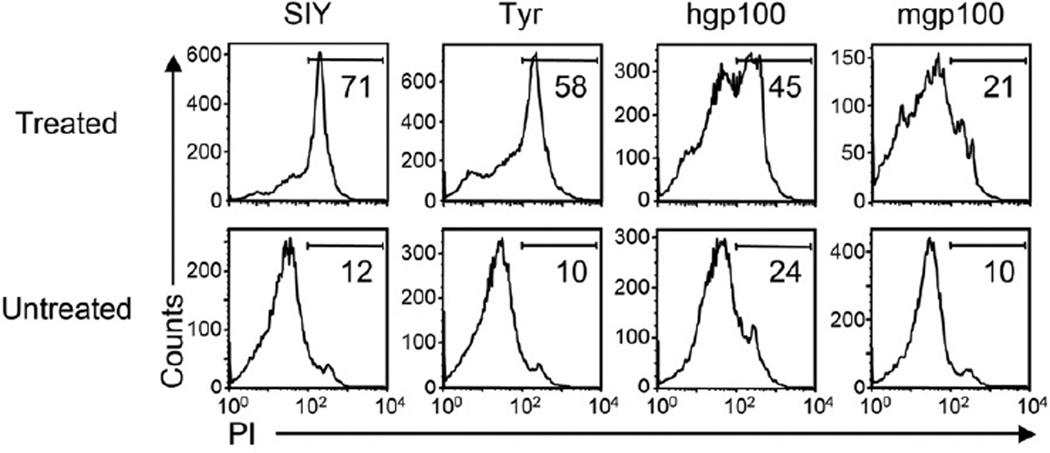
We analyzed regressing MC57 tumors to help us understand how tumor eradication correlated to peptide-MHC affinities. Tumors were dissected on day five after adoptive T cell transfer and we analyzed the viability of CD11b+ stromal cells. Stroma from tumors expressing SIY or Tyr369 showed a high percentage of dead cells (Figure 6), 6- and 5.8-fold increase over background, respectively. In accordance with the relapse of tumors from gp10025 peptide-expressing cells, death of tumor stroma was low, with only 1.9- and 2.2-fold increases over background for hgp10025 and mgp10025, respectively. In conclusion, tumors that were eradicated by cognate T cell therapy showed a high rate of stromal death, while relapsing tumors contain less dead CD11b+ stromal cells.
Figure 6. Death of stromal cells in T cell-treated SIY and Tyr369 expressing tumors.
MC57-SIY, -TyrHHD, -hgp100 and -mgp100 tumors were treated with 2C, AFH (non-self) or pmel T cells, respectively. Five days after adoptive transfer, single cell suspensions were generated from treated and untreated tumors and analyzed by flow cytometry. Histograms show CD11b+ T cells stained with propidium iodide (PI) to identify cell death. Numbers indicate the percentage of dead cells among CD11b+ cells. Data are representative for four independent experiments, with single mice per group.
Discussion
Our results have a direct impact on the design of adoptive immunotherapy. First, and most importantly, the affinity of the targeted peptide for the presenting MHC was highly predictable of success or failure of T cell therapy, indicating that this is a key variable. Only high-affinity peptides that were efficiently presented by cancer cells and/or stroma induced cytokine secretion by T cells, stromal death and relapse-free regression of tumors. Second, targeting self-antigens on tumors did not preclude eradication of large cancers even though the treated mice developed vitiligo (data not shown). Such autoimmunity was also observed in patients treated with anti-self T cells (Morgan et al., 2010; Palmer et al., 2008; Parkhurst et al., 2011; Yee et al., 2000). As might be expected, we found that the self-peptide with the higher affinity for the presenting MHC molecule was associated with stronger autoimmunity (mTyr369 as opposed to mgp10025). Vitiligo was also detected earlier in mice transgenic for the FH TCR compared to pmel (Figure S1 and (Gregg et al., 2010)).
We have analyzed the potential of the different peptides to be cross-presented by tumor stroma. This is an effective readout to evaluate different affinities of peptides for MHC and more sensitive than direct presentation by cancer cells. All peptides were overexpressed by the cancer lines, therefore, no differences in direct presentation were detected in killing and cytokine secretion assays, but cross-presentation reflected the results obtained from the cell-free affinity measurements. Death of stroma correlated with the amount of cross-presentation and tumor relapse. Though it seems to be required for tumor eradication, we do not know whether cross-presentation is essential for stromal death. For example, direct T cell stimulation provided by cancer cells expressing peptides with high affinity for MHC can lead to strong cytokine production, Fas ligand upregulation, and bystander killing (Wang et al., 1996), which could destroy stroma. Stromal cross-presentation may also not be needed when an essential oncogene on the cancer is targeted (Anders et al., 2011; Listopad et al., 2013).
