Summary
Approximately one-third of the U.S. population has nonalcoholic fatty liver disease (NAFLD), a condition closely associated with insulin resistance and increased risk of liver injury. Dysregulated mitochondrial metabolism is central in these disorders, but the manner and degree of dysregulation are disputed. This study tested whether humans with NAFLD have abnormal in vivo hepatic mitochondrial metabolism. Subjects with low (3.0%) and high (17%) intrahepatic triglyceride (IHTG) were studied using 2H and 13C tracers to evaluate systemic lipolysis, hepatic glucose production, and mitochondrial pathways (TCA cycle, anaplerosis, and ketogenesis). Individuals with NAFLD had 50% higher rates of lipolysis and 30% higher rates of gluconeogenesis. There was a positive correlation between IHTG content and both mitochondrial oxidative and anaplerotic fluxes. These data indicate that mitochondrial oxidative metabolism is ∼2-fold greater in those with NAFLD, providing a potential link between IHTG content, oxidative stress, and liver damage.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is associated with obesity and insulin resistance and affects approximately one-third of the U.S. population (Browning and Horton, 2004; Browning et al., 2004; Clark et al., 2002). Although NAFLD can remain benign, up to 50% of affected individuals will develop an insidious form of liver disease known as nonalcoholic steatohepatitis (NASH) that can progress to cirrhosis and liver failure (Browning et al., 2004; Neuschwander-Tetri and Caldwell, 2003). The pathogenesis and progression of NAFLD involve multiple pathologic states that appear to be associated with elevated liver fat (Day and James, 1998). However, the precise cellular and/or metabolic events that accompany steatosis and threaten liver function remain unclear.
Accumulation of triglyceride (TG) is characteristic of most insulin-resistant tissues (Muoio and Newgard, 2008; Savage et al., 2007). The connection between lipid metabolism and insulin resistance hinges not on TG accumulation, per se, but on the pathologic catabolism of TG and generation of lipid-derived signaling molecules which impair insulin action. This process originates with the impaired (Savage et al., 2007) or incomplete mitochondrial catabolism (Muoio and Newgard, 2008) of intracellular lipid in insulin-resistant skeletal muscle. A similar limitation of mitochondrial fatty acid oxidation in liver (Bickerton et al., 2008) would provide a plausible basis for the development of NAFLD during insulin resistance (Savage et al., 2007). However, NAFLD appears to result predominantly from increased adipose lipolysis, de novo lipogenesis, and perhaps saturated lipoprotein TG export (Choi and Ginsberg, 2011; Donnelly et al., 2005; Fabbrini et al., 2008), with sparse in vivo data to address the role of mitochondrial metabolism in the pathogenesis or progression of NAFLD.
Mitochondrial metabolism is indispensible for liver function (Kucejova et al., 2011). Mitochondrial β-oxidation is upregulated 10-fold to accommodate large differentials in lipid influx and insulin action (McGarry and Foster, 1980) during fasting. Induction of lipid oxidation is required for the endergonic steps of gluconeogenesis and ureagenesis, pathways that are partially localized in liver mitochondria and constitutively upregulated during insulin resistance. Thus, unlike skeletal muscle, the insulin-resistant and fatty liver may activate oxidative metabolism (Iozzo et al., 2010; Sanyal et al., 2001). Chronic activation of mitochondria in the setting of lipid overload could predispose the liver to oxidative stress and cellular damage, events thought to drive the development of NASH (Browning and Horton, 2004). In fact, defects in hepatic mitochondria or the function of oxidative phosphorylation similar to skeletal muscle are typically observed only during NASH or diabetes (Caldwell et al., 2009; Cortez-Pinto et al., 1999; Pérez-Carreras et al., 2003; Szendroedi et al., 2009). Thus, mitochondrial metabolism almost certainly plays a role in hepatic insulin resistance and NAFLD (Wei et al., 2008), but the nature of this role is poorly understood.
