Abstract
Few nonsurgical treatment options are available to the patient with debilitating knee osteoarthritis (OA) that is refractory to conservative care. The KineSpring® System joint unloading implant is a unique device that reduces the load carried by the medial compartment of the knee joint by up to 13 kilograms during the stance phase of gait. We report a case of a male patient who underwent implant with the KineSpring System for symptomatic knee OA but subsequently required revision due to local infection. We performed a novel two-stage revision procedure where the absorber unit was removed in the first phase and a new absorber was placed 3 months later after the infection resolved. A key finding of this case was that knee OA pain resolved with the KineSpring System, returned following explant of the absorber unit, and resolved again following implant of the new absorber. Another important aspect of this case was that the femoral and tibial bases of the KineSpring System remained in situ, which simplified each phase of the revision procedure.
Keywords: KineSpring, knee, osteoarthritis, revision, unloading
Introduction
Patients with debilitating knee osteoarthritis (OA) refractory to conservative treatments are often referred for total knee arthroplasty (TKA). However, due to the invasive procedure, extended recovery period, and complication risk, only 9% to 33% of patients are willing to consider TKA.1–3 Consequently, an estimated 3.6 million knee OA patients in the United States linger in the treatment gap, characterized by failure of conservative therapies and refusal to undergo TKA.4
The KineSpring® Knee Implant System (Moximed, Inc, Hayward, CA) is a unique, joint preserving, unloading implant with potential to fill the therapeutic void between conservative and surgical knee OA treatments. The key component of the KineSpring System is a covered cobalt/cobalt-chrome alloy absorber that reduces the load carried by the affected medial compartment by up to 13 kilograms during the stance phase of gait. The clinical utility of the KineSpring System is postulated since excessive knee joint loading is a known potent stimulus for knee OA development5–7 while therapies that reduce loading forces across the knee joint may halt OA progression8,9 or even allow healing.10 The KineSpring System absorber resides in the subcutaneous tissue and is attached to titanium alloy femoral and tibial bases (Figure 1). The entire device is extra-capsular and extra-articular with no removal of bone, ligament, or cartilage required. Despite the simplicity of the implant procedure, surgeons must be intimately familiar with appropriate revision techniques should the need arise. We report a case that involved a two-stage revision procedure to treat infection following KineSpring System implant.
Figure 1.
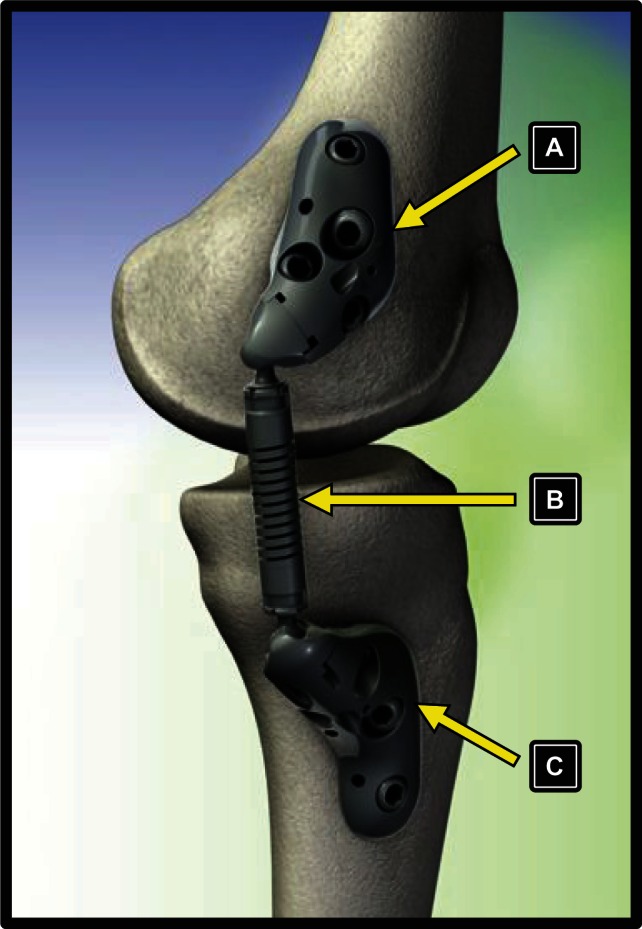
Components of the KineSpring® Knee Implant System. (A) femoral base, (B) absorber unit, (C) tibial base.
