Abstract
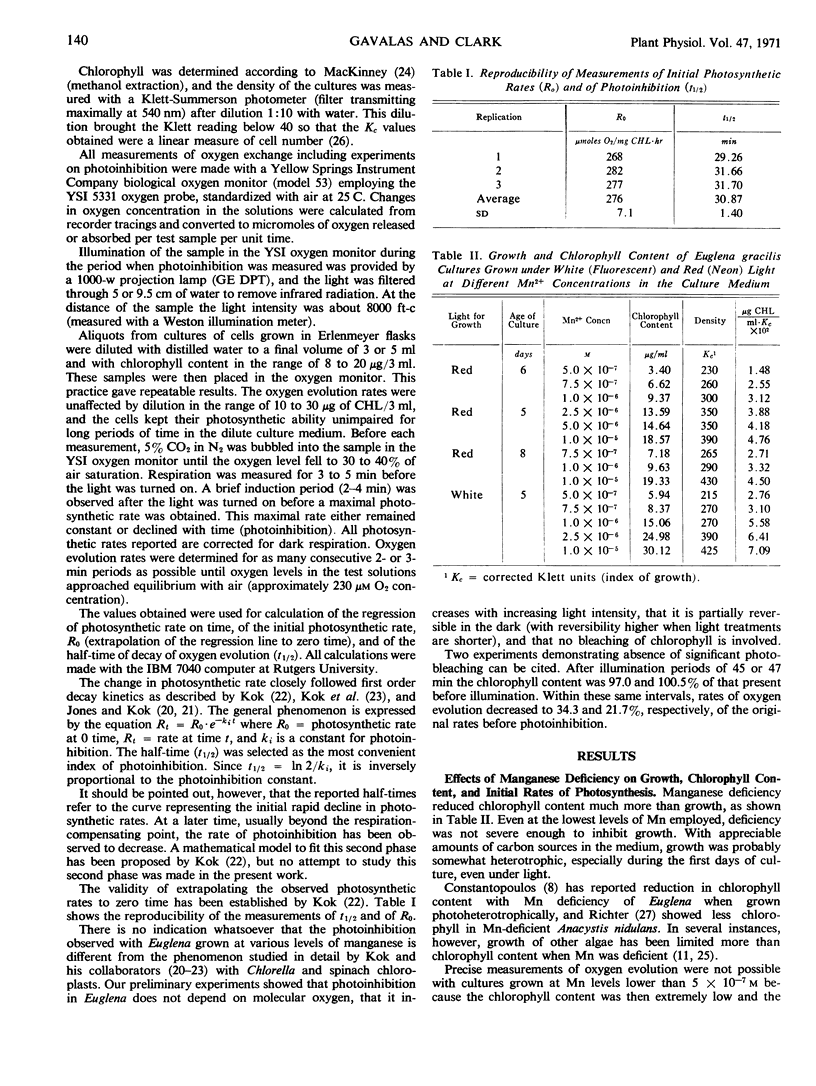
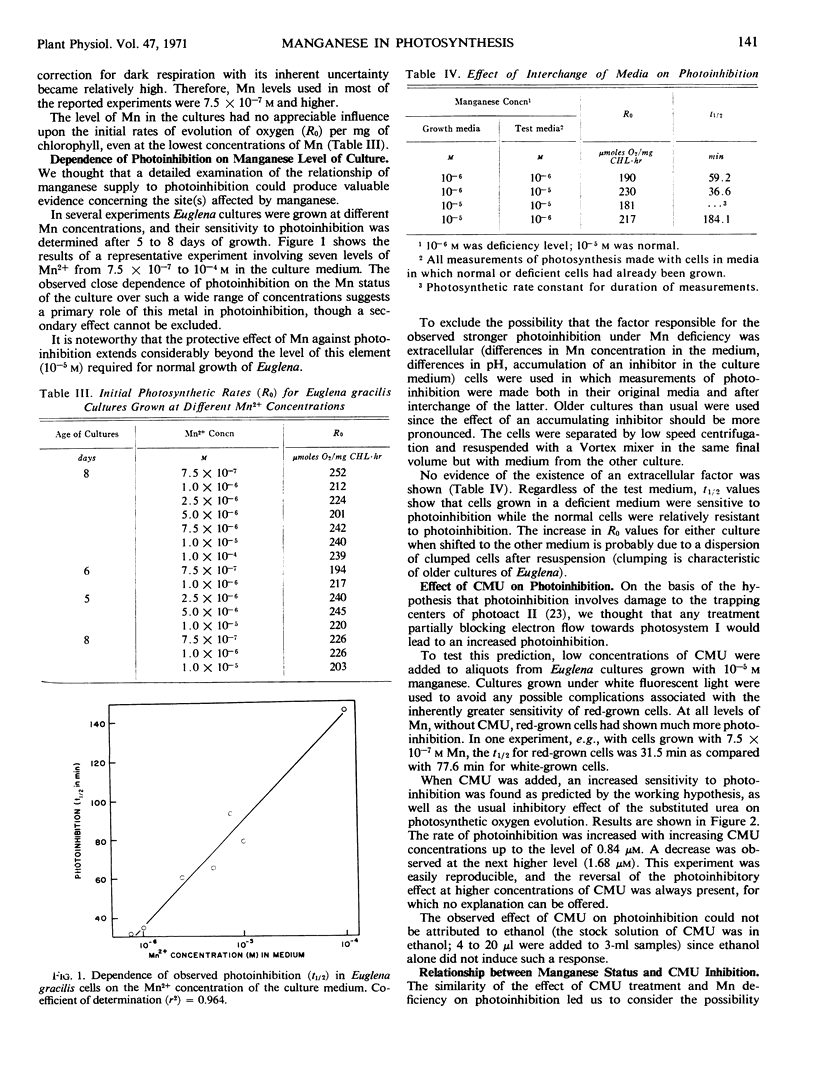
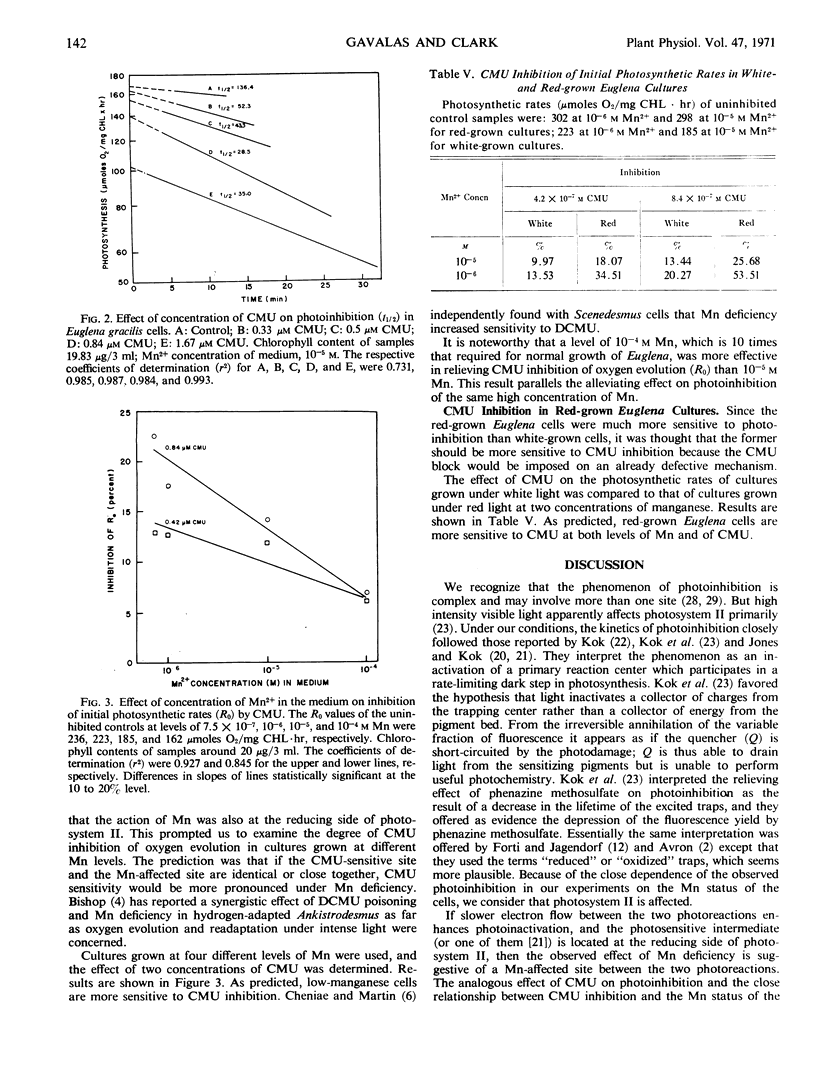
Euglena gracilis (Klebs) cultures were grown under conditions where limitation in supply of manganese limited chlorophyll content much more than growth. Although the initial rates of photosynthetic oxygen evolution were not affected by the level of manganese, photoinhibition in high intensity light was markedly influenced. All cultures showed first order kinetics for photoinhibition, with the half-time exponentially related to the Mn concentration in the medium. Treatment with 3-(4-chlorophenyl)-1, 1-dimethylurea (CMU) also increased the rate of photoinhibition. Manganese-deficient cells were also more sensitive to CMU inhibition of photosynthesis. The similar effects on photoinhibition of manganese deficiency and of CMU treatment and the protective action of manganese against photoinhibition and CMU poisoning are interpreted to indicate a site of action of manganese on the reducing side of photosystem II, close to the CMU-sensitive site. This manganese-affected site may represent a secondary structural or metabolic consequence of manganese deficiency, not necessarily involved in quantum yields of oxygen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Light inactivation of photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 Oct 21;44:41–48. doi: 10.1016/0006-3002(60)91520-1. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Thorne S. W. The fluorescence properties of manganese-deficient spinach chloroplasts. Biochim Biophys Acta. 1968 Jul 16;162(1):122–134. doi: 10.1016/0005-2728(68)90220-x. [DOI] [PubMed] [Google Scholar]
- BISHOP N. I. The influence of the herbicide, DCMU, on the oxygen-evolving system of photosynthesis. Biochim Biophys Acta. 1958 Jan;27(1):205–206. doi: 10.1016/0006-3002(58)90313-5. [DOI] [PubMed] [Google Scholar]