The present study examined hepatic mitochondrial pathways in humans with low or high liver fat. Liver fat was quantified by MRS, and subjects were administered 2H and 13C tracers to evaluate gluconeogenesis, hepatic TCA cycle activity, ketogenesis, and systemic lipolysis. The data reveal that hepatic mitochondrial oxidative and anaplerotic TCA cycle activities are elevated in subjects with NAFLD and correlate strongly with liver fat content. We conclude that NAFLD develops in the presence of chronic oxidative metabolism, a potential contributing factor to the progression from benign steatosis to NASH.
Results
Subject Characteristics
Subjects had a wide range of IHTG content by 1H MRS (0%–21%) and were thus separated equally into a low IHTG and a high IHTG group (p < 0.001) (Table 1). The low IHTG group was composed of subjects with IHTG ≤6% (3.0% ± 2.4%; n = 8), and the high IHTG group was composed of subjects with IHTG >6% (17.4% ± 2.8%; n = 8). As expected from a previous population study (Guerrero et al., 2009; Browning et al., 2004), the low IHTG group consisted mostly of African Americans (five out of eight), while the high IHTG group consisted of mostly Hispanics (six out of eight), though the relatively low number of subjects precluded an analysis based on ethnicities. High and low IHTG groups were matched for age, body mass index, and body fat content (Table 1). Plasma chemistries, including fasting blood glucose and HbA1c, were not different between the groups. However, plasma insulin concentration (p = 0.07) tended to be higher, and hepatic insulin sensitivity (p = 0.01), but not whole-body insulin sensitivity (p = 0.17), index was impaired in the high IHTG group (Table 1). These data demonstrate that the primary differences between the low and high IHTG groups were IHTG content and hepatic insulin sensitivity.
Table 1. Clinical and Laboratory Characteristics of Human Subjects.
| Subject Characteristics | Low IHTG (≤ 6%) | High IHTG (>6%) | P Value |
|---|---|---|---|
| Intrahepatic TG (%) | 3.0 ± 2.4 (0.0–6.0) | 17.4 ± 2.8* (12.8–21.3) | <0.001 |
| Hispanic/African American | 3/5 | 6/2 | |
| Age (years) | 44 ± 6 (36–51) | 50 ± 11 (30–67) | 0.210 |
| Body weight (kg) | 105.6 ± 24.8 (83.4–148.6) | 88.4 ± 17.4 (59.2–116.8) | 0.131 |
| Lean body weight (kg) | 58.4 ± 7.0 (52.9–73.5) | 48.9 ± 10.4 (30.5–66.9) | 0.075 |
| Body fat (%) | 39.5 ± 11.6 (22.9–55.7) | 45.4 ± 9.3 (32.5–63.5) | 0.282 |
| BMI (kg/m2) | 36.0 ± 9.0 (25.1–50.6) | 34.8 ± 5.8 (28.1–42.5) | 0.754 |
| AST (U/L) | 42 ± 30 (14–102) | 62 ± 46 (22–121) | 0.329 |
| ALT (U/L) | 50 ± 40 (15–117) | 75 ± 52 (28-166) | 0.306 |
| Plasma glucose (mg/dL) | 92.5 ± 9.2 (75.3–107.5) | 99.5 ± 19.7 (68.4–122.5) | 0.377 |
| HbA1c (%) | 5.9 ± 0.2 (5.6–6.1) | 6.0 ± 0.3 (5.7–6.4) | 0.171 |
| Plasma insulin (mU/L) | 8 ± 5 (4–17) | 14 ± 6 (6–25) | 0.065 |
| Hepatic insulin sensitivity index (102 umol−1 min Kg mU−1 middot;L) | 1.5 ± 0.29 (0.37–2.7) | 0.62 ± 0.35 (0.25–0.83) | 0.011 |
| Insulin sensitivity index (10−4 min−1 per μU/mL) | 3.2 ± 1.6 (1.6–6.3) | 2.1 ± 1.3 (0.9–3.1) | 0.172 |
| Fasting FFA (mmol/L) | 0.58 ±0.17 (0.29–0.86) | 0.69 ± 0.11 (0.56–0.91) | 0.155 |
| Fasting plasma TG (mmol/L) | 1.31 ± 0.73 (0.50–2.79) | 1.22 ± 0.34 (0.71–1.70) | 0.751 |
Values are represented as means ±SD with the range in parentheses. Each mean is an average of n = 8 human subjects. *p < 0.05 between subjects with liver fat ≤6% and liver fat >6%.