Case details
Patient evaluation
A 46-year-old male patient presented to the clinic in April 2010 complaining of medial pain and catching in the left knee when playing tennis. He also reported intermittent mild medial pain with weight-bearing over the past 2 years. Physical examination revealed mild correctable varus and medial joint line tenderness. Weight-bearing radiographs demonstrated moderate narrowing of the medial joint space (Figure 2). Magnetic resonance imaging confirmed a medial meniscal tear, herniation from the joint space with bone edema within the medial tibial plateau, and osteochondral damage on the medial femoral articular surface (Figure 3).
Figure 2.
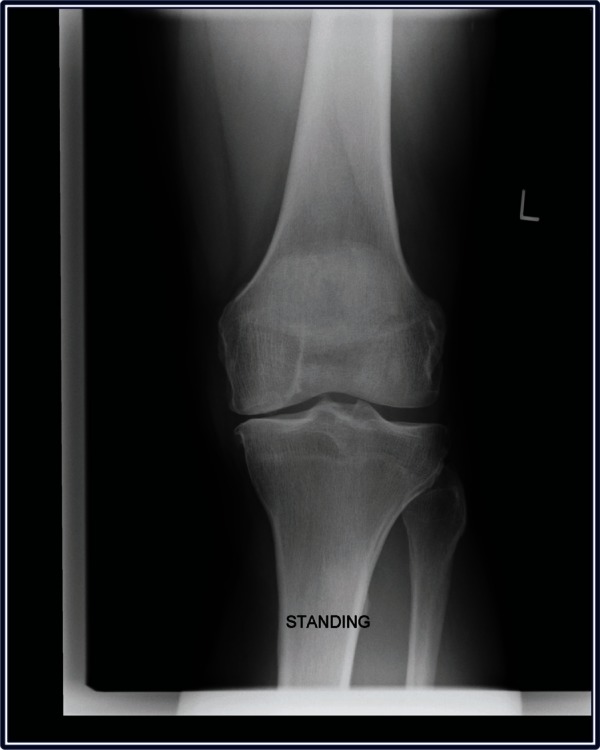
Weight-bearing radiograph of left knee demonstrating moderate narrowing of the medial joint space.
Figure 3.
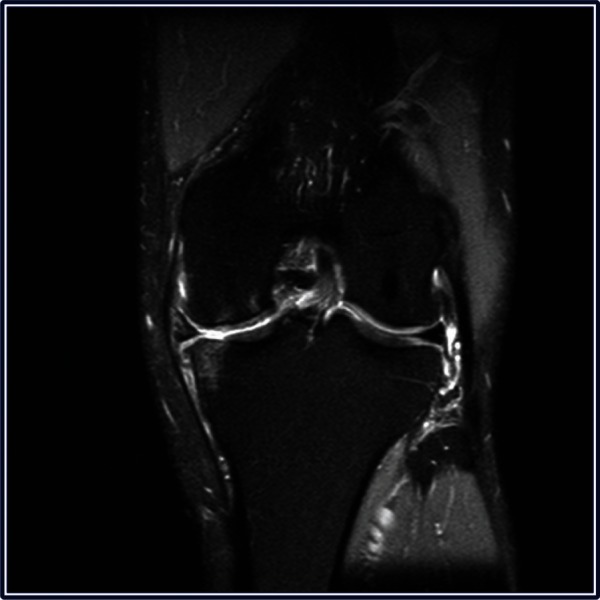
Magnetic resonance imaging of the left knee demonstrating a medial meniscal tear, herniation from the joint space with bone edema within the medial tibial plateau, and osteochondral damage on the medial femoral articular surface.
Arthroscopy and meniscectomy
The patient underwent arthroscopy in September 2010, which revealed a degenerated medial meniscal tear, grade IV anteromedial lesion on the proximal tibia, and a grade 3 lesion on the opposing medial femoral condyle. A partial medial meniscectomy was undertaken with chondroplasty of the unstable chondral femoral flap. The patient returned 3 months later reporting that knee joint catching had improved although medial pain in extension during weight-bearing activity persisted. He also reported discomfort when walking distances and stated that he could not return to tennis because of these symptoms. Surgical options were discussed with the patient including osteotomy, unicompartmental knee arthroplasty, and KineSpring System joint unloading implant. After consideration of potential risks and benefits of each treatment, the patient opted for the KineSpring System unloading device due to lower anticipated morbidity and operative risks compared to other invasive surgical alternatives.