Targeted peptides that led to tumor eradication fell into a category of high-affinity MHC binders (IC50 < 10 nM), whereas affinities of peptides that led to relapse fell into a category of intermediate (IC50 between 50 and 500 nM) or low binders (IC50 > 500 nM). These data are consistent with the low nanomolar affinities needed to provide full protection against lethal Vaccinia virus infection (Moutaftsi et al., 2009). Low affinity peptides may allow perforin-mediated killing, which requires only two to three peptide/MHC complexes and brief T cell–target cell interactions (Purbhoo et al., 2004). However, the efficacy of adoptively transferred T cells to eradicate tumors does not depend on perforin (Garcia-Hernandez Mde et al., 2010; Listopad et al., 2013). The high affinity of peptides for MHC is probably needed for tumor eradication because this allows the formation of stable synapses between T cells and antigen-positive cancer cells and/or stromal cells cross-presenting the antigen. At least ten peptide/MHC complexes need to be engaged for the prolonged interactions required to stimulate T cells to secrete cytokines (Purbhoo et al., 2004), which are essential for tumor eradication (Garcia-Hernandez Mde et al., 2010; Listopad et al., 2013; Zhang et al., 2008). It appears that targeting peptides with affinities below a certain threshold will result in a level of stimulation of effector T cells that is insufficient to eradicate the cancer, resulting in relapse of antigen-positive or -negative cancer cells.
Several studies have tried to overcome relapse after adoptive T cell therapy. They show that the anti-tumor effects of adoptively transferred T cells can be enhanced by selecting for more effective T cell populations, multiple transfers of T cells, high-dose IL-2, vaccinations, and/or total body irradiation (Cheever et al., 1980; Cho et al., 2012; Dummer et al., 2002; Ho et al., 2003; Ly et al., 2010; Matsui et al., 2003; North, 1982; Overwijk et al., 2003). But even under these conditions, relapse was often observed when peptides with low affinities for MHC were targeted (Antony et al., 2005; Gattinoni et al., 2005; Gattinoni et al., 2009; Overwijk et al., 2003).
TCR affinity can undoubtedly be an important factor (Gottschalk et al., 2012). However, in the study presented here, the affinity of the peptides for MHC seemed to determine if T cells could eradicate tumors or not. As reasons for this, we propose that the affinities (KD) of the majority of natural TCRs, measured by surface plasmon resonance, are 1 to 100 µM (Davis et al., 1998; Williams et al., 1999). This is a very narrow range considering the affinity range from under 1 to more than 20,000 nM measured for the different peptides binding MHC. In the same line of argument, a study by Bowerman and colleagues demonstrated in vitro that the magnitude of T cell activity against peptide/MHC was influenced more by peptide binding to MHC than by binding of TCR to peptide/MHC, especially for higher affinity TCRs (Bowerman et al., 2009). Finally, T cells expressing the 2C TCR even when targeting a peptide/MHC complex with a 30-fold higher affinity could not prevent relapse in the absence of cross-presentation. Using 2C T cells to treat MC57-SIY and MC57-Ld tumors, SIY-expressing tumors were rejected (Figure 2B and (Spiotto et al., 2004); affinity of 2C TCR for SIY-Kb (KD = 30 µM)) while MC57-Ld cancer cells grew out as ALV ((Spiotto et al., 2004); affinity of 2C for QL9- and p2Ca-Ld (KD ≈ 1 µM) (Corr et al., 1994; Garcia et al., 1997; Holler and Kranz, 2003)). In contrast to SIY, QL9- and p2Ca-Ld, recognized by 2C as alloantigens, cannot be cross-presented, as the entire peptide/MHC complex would need to be taken up by the stromal cells and then be re-expressed on their surface.