Gluconeogenesis Is Elevated in Individuals with High IHTG Content
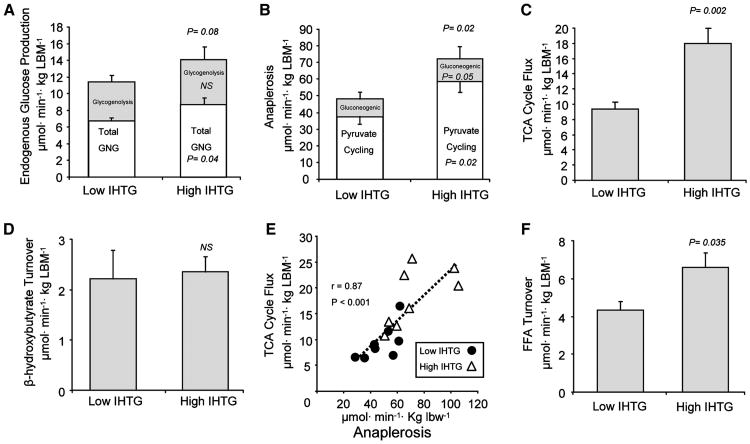
Inasmuch as NAFLD is associated with insulin resistance, we examined endogenous glucose production and gluconeogenesis following a standardized meal and overnight fast. Endogenous glucose production tended to be higher in subjects with high IHTG (p = 0.08; Figure 1A), and gluconeogenic flux was elevated by 25% in subjects with high IHTG (p = 0.05; Figure 1A). However, the contribution of glycogenolysis to endogenous glucose production was similar (p = 0.39) between the groups (Figure 1A). These data indicate that elevated IHTG content is associated with the dysregulation of gluconeogenesis, a finding that is consistent with impaired insulin action in liver.
Figure 1. Glucose and Mitochondrial Fat Metabolism in Human Subjects with Low or High IHTG Content.
2H and 13C isotopomer analysis of plasma glucose and β-hydroxybutyrate by NMR and FFA by GC-MS was used to determine hepatic flux in overnight fasted individuals. Shown are (A) endogenous glucose production and its contributions from gluconeogenesisand glycogenolysis in hexose units, (B) anaplerotic flux as an estimateofpyruvate carboxylase and PEPCK flux and its contribution to pyruvate cycling and gluconeogenesis, (C) hepatic TCA cyclefluxinacetyl-CoA units, (D) apparent β-hydroxybutyrate turnover as an estimate of ketogenesis; (E) correlation between TCA cycle flux and anaplerosis; and (F) FFA turnover as an estimate of systemic lipolysis. Data are presented as means ±SEM (n = 8) with significance declared at p ≤ 0.05 and p ≤ 0.1 considered a trend.
Mitochondrial Anaplerosis Is Increased in Individuals with Elevated IHTG
Anaplerosis is the nonoxidative flux of intermediates into the TCA cycle. In the liver this pathway is dominated by the actions of mitochondrial pyruvate carboxylase (Walter et al., 1966), which catalyzes the conversion of pyruvate to oxaloacetate and, combined with cataplerotic efflux through phosphoenolpyrvate carboxykinase (PEPCK), is the first step of gluconeogenesis. To assess hepatic anaplerosis, subjects were administered an oral dose of [U-13C3]propionate, which is metabolized to glucose after incorporation into the hepatic TCA cycle (Landau et al., 1993). The relative rate of anaplerosis, flux of TCA cycle carbons into gluconeogenesis, and TCA cycle turnover were detected by the 13C spin coupling patterns in the 13C NMR spectrum of plasma glucose (Browning et al., 2008; Jones et al., 2001). Hepatic anaplerotic flux was increased by 50% in subjects with high IHTG (p = 0.015; Figure 1B), indicating increased flux through the combined pathways of mitochondrial PC and PEPCK, and is consistent with the increased rate of gluconeogenesis observed in subjects with high IHTG. Pyruvate cycling, which is a futile cycle linking anaplerotic flux to the regeneration of pyruvate through pyruvate kinase or malic enzyme, was also elevated by 55% in subjects with high IHTG (p = 0.015; Figure 1B). Together, these data indicate that the anabolic pathways of the mitochondrial metabolism are elevated in individuals with high IHTG.