KineSpring System implant procedure and follow-up
The patient underwent implant with the KineSpring System in July 2011. Arthroscopy was undertaken prior to implant, which confirmed grade 4 lesions on the medial femoral condyle and the medial tibial plateau. The KineSpring procedure followed standard techniques that have been described elsewhere11 and was uneventful (Figure 4). The patient remained hospitalized for 2 days following the procedure. Although he was allowed to bear partial body weight and perform gentle range of motion exercises, he was instructed to walk with crutches for 2 weeks to encourage wound healing. The patient reported immediate arthritic pain relief in the postoperative period. At the 2-week follow-up, the wound was fully healed and range of motion was 90 degrees. He was encouraged to engage in only light activities until 6 weeks post-surgery. At 5 weeks, the patient expressed satisfaction with his progress. Knee range of motion was 110 degrees, all wounds were well healed, and arthritic symptoms were nonexistent. The patient was instructed to slowly resume physical activity as tolerated. At 6 weeks, the patient returned with redness and inflammation at the tibial wound following several days of prolonged physical activity. He was diagnosed with suspected bursitis, instructed to rest and ice, and placed on a precautionary 2-week course of antibiotics. At the 8-week follow-up, the wound appeared to have settled with only minor detectable inflammation. At 10 weeks, the tibial wound began discharging necrotic fat from a small sinus at the proximal end of the tibial wound. The patient was readmitted for surgery where the tibial wound was incised, cleaned, and debrided of necrotic tissue down to the tibial base. Neither the femoral wound, the knee joint, nor the unloader device was involved. Microbiological swabs confirmed the presence of antibiotic-sensitive coagulase-negative staphylococci. The patient was then offered one of four treatment options: (1) continuation of antibiotics, (2) device explant with antibiotic continuation, (3) a single-stage revision procedure with exchange of the absorber unit and bases, or (4) a two-stage revision procedure involving initial removal of the absorber unit while leaving the bases in situ and later returning to reinsert a new absorber after the infection resolved. The patient selected the two-stage revision procedure.
Figure 4.
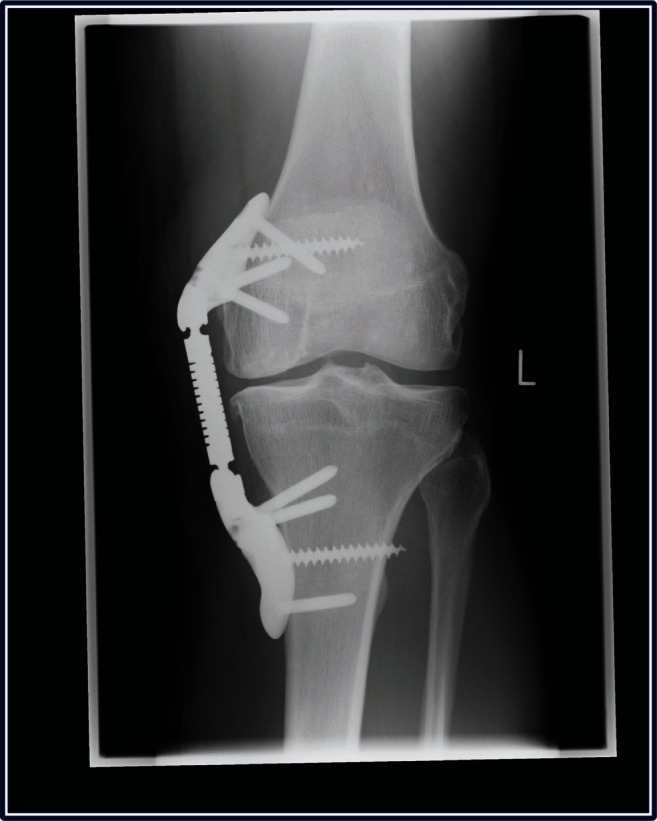
Weight-bearing radiograph of left knee demonstrating successful initial KineSpring® System.
First stage revision procedure and follow-up
The first-stage revision procedure was performed in September 2011, 3 months after the initial KineSpring procedure. The femoral and tibial wounds were reopened and the absorber unit was easily removed with the base units remaining in situ. A pre-soaked saline gauze was introduced into the resulting soft tissue voids and the wounds were thoroughly cleaned and closed. Intravenous antibiotics were administered for 48 hours with oral antibiotics prescribed for 6 weeks. Interestingly, following removal of the absorber unit, the patient reported that arthritic medial knee pain returned with weight-bearing.
Second stage revision procedure and follow-up
The second stage of the revision procedure was performed 3 months after the first stage. Two incisions were made through the original exposure sites and a new absorber unit was fitted onto the pre-existing bases and subsequently activated (Figure 5). The procedure was completed in 30 minutes with no complications. Postoperatively, the patient was instructed to gradually increase ambulation with the assistance of crutches while using minimal knee bending. The patient returned for follow-up visits weekly, which included visual inspection and microbial swabs of the wounds, until complete wound healing was realized. At 6 weeks, there was no evidence of infection, the wounds were well healed, and radiographs were unremarkable. At 3 months, the patient reported no wound problems, complete resolution of arthritic pain, and 120 degree range of motion with normal ambulation. Despite residual muscle weakness, the patient regularly engaged in moderate physical activity including walking, cycling, and tennis with no reported complications, pain, or joint dysfunction.