While we did not study the influence of several TCRs with different affinities to one (same) peptide MHC complex, the influence of one TCR (pmel) on tumors expressing three peptides with different affinities for MHC was studied here. The affinities of the pmel TCR for the three peptide/MHC complexes studied, mgp10025, EGP and hgp10025 binding Db, are not known; however, alanine-scans of murine and human gp10025 suggest similar affinities since the first three amino acids, which harbor the only differences between the three peptides, do not contribute to the binding of the peptide/MHC complex to the TCR (but are important for the binding of the peptides to MHC (Overwijk et al., 1998)). Also, structural studies of these peptides (van Stipdonk et al., 2009) in complexes with Db indicate that the two positions that influenced binding to Db (p2 and p3) are both pointing down into the MHC pocket. In fact, the authors did not see significant differences in any of the exposed regions of the peptides, which would be in contact with the TCR. Nevertheless, only cancer cells expressing the peptide with the highest affinity for Db (hgp10025) were effectively killed in vivo, tumors expressing the other peptides relapsed being antigen positive. As none of the three peptides supported complete tumor eradication, we further analyzed their affinity for MHC in detail, which has given us insight into the importance of the stability of peptide–MHC interactions. In the original description of EGP (van Stipdonk et al., 2009), two different RMA-S cell-based assays were employed to determine the relative affinity of EGP for Db compared to the murine gp100 peptide (EGS) and human gp100 peptide (KVP). These assays were: first, a binding assay that measured cell surface Db levels as a function of peptide concentration; in this assay EGP was almost 100-fold better than hgp10025 (KVP) and 1000-fold better than mgp10025 (EGS). Second, a stabilization assay that measured the cell surface lifetimes of the peptide/Db complexes. In this stabilization assay EGP and hgp10025 (KVP) showed similar lifetimes, whereas mgp10025 (EGS) had a considerable shorter lifetime (i.e. the complex was less stable). Consistent with their results, we observed a 50-fold increased affinity of EGP over mgp10025, and this affinity of EGP was similar to that of hgp10025 (454 nM and 186 nM, respectively). We used a cell-free competition binding assay, which is influenced by on- and off-rates (giving an approximation of the dissociation constant (KD)). Taken together, EGP demonstrated similar MHC stabilization compared to hgp10025 (van Stipdonk et al., 2009). Indeed, like hgp10025, the affinity of EGP for Db was insufficient to allow for tumor eradication.
Heteroclitic peptides can induce strong T cell responses that include TCRs with high affinities (Gold et al., 2003; van Stipdonk et al., 2009). However, these T cells will not be able to eradicate tumors if the targeted tumor antigen has low affinity for its presenting MHC. An example is a recent clinical trial that showed no improvement of anti-melanoma effects by addition of vaccinations with heteroclitic gp100 peptides to the immune stimulating anti-CTLA-4 antibody (Hodi et al., 2010). The affinities of the corresponding natural peptides are gp100209: 83 – 172 nM and gp100280: 94 – 455 nM (Kawakami et al., 1995; Parkhurst et al., 1996; Tsai et al., 1997)).
Since our data show that high affinity of peptide for MHC results in tumor eradication along with strong stimulation of T cells to secrete cytokines, future studies should concentrate on targeting peptides that have high affinities for presenting MHC class I. There are several algorithms that are constantly being improved to give a fairly reliable prediction of peptide affinities for MHC (e.g. Immune Epitope Database Analysis Resource). Nevertheless, the predicted affinities of the peptides show 2 to 20-fold differences when compared to the measured affinities (as analyzed here for SIY and OVA257, respectively). While the affinities of peptides for MHC can be accurately measured in standardized cell-free assays, natural processing and presentation of these putative peptides needs also to be confirmed before selecting a peptide as a therapeutic target (Popovic et al., 2011). Together, it should be possible to identify optimal targets for T cell therapies when analysis of peptide-MHC affinity is included.
