Abstract
The synergistic inhibition of the growth of Marchantia polymorpha gemmalings by lysine and threonine and its prevention by methionine has been investigated utilizing 14C-labeled amino acids. Experiments involving the uptake of 14C-lysine or 14C-threonine in the presence or absence of methionine indicated that the synergistic growth effects were not a result of altered amino acid uptake. These data, as well as direct chemical analysis, indicated that growth inhibition was correlated with an inhibition of protein synthesis. Experiments utilizing 14C-aspartic acid revealed that the presence of lysine and threonine resulted in increased 14CO2 production and an accumulation of soluble 14C-aspartic acid and labeled ninhydrin-positive compounds. These metabolic alterations were prevented when methionine was also included in the growth media. A model depicting a sequence of events which involve the interaction of regulatory mechanisms is suggested to account for the effects of specific amino acids on plant growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURLANT L., DATTA P., GEST H. CONTROL OF ENZYME ACTIVITY IN GROWING BACTERIAL CELLS BY CONCERTED FEEDBACK INHIBITION. Science. 1965 Jun 4;148(3675):1351–1353. doi: 10.1126/science.148.3675.1351. [DOI] [PubMed] [Google Scholar]
- Billen D., Hewitt R. Influence of starvation for methionine and other amino acids on subsequent bacterial deoxyribonucleic acid replication. J Bacteriol. 1966 Sep;92(3):609–617. doi: 10.1128/jb.92.3.609-617.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J. K. Studies on the catalytic and regulatory properties of homoserine dehydrogenase of Zea mays roots. Biochim Biophys Acta. 1969 Feb 11;171(2):205–216. doi: 10.1016/0005-2744(69)90154-5. [DOI] [PubMed] [Google Scholar]
- Cohen G. N., Stanier R. Y., Le Bras G. Regulation of the biosynthesis of amino acids of the aspartate family in Coliform bacteria and Pseudomonads. J Bacteriol. 1969 Sep;99(3):791–801. doi: 10.1128/jb.99.3.791-801.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATTA P., GEST H. CONTROL OF ENZYME ACTIVITY BY CONCERTED FEEDBACK INHIBITION. Proc Natl Acad Sci U S A. 1964 Oct;52:1004–1009. doi: 10.1073/pnas.52.4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P. Regulation of branched biosynthetic pathways in bacteria. Science. 1969 Aug 8;165(3893):556–562. doi: 10.1126/science.165.3893.556. [DOI] [PubMed] [Google Scholar]
- Dougall D. K., Fulton M. M. Biosynthesis of Protein Amino Acids in Plant Tissue Culture IV Isotope Competition Experiments using Glucose-U-C and Potential Intermediates. Plant Physiol. 1967 Jul;42(7):941–945. doi: 10.1104/pp.42.7.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