Figure 5.
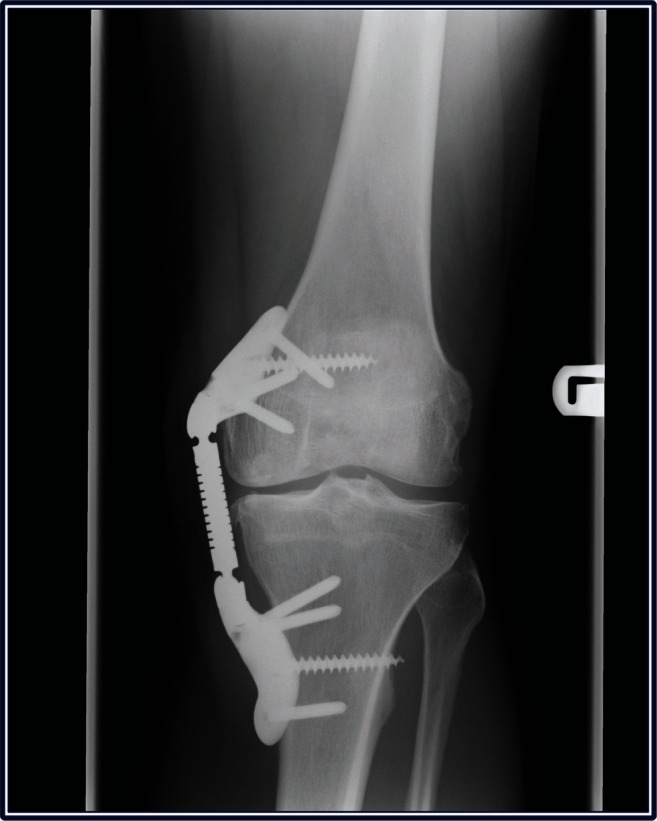
Weight-bearing radiograph of left knee demonstrating successful KineSpring® System following two-stage revision procedure.
Discussion
Knee OA treatments that delay or prevent TKA have potential to yield tremendous clinical, economic, and societal benefits. The KineSpring System is a unique joint unloading device that is appropriate for patients with symptomatic knee OA unresponsive to conservative care who refuse to undergo TKA. The effectiveness of this implant was clearly demonstrated by relief of arthritic symptoms with the implant and return of symptoms with removal of the absorber unit. The novelty of this case, however, is the unique two-stage revision procedure that was utilized. Unlike invasive surgical procedures such as unicompartmental knee arthroplasty, TKA and high tibial osteotomy that permanently alter the knee anatomy, the KineSpring System can be easily implanted and, if needed, easily explanted via the same access sites without limiting future surgical options since all surrounding anatomical structures (eg, bone, muscles, and ligaments) remain intact. The main advantage of the two-stage revision procedure is that the femoral and tibial bases were able to remain in situ. Consequently, each stage of the procedure required only manipulation of the absorber with no need to remove and reinsert bases. This is advantageous since the revision procedure was simpilified and because the resulting osseous voids following removal of the base screws may limit the available trajectories for future base screw insertions if another KineSpring System is implanted later. This two-stage revision procedure is distinctly different than that required for TKA revision. For example, early infections after TKA are managed by removal of the articulating spacer, thoroughly irrigating the joint, reinserting a new spacer, and employing aggressive antibiotic therapy. Late infections after TKA are managed with a complex two-stage open revision requiring removal of all implants at the first stage and, following antibiotic therapy and complete resolution of the infection, reinsertion of all components at the second stage. Our approach greatly simplifies each procedural stage while also salvaging the base components of the device.
Although we cannot definitively confirm the cause of infection, this case highlights the importance of careful wound management and slow return to vigorous or sporting activities during the postoperative period with the KineSpring System. Following the initial implant, the combination of significant symptom relief and excellent wound healing persuaded the patient to rapidly increase physical activity workload, which led to inflammation of an immature wound.