Mitochondrial Oxidative Metabolism Is Increased in Liver of Subjects with Elevated IHTG
Lipid oxidation is the principal source of energy generation in liver, and its dysregulation represents a potential metabolic link between NAFLD, mediators of insulin resistance, and hepatocellular damage. Subjects with NAFLD had an ∼2-fold induction in oxidative flux through TCA cycle (p = 0.002; Figure 1C). Liver TG content was positively correlated with hepatic TCA cycle flux (r = 0.71; p = 0.002), indicating that oxidative metabolism in the mitochondrial TCA cycle is not impaired even at very high IHTG levels in humans. However, ketone production assessed by tracer dilution of β-hydroxybutyrate was not different between subjects with low and high IHTG (Figure 1D). These data indicate that excess acetyl-CoA was selectively partitioned to oxidation in the TCA cycle rather than ketogenesis, perhaps due to increased energy requirements of the fatty liver. There was a strong correlation between TCA cycle flux and PC/PEPCK (anaplerosis and cataplerosis) (r = 0.87; p < 0.001; Figure 1E), consistent with the induction of gluconeogenesis by fat oxidation (Chen et al., 1999; Roden et al., 2000; Staehr et al., 2003), and also suggesting that part of the increased energy demand/production of the fatty liver is associated with increased gluconeogenesis.
FFA Delivery Is Increased in NAFLD
Since dysregulated fatty acid homeostasis contributes to the pathophysiology of insulin-resistant liver, we evaluated systemic free fatty acid (FFA) metabolism. Plasma FFA concentration was insignificantly increased (Table 1), but systemic lipolysis assessed by 13C FFA turnover (RaFFA) was 2-fold higher in subjects with elevated IHTG content compared to subjects with low IHTG (p = 0.035; Figure 1F). RaFFA was also closely associated with β-hydroxybutyrate turnover (r = 0.58; p = 0.039). These findings agree with earlier reports (Fabbrini et al., 2008) and are consistent with a role for increased FFA delivery in the induction of hepatic mitochondrial oxidative metabolism during NAFLD.
Discussion
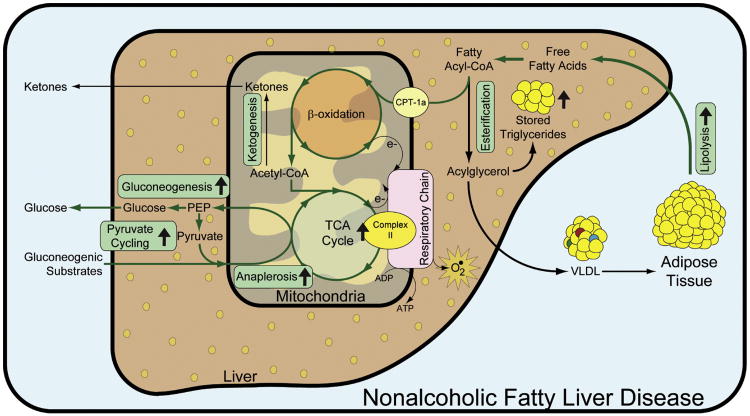
Hepatic steatosis occurs because of an imbalance between lipid accrual (uptake, synthesis) and disposal (secretion, oxidation). The contributions of dietary lipid intake, lipogenesis, lipolysis, and lipoprotein secretion to the development of NAFLD have all been studied in humans (Choi and Ginsberg, 2011; Diraison et al., 2003; Donnelly et al., 2005; Fabbrini et al., 2008). Mitochondrial metabolism occupies the nexus connecting insulin resistance, steatosis, oxidative stress, and the dysregulation of metabolic pathways like gluconeogenesis (Muoio and Newgard, 2008; Savage et al., 2007), but its role in the pathogenesis of NAFLD has not been systematically evaluated in humans. We report that the pathophysiology of NAFLD is marked by increased hepatic mitochondrial metabolism, as illustrated in Figure 2. This conclusion is supported by a 2-fold increase in hepatic TCA cycle flux in subjects with elevated IHTG content and a strong correlation between TCA cycle flux and IHTG among all subjects. Mitochondrial anaplerosis (predominantly pyruvate carboxylase flux [Walter et al., 1966]) was also increased by roughly 50%, contributing to elevated rates of gluconeogenesis and partially accounting for the attendant increase in hepatic energy demand in people with high IHTG. We conclude that hepatic steatosis develops in the presence of elevated mitochondrial oxidative and anabolic metabolism in the liver.