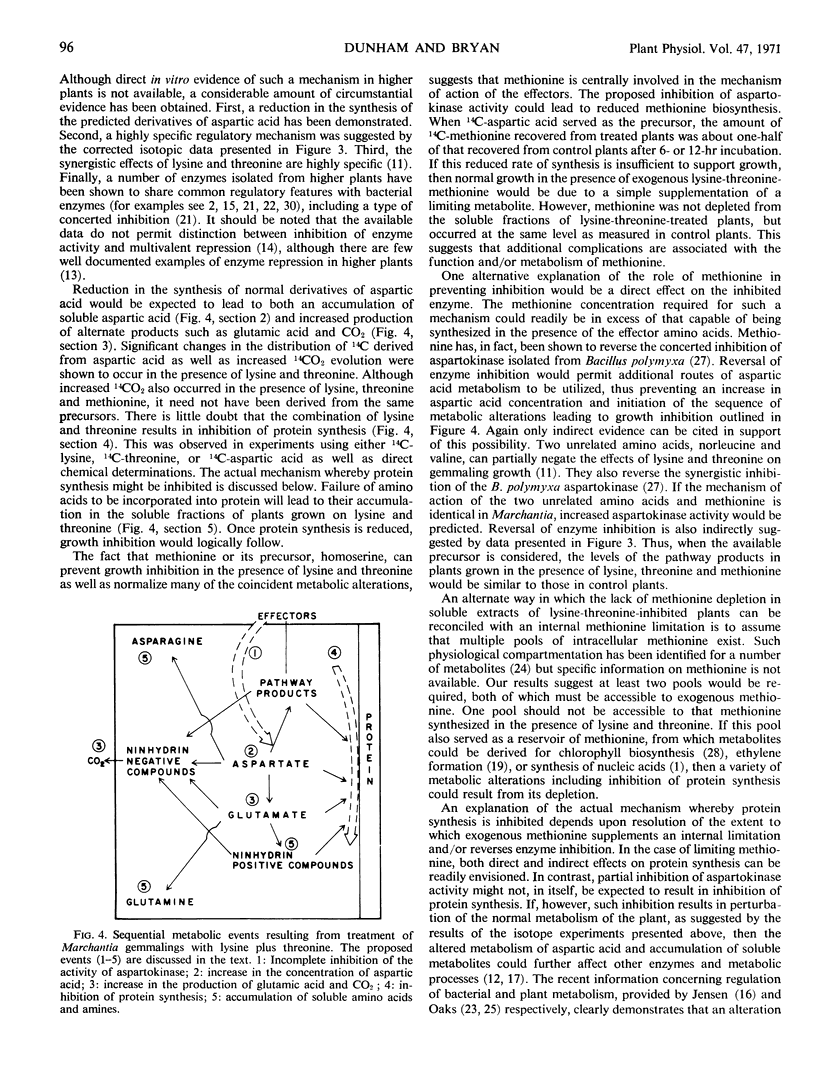
- Dunham V. L., Bryan J. K. Synergistic effects of metabolically related amino acids on the growth of a multicellular plant. Plant Physiol. 1969 Nov;44(11):1601–1608. doi: 10.1104/pp.44.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREUNDLICH M. Multivalent repression in the biosynthesis of threonine in Salmonella typhimurium and Escherichia coli. Biochem Biophys Res Commun. 1963 Feb 6;10:277–282. doi: 10.1016/0006-291x(63)90430-3. [DOI] [PubMed] [Google Scholar]
- Filner P. Regulation of nitrate reductase in cultured tobacco cells. Biochim Biophys Acta. 1966 May 5;118(2):299–310. doi: 10.1016/s0926-6593(66)80038-3. [DOI] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Jensen R. A. Metabolic interlock. Regulatory interactions exerted between biochemical pathways. J Biol Chem. 1969 Jun 10;244(11):2816–2823. [PubMed] [Google Scholar]
- Joy K. W. Nitrogen metabolism of Lemna minor. I. Growth, nitrogen sources and amino acid inhibition. Plant Physiol. 1969 Jun;44(6):845–848. doi: 10.1104/pp.44.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. T. Ethylene production from methionine. Biochem J. 1965 Nov;97(2):449–459. doi: 10.1042/bj0970449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks A. Asparagine synthesis in Zea mays. Biochim Biophys Acta. 1967 Jul 25;141(2):436–439. doi: 10.1016/0304-4165(67)90122-5. [DOI] [PubMed] [Google Scholar]
- Oaks A. The synthesis of leucine in maize embryos. Biochim Biophys Acta. 1965 Nov 15;111(1):79–89. doi: 10.1016/0304-4165(65)90474-5. [DOI] [PubMed] [Google Scholar]
- PAULUS H., GRAY E. MULTIVALENT FEEDBACK INHIBITION OF ASPARTOKINASE IN BACILLUS POLYMYXA. J Biol Chem. 1964 Nov;239:PC4008–PC4009. [PubMed] [Google Scholar]
- Paulus H., Gray E. Multivalent feedback inhibition of aspartokinase in Bacillus polymyxa. I. Effect of nonpolar L-amino acids. J Biol Chem. 1968 Apr 10;243(7):1349–1355. [PubMed] [Google Scholar]
- Radmer R. J., Bogorad L. (Minus) S-adenosyl-L-methionine-magnesium protoporphyrin methyltransferase, an enzyme in the biosynthetic pathway of chlorophyll in Zea mays. Plant Physiol. 1967 Mar;42(3):463–465. doi: 10.1104/pp.42.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala J., Patrick C., Macbeth G. FDPases of the castor bean endosperm and leaf: properties and partial purification. Arch Biochem Biophys. 1968 Sep 20;127(1):576–584. doi: 10.1016/0003-9861(68)90265-8. [DOI] [PubMed] [Google Scholar]