Experimental Procedures
Cell lines
Phoenix-ampho (Fujita et al., 1992) cells were cultured in DMEM (Mediatech, Manassas, VA), 10% non-heat inactivated FCS (Sigma-Aldrich, St. Louis, MO) at 37°C in a 5% CO2 humidified incubator. Cancer cells lines were cultured in DMEM, 5% FCS (Gemini Bio-Products, West Sacramento, CA) at 37°C in a 10% CO2 dry incubator. 8101 originated in a UV-treated C57BL/6 and has been described (Dubey et al., 1997; Schreiber et al., 2001). P. Ohashi (University of Toronto, Toronto, Ontario, Canada), with permission of H. Hengartner (University Hospital Zurich, Zurich, Switzerland), provided the MC57G methylcholanthrene-induced, C57BL/6-derived fibrosarcoma (MC57). Its transfectant MC57-SIY-1 (MC57-SIY) has been described previously (Spiotto et al., 2002). The new cell lines 8101- and MC57-hgp100, 8101- and MC57-mgp100, 8101-SIY, MC57-EGP and MC57-OVA were generated by transductions of 8101 or MC57 with MFG retroviral vectors expressing peptide-EGFP fusion genes (see Supplemental Experimental Procedures for details on retroviral vectors and transductions). MC57-mgp100/SIY was derived from MC57 by subsequent transduction with MFG-(SIY)3-Cerulean and MFG-mgp100-EGFP. MC57-TyrHHD was obtained by sequential transductions with MFG-Tyr-EGFP and MP71-HHD, encoding a fusion protein of a HLA-A2/Dd chimera and human β2m (Pascolo et al., 1997).
Mice
A list of the pairs of mice used as hosts of tumors and donors of T cell can be found in Table 1. OVA257-Kb-specific TCR-transgenic OT-I mice were provided by M. Mescher (University of Minnesota, Twin Cities, MN), the SIY-Kb-specific TCR-transgenic 2C mice were provided by J. Chen (Massachusetts Institute of Technology, Cambridge, MA), and the human and murine gp10025-Db-specific pmel-1 (referred to as pmel) were provided by N. Restifo (National Cancer Institute, Bethesda, MD) (Overwijk et al., 2003). Other TCR-transgenic mice used in this study are the murine Tyr369-A2-specific AFH mouse (Nichols et al., 2007), which is also AAD-transgenic (HLA-A2 and Db chimera (Newberg et al., 1996)) and albino (Tyr-deficient) (Colella et al., 2000). It is important to note that the FH TCR used for targeting the self-peptide mTyr369 has been derived in a non-self setting. The TCR was obtained from a Tyr-deficient albino mouse (Nichols et al., 2007), while the mouse from which pmel was obtained expressed mgp10025 (Overwijk et al., 2003). This does not imply that the pmel TCR specific for mgp10025 is of lower affinity, but rather that TCRs with a certain affinity for peptide/MHC can only be found naturally, if the target peptide is not expressed (FH) or of low affinity for MHC (pmel). The tyrosinase-positive, Tyr369-A2-specific, AAD-transgenic FH mice were generated by crossing AFH to C57BL/6J (The Jackson Laboratory, Bar Harbor, ME) and selecting black (Tyr+) mice. The OT-I-, Thy1.1- and AAD-transgenic strains AOTA (albino, Tyr−) and OTA (Tyr+) were obtained by crossing ATA (Nichols et al., 2007) to OT-I/Thy1.1 (Thy1a; provided by T. Gajewski, The University of Chicago, Chicago, IL) and selecting for mice with white or black fur color, respectively. All colonies, including Rag1−/−(B6.129S7-Rag1tm1Mom/J, The Jackson Laboratory), were maintained at the University of Chicago facilities. The Institutional Animal Care and Use Committee at the University of Chicago approved all animal experiments and all experiments were performed conform to the relevant regulatory standards.
Peptides
The peptides EGP (EGPRNQDWL), hgp10025 (KVPRNQDWL), mgp10025 (EGSRNQDWL), OVA257 (SIINFEKL), SIY (SIYRYYGL), and Tyr369 (FMDGTMSQV) were made by solid-phase peptide synthesis using standard FMOC chemistry (see Supplemental Experimental Procedures for details).
T cell cultures
NH4Cl-treated splenocytes were cultured at 4 × 106 cells/ml, 3 ml per well of a 6-well plate in RPMI, 10% FCS (Sigma-Aldrich), 2 mM glutamine, 50 µM β-mercaptoethanol, 1 mM Hepes, 1 mM sodium pyruvate, 1x non-essential amino acids, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µg/ml gentamicin (all Gibco/Invitrogen, Carlsbad, CA). T cells were activated with 1 µg/ml anti-CD3 (145-2C11) and anti-CD28 (37.51, both eBioscience, San Diego, CA) for killing assays in vitro or 1 µM cognate peptide: SIY for 2C, Tyr369 for AFH and FH, OVA257 for OT-I, and hgp10025 for pmel. Activated T cells were used for adoptive transfer after 3 days and for assays in vitro after 4 days of culture.
