Abstract
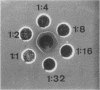
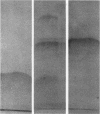
Glucanase (endo-β-1, 3-glucan 3-glucanohydrolase, EC 3.2.1.6, laminarinase, callase) and chitinase (poly-β-1, 4-[2-acetamido-2-deoxy] -d-glucoside glycanohydrolase, EC 3.2.1.14) were extracted from ethylene-treated bean (Phaseolus vulgaris L. cv. Red Kidney) leaves and purified on hydroxyapatite and carboxymethyl Sephadex columns. The glucanase prepared was homogeneous as judged by analytical centrifugation data, electrophoresis, and antibody-antigen reactions. On the basis of gel filtration, antibody-antigen reactions, and amino acid analysis, the molecular weight was estimated to be between 11,500 and 12,500. However, ultracentrifugation gave a higher estimate of 34,000. The glucanase had an isoelectric point near pH 11 and was specific for β-1, 3-linkages. The chitinase was only partially purified as judged by electrophoretic behavior.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Forrence L. E. Temporal and hormonal control of beta-1,3-glucanase in Phaseolus vulgaris L. Plant Physiol. 1970 Apr;45(4):395–400. doi: 10.1104/pp.45.4.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl O. P., Agrawal K. M. Glycosidases of Phaseolus vulgaris. I. Isolation and characterization of beta-N-acetylglucosaminidase. J Biol Chem. 1968 Jan 10;243(1):98–102. [PubMed] [Google Scholar]
- Bull A. T., Chesters C. G. The biochemistry of laminarin and the nature of laminarinase. Adv Enzymol Relat Areas Mol Biol. 1966;28:325–364. doi: 10.1002/9780470122730.ch5. [DOI] [PubMed] [Google Scholar]
- Chalutz E., Stahmann M. A. Induction of pisatin by ethylene. Phytopathology. 1969 Dec;59(12):1972–1973. [PubMed] [Google Scholar]
- Cleland R. Auxin and wall extensibility: reversibility of auxin-induced wall-loosening process. Science. 1968 Apr 12;160(3824):192–194. doi: 10.1126/science.160.3824.192. [DOI] [PubMed] [Google Scholar]
- FEINGOLD D. S., NEUFELD E. F., HASSID W. Z. Synthesis of a beta-1, 3-linked glucan by extracts of Phaseolus aureus seedlings. J Biol Chem. 1958 Oct;233(4):783–788. [PubMed] [Google Scholar]
- FOSTER A. B., WEBBER J. M. Chitin. Adv Carbohydr Chem. 1960;15:371–393. doi: 10.1016/s0096-5332(08)60192-7. [DOI] [PubMed] [Google Scholar]
- FREEBAIRN H. T., BUDDENHAGEN I. W. ETHYLENE PRODUCTION BY PSEUDOMONAS SOLANACEARUM. Nature. 1964 Apr 18;202:313–314. doi: 10.1038/202313a0. [DOI] [PubMed] [Google Scholar]
- Frankel R., Izhar S., Nitsan J. Timing of callase activity and cytoplasmic male sterility in Petunia. Biochem Genet. 1969 Oct;3(5):451–455. doi: 10.1007/BF00485605. [DOI] [PubMed] [Google Scholar]
- Heyn A. N. Glucanase activity in coleoptiles of Avena. Arch Biochem Biophys. 1969 Jul;132(2):442–449. doi: 10.1016/0003-9861(69)90387-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakagaki Y., Hirai T., Stahmann M. A. Ethylene production by detached leaves infected with tobacco mosaic virus. Virology. 1970 Jan;40(1):1–9. doi: 10.1016/0042-6822(70)90372-7. [DOI] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Skujins J. J., Potgieter H. J., Alexander M. Dissolution of fungal cell walls by a streptomycete chitinase and beta-(1-3) glucanase. Arch Biochem Biophys. 1965 Aug;111(2):358–364. doi: 10.1016/0003-9861(65)90197-9. [DOI] [PubMed] [Google Scholar]
- Stahmann M. A., Clare B. G., Woodbury W. Increased disease resistance and enzyme activity induced by ethylene and ethylene production of black rot infected sweet potato tissue. Plant Physiol. 1966 Nov;41(9):1505–1512. doi: 10.1104/pp.41.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]