- Bennoun P., Joliot A. Etude de la photooxydation de l'hydroxylamine par les chloroplastes d'epinards. Biochim Biophys Acta. 1969 Sep 16;189(1):85–94. doi: 10.1016/0005-2728(69)90229-1. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Site of manganese function in photosynthesis. Biochim Biophys Acta. 1968 May 28;153(4):819–837. doi: 10.1016/0005-2728(68)90009-1. [DOI] [PubMed] [Google Scholar]
- Cheniae G. M., Martin I. F. Sites of function of manganese within photosystem II. Roles in O2 evolution and system II. Biochim Biophys Acta. 1970 Mar 3;197(2):219–239. doi: 10.1016/0005-2728(70)90033-2. [DOI] [PubMed] [Google Scholar]
- Constantopoulos G. Lipid metabolism of manganese-deficient algae. I. Effect of manganese deficiency on the greening and the lipid composition of Euglena gracilis Z. Plant Physiol. 1970 Jan;45(1):76–80. doi: 10.1104/pp.45.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyster C., Brown T. E., Tanner H. A., Hood S. L. Manganese Requirement with Respect to Growth, Hill Reaction and Photosynthesis. Plant Physiol. 1958 Jul;33(4):235–241. doi: 10.1104/pp.33.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORTI G., JAGENDORF A. T. Inactivation by light of the phosphorylative activity of chloroplasts. Biochim Biophys Acta. 1960 Oct 21;44:34–40. doi: 10.1016/0006-3002(60)91519-5. [DOI] [PubMed] [Google Scholar]
- Gingras G., Lemasson C. A study of the mode of action of 3-(4-chlorophenyl)-1,1-dimethylurea on photosynthesis. Biochim Biophys Acta. 1965 Sep 27;109(1):67–78. doi: 10.1016/0926-6585(65)90091-9. [DOI] [PubMed] [Google Scholar]
- Heath R. L., Hind G. On the functional site of manganese in photosynthetic oxygen evolution. Biochim Biophys Acta. 1969 Oct 21;189(2):222–233. doi: 10.1016/0005-2728(69)90049-8. [DOI] [PubMed] [Google Scholar]