Cytotoxicity assay
Cell-mediated lysis of target cells by activated T cells was determined by standard 4.5-h 51Cr-release assay. Briefly, target cells were labeled for 1 h with 100 µCi sodium chromate-51 (Perkin Elmer, Waltham, MA) and incubated with T cells using E:T ratios from 50:1 to 1.3:1, using 5 × 103 target cells. The 51Cr-released was measured using a gamma counter (Titertek, Huntsville, AL). The percentage of specific lysis was calculated as: % specific lysis = [(experimental release-spontaneous release)/(maximum release - spontaneous release)] × 100.
Tumor challenge and treatment
Cultured cancer cells were trypsinized and washed with PBS. Cancer cells in suspension (MC57: 2 × 106 / 200 µl, 8101: 5 to 10 × 106 / 200 µl) were injected subcutaneously onto the shaved back of mice. Tumor volumes were measured along three orthogonal axes (a, b, and c) every 3 to 4 days and tumor volume calculated as abc/2. MC57 tumors were treated after at least 14 days, when tumors reached approximately 500 mm3; 8101 tumors were treated after at least 5 weeks, when tumors reached approximately 300 mm3. Mice were treated with 3-day activated T cells, one spleen per recipient. We injected 5.5 ± 1.3 × 107 activated 2C T cells, 5.3 ± 2.4 × 107 activated FH T cells and 6.9 ± 2.2 × 107 activated pmel T cells (Numbers were derived from six independent experiments). 8101 tumors were treated with cells from half a spleen only, and with naïve T cells in some of the experiments (see Table S2). T cell suspensions of one spleen were injected into the recipient via the retro orbital plexus in two doses of 150 µl. For tumor protection, T cells were injected on the day of tumor challenge or 3 days later, as indicated.
Isolation of stromal and cancer cells from tumors
Two-week old, untreated tumors were used for functional analysis; tumors of mice treated with T cells 4 or 5 days prior were used for flow cytometric analysis of T cells and stromal death, respectively. Tumors were surgically excised and single cell suspensions generated by enzymatic digestion (see Supplemental Experimental Procedures). For stromal cross-presentation, CD11b+ cells were enriched using magnetic beads (Dynabeads FlowComp Flexi (Invitrogen Dynal, Oslo, Norway) and anti-CD11b antibody (M1/70, BD Bioscience, Franklin Lakes, NJ)).
To analyze antigen loss, relapsed tumors were surgically excised under sterile conditions and placed in DMEM on ice. Tumors were minced to 1 – 2 mm pieces and seeded in DMEM, 10% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µg/ml gentamicin and 50 µg/ml nystatin. Cells and fragments in the flask were not moved for the initial three days and then cultured normally.
Cytokine release assay
T cells activated in vitro for 4 days were incubated with cancer cells cultured in vitro or tumor stromal cells obtained ex vivo. 1 × 105 responders were cultured with 1 × 105 stimulators per well of a 96-well U-bottom plate for 24 h. Wells coated with 1 µg/ml of anti-CD3 (145-2C11) and anti-CD28 (37.51, eBioscience) served as positive controls and maximal stimulation. All supernatants were removed and tested for IFN-γ and TNF-α using ELISA Kits (‘Femto-HS’ High Sensitivity, eBioscience) according to the manufacturer’s protocol.
Flow cytometry
Cells were stained using directly labeled antibodies (see Supplemental Experimental Procedures). Flow cytometry data were acquired on FACSCalibur or FACSCanto machines (BD) and data were analyzed using FlowJo (Tree Star, Ashland, OR) software. Cell sorting was performed using FACSAria (BD) or MoFlo-HTS (Beckman Coulter, Brea, CA) at the Flow Cytometry Facility of The University of Chicago.
MHC peptide binding assays
MHC purification, and quantitative assays to measure the binding affinity of peptides to purified H2-Kb, H2-Db, and HLA-A*0201 molecules were performed as previously described (Assarsson et al., 2007; Sidney et al., 2001) (see Supplemental Experimental procedures for details). Under the conditions used, where [label] < [MHC] and IC50 ≥ (MHC), the measured IC50 values are reasonable approximations of the true KD values.
Statistical analysis
Results of treatment of small groups of mice were analyzed using the two-tailed probability calculated by the Fisher’s exact probability test (p ≤ 0.05 is considered significant, p ≤ 0.01 highly significant).
Supplementary Material
Highlights.