The postoperative rehabilitation program following KineSpring implant consists of three distinct stages. Phase 1 ranges from 0 to 2 weeks and focuses on wound healing. This stage consists of regular wound control to hasten healing; pain medication such as femoral nerve block or analgesics, elevation, ice, and lymph drainage to minimize edema; limits on physical activity and knee flexion to protect the wounds; and walking with crutches to further minimize soft tissue irritation. Phase 2 ranges from 3 to 6 weeks and focuses on increasing range of motion and returning to activities of daily living. During this period, the use of crutches should be discontinued; if tolerated, exercises to improve flexibility and strength are incorporated. Examples of such exercises include cycling on a stationary bike with no resistance, assisted single leg balancing, slow to normal speed walking, isometric quadriceps strengthening exercises, and functional exercises including core stabilization movements. Phase 3 continues beyond 6 weeks and focuses on increasing strength and, potentially, returning to sport. Patients are encouraged to increase the intensity of strengthening exercises and to begin sport-specific training in a gradual manner. General guidelines for returning to sport is to wait at least 4 to 6 weeks before swimming, cycling, or golfing, 2 to 3 months before jogging, 3 to 6 months before playing racquet sports, and to wait at least 6 months before skiing. The benefits of a gradual return to physical activity were realized with excellent outcomes following the two-stage revision procedure.
Five clinical trials have been conducted to evaluate the safety and effectiveness of the KineSpring System to date. Peer-reviewed reports with this device are anxiously awaited. In a prospective single-arm clinical trial of patients treated with the KineSpring System and followed for 3 years, knee pain severity improved 76% (P < 0.001) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) subscores significantly improved with an 81% improvement in pain, 81% improvement in function, and a 73% improvement in stiffness (all P < 0.001) (unpublished data). Potential risks with the KineSpring System include deep infection, device separation including ball socket disassembly or loosening of cortical screws, bursitis, and unresolved OA symptoms.
Conclusion
The widespread adoption of orthopedic implants requires the surgeon to become knowledgeable about appropriate revision techniques. The case presented here demonstrates a novel technique that may be utilized for revision of a KineSpring System that simplifies the procedure while salvaging the femoral and tibial bases.
Footnotes
Disclosure
Moximed, Inc, (Hayward, CA) provided financial support for development of this manuscript. The authors report no other conflicts of interest in this work.
References
- 1.Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212–3220. doi: 10.1002/art.22146. [DOI] [PubMed] [Google Scholar]
- 2.Hawker GA, Wright JG, Badley EM, Coyte PC. Perceptions of, and willingness to consider, total joint arthroplasty in a population-based cohort of individuals with disabling hip and knee arthritis. Arthritis Rheum. 2004;51(4):635–641. doi: 10.1002/art.20524. [DOI] [PubMed] [Google Scholar]
- 3.Hawker GA, Wright JG, Coyte PC, et al. Determining the need for hip and knee arthroplasty: the role of clinical severity and patients’ preferences. Med Care. 2001;39(3):206–216. doi: 10.1097/00005650-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 4.London NJ, Miller LE, Block JE. Clinical and economic consequences of the treatment gap in knee osteoarthritis management. Med Hypotheses. 2011;76(6):887–892. doi: 10.1016/j.mehy.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 5.Wilson JF. To stop osteoarthritis, fixing cartilage may not be enough. Ann Intern Med. 2007;147(6):437–439. doi: 10.7326/0003-4819-147-6-200709180-00024. [DOI] [PubMed] [Google Scholar]
- 6.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 7.Thorp LE, Sumner DR, Block JA, Moisio KC, Shott S, Wimmer MA. Knee joint loading differs in individuals with mild compared with moderate medial knee osteoarthritis. Arthritis Rheum. 2006;54(12):3842–3849. doi: 10.1002/art.22247. [DOI] [PubMed] [Google Scholar]
- 8.Block JA, Shakoor N. The biomechanics of osteoarthritis: implications for therapy. Curr Rheumatol Rep. 2009;11(1):15–22. doi: 10.1007/s11926-009-0003-7. [DOI] [PubMed] [Google Scholar]
- 9.Lafeber F P, Intema F, Van Roermund PM, Marijnissen AC. Unloading joints to treat osteoarthritis, including joint distraction. Curr Opin Rheumatol. 2006;18(5):519–525. doi: 10.1097/01.bor.0000240366.54960.a1. [DOI] [PubMed] [Google Scholar]
- 10.Radin EL, Burr DB. Hypothesis: joints can heal. Semin Arthritis Rheum. 1984;13(3):293–302. doi: 10.1016/0049-0172(84)90031-3. [DOI] [PubMed] [Google Scholar]
- 11.Clifford A, O’Connell M, Gabriel S, Miller LE, Block JE. The KineSpring load absorber implant: rationale, design and biomechanical characterization. J Med Eng Technol. 2011;35(1):65–71. doi: 10.3109/03091902.2010.535592. [DOI] [PubMed] [Google Scholar]