- Homann P. H. Effects of manganese on the fluorescence of chloroplasts. Biochem Biophys Res Commun. 1968 Oct 24;33(2):229–234. doi: 10.1016/0006-291x(68)90773-0. [DOI] [PubMed] [Google Scholar]
- Ito M., Yamashita K., Nishi T., Konishi K., Shibata K. The site of manganese function in photosynthetic electron transport system. Biochim Biophys Acta. 1969 Aug 5;180(3):509–519. doi: 10.1016/0005-2728(69)90029-2. [DOI] [PubMed] [Google Scholar]
- Joliot A. Actions du chlorométhylurée et de l'hydroxylamine sur la réaction photochimique d'émission d'oxygène (système II) Biochim Biophys Acta. 1966 Nov 8;126(3):587–590. [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of Chloroplast Reactions. II. Multiple Effects. Plant Physiol. 1966 Jun;41(6):1044–1049. doi: 10.1104/pp.41.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiol. 1966 Jun;41(6):1037–1043. doi: 10.1104/pp.41.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOK B. On the inhibition of photosynthesis by intense light. Biochim Biophys Acta. 1956 Aug;21(2):234–244. doi: 10.1016/0006-3002(56)90003-8. [DOI] [PubMed] [Google Scholar]
- Kok B., Gassner E. B., Rurainski H. J. Photoinhibition of chloroplast reactions. Photochem Photobiol. 1966 Mar;4(2):215–227. doi: 10.1111/j.1751-1097.1965.tb05739.x. [DOI] [PubMed] [Google Scholar]
- Price C. A., Vallee B. L. Euglena gracilis, A Test Organism for Study of Zinc. Plant Physiol. 1962 May;37(3):428–433. doi: 10.1104/pp.37.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichler-Zallen D. The effect of manganese on chloroplast structure and photosynthetic ability of Chlamydomonas reinhardi. Plant Physiol. 1969 May;44(5):701–710. doi: 10.1104/pp.44.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]