Tumor relapse versus eradication is determined by affinity of peptide for MHC
Outcome of adoptive T cell therapy is determined by affinity of peptide for MHC
Stroma is only destroyed in tumors expressing peptides with high affinity for MHC
Efficient cross-presentation is dependent on high peptide-MHC affinity
Significance.
Cancer relapse remains the greatest obstacle to virtually any cancer therapy. Our data show that high affinity of the targeted peptides for MHC is required for strong stimulation of T cells to secrete cytokines and cause relapse-free tumor eradication. Adoptive T cell transfer therapies should, therefore, target peptides that have high affinities for the presenting MHC class I.
Acknowledgements
We thank Dr. Theodore Karrison (The University of Chicago) for help with statistical analysis, Zhang Yi for generating the cancer lines MC57-hgp100 and MC57-mgp100 and the University of Chicago Flow Cytometry Core Facility. We also thank Ainhoa Arina and Christian Idel for critical review of the manuscript.
This work was supported by a Research Fellowship of the DFG to BE (EN 703/3-1), NIH grants P01-CA97296, R01-CA22677 and R01-CA37516 to HS and the Cancer Center at the University of Chicago.
Nonstandard abbreviations used
- ALV
antigen-loss variant
- nM
nanomolar
- Tyr
tyrosinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflict of interests.
References
- Anders K, Buschow C, Herrmann A, Milojkovic A, Loddenkemper C, Kammertoens T, Daniel P, Yu H, Charo J, Blankenstein T. Oncogene-targeting T cells reject large tumors while oncogene inactivation selects escape variants in mouse models of cancer. Cancer Cell. 2011;20:755–767. doi: 10.1016/j.ccr.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assarsson E, Sidney J, Oseroff C, Pasquetto V, Bui HH, Frahm N, Brander C, Peters B, Grey H, Sette A. A quantitative analysis of the variables affecting the repertoire of T cell specificities recognized after vaccinia virus infection. J Immunol. 2007;178:7890–7901. doi: 10.4049/jimmunol.178.12.7890. [DOI] [PubMed] [Google Scholar]
- Bowerman NA, Colf LA, Garcia KC, Kranz DM. Different strategies adopted by K(b) and L(d) to generate T cell specificity directed against their respective bound peptides. J Biol Chem. 2009;284:32551–32561. doi: 10.1074/jbc.M109.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhu S, Loike JD, Pandolfi A, Han S, Catalano G, Constantinescu A, Clynes R, Silverstein SC. CD8+ T cell concentration determines their efficiency in killing cognate antigen-expressing syngeneic mammalian cells in vitro and in mouse tissues. J Exp Med. 2010;207:223–235. doi: 10.1084/jem.20091279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschow C, Charo J, Anders K, Loddenkemper C, Jukica A, Alsamah W, Perez C, Willimsky G, Blankenstein T. In vivo imaging of an inducible oncogenic tumor antigen visualizes tumor progression and predicts CTL tolerance. J Immunol. 2010;184:2930–2938. doi: 10.4049/jimmunol.0900893. [DOI] [PubMed] [Google Scholar]
- Chapiro J, Claverol S, Piette F, Ma W, Stroobant V, Guillaume B, Gairin JE, Morel S, Burlet-Schiltz O, Monsarrat B, et al. Destructive cleavage of antigenic peptides either by the immunoproteasome or by the standard proteasome results in differential antigen presentation. J Immunol. 2006;176:1053–1061. doi: 10.4049/jimmunol.176.2.1053. [DOI] [PubMed] [Google Scholar]
- Cheever MA, Greenberg PD, Fefer A. Specificity of adoptive chemoimmunotherapy of established syngeneic tumors. J Immunol. 1980;125:711–714. [PubMed] [Google Scholar]
- Cho HI, Reyes-Vargas E, Delgado JC, Celis E. A potent vaccination strategy that circumvents lymphodepletion for effective antitumor adoptive T-cell therapy. Cancer Res. 2012;72:1986–1995. doi: 10.1158/0008-5472.CAN-11-3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella TA, Bullock TN, Russell LB, Mullins DW, Overwijk WW, Luckey CJ, Pierce RA, Restifo NP, Engelhard VH. Self-tolerance to the murine homologue of a tyrosinase-derived melanoma antigen: implications for tumor immunotherapy. J Exp Med. 2000;191:1221–1232. doi: 10.1084/jem.191.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr M, Slanetz AE, Boyd LF, Jelonek MT, Khilko S, al-Ramadi BK, Kim YS, Maher SE, Bothwell AL, Margulies DH. T cell receptor-MHC class I peptide interactions: affinity, kinetics, and specificity. Science. 1994;265:946–949. doi: 10.1126/science.8052850. [DOI] [PubMed] [Google Scholar]
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- Dubey P, Hendrickson RC, Meredith SC, Siegel CT, Shabanowitz J, Skipper JC, Engelhard VH, Hunt DF, Schreiber H. The immunodominant antigen of an ultraviolet-induced regressor tumor is generated by a somatic point mutation in the DEAD box helicase p68. J Exp Med. 1997;185:695–705. doi: 10.1084/jem.185.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Nolan GP, Ghosh S, Baltimore D. Independent modes of transcriptional activation by the p50 and p65 subunits of NF-kappa B. Genes Dev. 1992;6:775–787. doi: 10.1101/gad.6.5.775. [DOI] [PubMed] [Google Scholar]
- Garcia KC, Tallquist MD, Pease LR, Brunmark A, Scott CA, Degano M, Stura EA, Peterson PA, Wilson IA, Teyton L. Alphabeta T cell receptor interactions with syngeneic and allogeneic ligands: affinity measurements and crystallization. Proc Natl Acad Sci U S A. 1997;94:13838–13843. doi: 10.1073/pnas.94.25.13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hernandez Mde L, Hamada H, Reome JB, Misra SK, Tighe MP, Dutton RW. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol. 2010;184:4215–4227. doi: 10.4049/jimmunol.0902995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JS, Ferrone CR, Guevara-Patino JA, Hawkins WG, Dyall R, Engelhorn ME, Wolchok JD, Lewis JJ, Houghton AN. A single heteroclitic epitope determines cancer immunity after xenogeneic DNA immunization against a tumor differentiation antigen. J Immunol. 2003;170:5188–5194. doi: 10.4049/jimmunol.170.10.5188. [DOI] [PubMed] [Google Scholar]
- Gottschalk RA, Hathorn MM, Beuneu H, Corse E, Dustin ML, Altan-Bonnet G, Allison JP. Distinct influences of peptide-MHC quality and quantity on in vivo T-cell responses. Proc Natl Acad Sci U SA. 2012;109:881–886. doi: 10.1073/pnas.1119763109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RK, Nichols L, Chen Y, Lu B, Engelhard VH. Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J Immunol. 2010;184:1909–1917. doi: 10.4049/jimmunol.0902778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- Hensley SE, Das SR, Bailey AL, Schmidt LM, Hickman HD, Jayaraman A, Viswanathan K, Raman R, Sasisekharan R, Bennink JR, Yewdell JW. Hemagglutinin receptor binding avidity drives influenza A virus antigenic drift. Science. 2009;326:734–736. doi: 10.1126/science.1178258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18:255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, Robbins PF, Sette A, Appella E, Rosenberg SA. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–3968. [PubMed] [Google Scholar]
- Li Y, Karlin A, Loike JD, Silverstein SC. A critical concentration of neutrophils is required for effective bacterial killing in suspension. Proc Natl Acad Sci U S A. 2002;99:8289–8294. doi: 10.1073/pnas.122244799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Karlin A, Loike JD, Silverstein SC. Determination of the critical concentration of neutrophils required to block bacterial growth in tissues. J Exp Med. 2004;200:613–622. doi: 10.1084/jem.20040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listopad JJ, Kammertoens T, Anders K, Silkenstedt B, Willimsky G, Schmidt K, Kuehl AA, Loddenkemper C, Blankenstein T. Fas expression by tumor stroma is required for cancer eradication. Proc Natl Acad Sci U S A. 2013;110:2276–2281. doi: 10.1073/pnas.1218295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly LV, Sluijter M, Versluis M, Luyten GP, van Stipdonk MJ, van der Burg SH, Melief CJ, Jager MJ, van Hall T. Peptide vaccination after T-cell transfer causes massive clonal expansion, tumor eradication, and manageable cytokine storm. Cancer Res. 2010;70:8339–8346. doi: 10.1158/0008-5472.CAN-10-2288. [DOI] [PubMed] [Google Scholar]
- Matsui K, O'Mara LA, Allen PM. Successful elimination of large established tumors and avoidance of antigen-loss variants by aggressive adoptive T cell immunotherapy. Int Immunol. 2003;15:797–805. doi: 10.1093/intimm/dxg078. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutaftsi M, Salek-Ardakani S, Croft M, Peters B, Sidney J, Grey H, Sette A. Correlates of protection efficacy induced by vaccinia virus-specific CD8+ T-cell epitopes in the murine intranasal challenge model. Eur J Immunol. 2009;39:717–722. doi: 10.1002/eji.200838815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberg MH, Smith DH, Haertel SB, Vining DR, Lacy E, Engelhard VH. Importance of MHC class 1 alpha2 and alpha3 domains in the recognition of self and non-self MHC molecules. J Immunol. 1996;156:2473–2480. [PubMed] [Google Scholar]
- Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of "self"-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DC, Chan CC, Gattinoni L, Wrzesinski C, Paulos CM, Hinrichs CS, Powell DJ, Jr., Klebanoff CA, Finkelstein SE, Fariss RN, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci U S A. 2008;105:8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA, Davis JL, Morgan RA, Merino MJ, Sherry RM, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19:620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med. 1997;185:2043–2051. doi: 10.1084/jem.185.12.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic J, Li LP, Kloetzel PM, Leisegang M, Uckert W, Blankenstein T. The only proposed T-cell epitope derived from the TEL-AML1 translocation is not naturally processed. Blood. 2011;118:946–954. doi: 10.1182/blood-2010-12-325035. [DOI] [PubMed] [Google Scholar]
- Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- Schreiber K, Wu TH, Kast WM, Schreiber H. Tracking the common ancestry of antigenically distinct cancer variants. Clin Cancer Res. 2001;7:871s–875s. [PubMed] [Google Scholar]
- Schultz ES, Chapiro J, Lurquin C, Claverol S, Burlet-Schiltz O, Warnier G, Russo V, Morel S, Levy F, Boon T, et al. The production of a new MAGE-3 peptide presented to cytolytic T lymphocytes by HLA-B40 requires the immunoproteasome. J Exp Med. 2002;195:391–399. doi: 10.1084/jem.20011974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidney J, Southwood S, Oseroff C, del Guercio MF, Sette A, Grey HM. Measurement of MHC/peptide interactions by gel filtration. Curr Protoc Immunol Chapter. 2001;18:18–13. doi: 10.1002/0471142735.im1803s31. Unit. [DOI] [PubMed] [Google Scholar]
- Singh S, Ross SR, Acena M, Rowley DA, Schreiber H. Stroma is critical for preventing or permitting immunological destruction of antigenic cancer cells. J Exp Med. 1992;175:139–146. doi: 10.1084/jem.175.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper HE. On mathematical modeling of critical variables in cancer treatment (goals: better understanding of the past and better planning in the future) Bull Math Biol. 1986;48:253–278. doi: 10.1007/BF02459681. [DOI] [PubMed] [Google Scholar]
- Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu YX, Schreiber H. Increasing tumor antigen expression overcomes "ignorance" to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- Tsai V, Southwood S, Sidney J, Sakaguchi K, Kawakami Y, Appella E, Sette A, Celis E. Identification of subdominant CTL epitopes of the GP100 melanoma-associated tumor antigen by primary in vitro immunization with peptide-pulsed dendritic cells. J Immunol. 1997;158:1796–1802. [PubMed] [Google Scholar]
- van Stipdonk MJ, Badia-Martinez D, Sluijter M, Offringa R, van Hall T, Achour A. Design of agonistic altered peptides for the robust induction of CTL directed towards H-2Db in complex with the melanoma-associated epitope gp100. Cancer Res. 2009;69:7784–7792. doi: 10.1158/0008-5472.CAN-09-1724. [DOI] [PubMed] [Google Scholar]
- Wang R, Rogers AM, Ratliff TL, Russell JH. CD95-dependent bystander lysis caused by CD4+ T helper 1 effectors. J Immunol. 1996;157:2961–2968. [PubMed] [Google Scholar]
- Williams CB, Engle DL, Kersh GJ, Michael White J, Allen PM. A kinetic threshold between negative and positive selection based on the longevity of the T cell receptor-ligand complex. J Exp Med. 1999;189:1531–1544. doi: 10.1084/jem.189.10.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, Kenyon K, Davis MM, Riddell SR, Greenberg PD. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley DA, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-gamma-and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest. 2008;118:1398–1404. doi: 10.1172/JCI33522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.