Figure 2. Hepatic Mitochondrial Metabolism Is Increased in Subjects with Nonalcoholic Fatty Liver Disease.
Subjects with increased IHTG had elevated adipose lipolysis which contributed to increased lipid delivery to liver. Hepatic TCA cycle flux was increased, indicating upregulated mitochondrial respiration (at least via complex II) and suggesting increased flux of acetyl-CoA from β-oxidation. Mitochondrial anaplerosis was also increased and provided substrate for the increased rate of gluconeogenesis observed in subjects with high IHTG. Pathways that are increased during high IHTG are designated by ↑.
Two principal hypotheses prevail regarding the function of mitochondrial metabolism during insulin resistance and NAFLD. First is the concept that impaired mitochondrial capacity results in the pathologic formation of lipid metabolites which deactivate the insulin signaling cascade through a PKC-dependent mechanism (Samuel et al., 2004; Savage et al., 2007). This hypothesis is compelling, because it addresses both the underlying etiology of insulin resistance and the concurrent intracellular lipid accumulation. Mitochondrial dysfunction has been reported in insulin-resistant skeletal muscle as morphologic defects, decreased mitochondrial content, respiration, ATP synthesis, and TCA cycle turnover (Kelley et al., 2002; Petersen et al., 2004; Sparks et al., 2005). In liver, several reports indicate defects in in vivo ATP synthesis in individuals with NASH (Cortez-Pinto et al., 1999) or diabetes (Szendroedi et al., 2009), but the majority of evidence is indirect. Hepatic lipid overload by short-term high-fat feeding (Samuel et al., 2004), lipid infusion (Lam et al., 2002), or inhibition of fat oxidation (Zhang et al., 2007) results in hepatic insulin resistance, while increasing mitochondrial fat oxidation is sufficient to suppress liver PKC (Samuel et al., 2004) and improve insulin sensitivity. Certainly the data in the present study support increased hepatic lipid burden, as indicated by elevated lipolysis, but surprisingly, the data revealed no indication of a mitochondrial insufficiency.
A second hypothesis holds that chronic lipid overload in the liver of individuals with NAFLD induces mitochondrial oxidation, resulting in oxidative stress and eventual damage to cellular components including mitochondria, ushering in inflammation, cell death, and the progression from benign steatosis to NASH (Browning and Horton, 2004; Day and James, 1998; Diehl et al., 2005; Pessayre and Fromenty, 2005). Indeed, individuals with NASH have elevated circulating ketones (Sanyal et al., 2001), and obese humans have a 2-fold increase in hepatic 11C-palmitate oxidation by positron emission tomography (Iozzo et al., 2010). We found that increased lipolytic rates were associated with elevated β-hydroxybutyrate turnover, although no differences were detected between the low and high IHTG groups. This might be related to the fact that we were unable to determine acetoacetate contribution to ketone turnover because of unexpectedly low enrichments and concentrations. Nonetheless, elevated hepatic TCA cycleflux demonstrated that mitochondrial oxidative activity is increased in people with high liver fat. Although histology was not characterized in these subjects, elevated oxidative metabolism may be an important contributor to the oxidative stress that attends the progression to NASH.
Inasmuch as mitochondrial TCA cycle activity is closely governed by energy demand, we interpret this finding to indicate increased energy demand during NAFLD. This interpretation is distinct from lipid overload, per se, because it implies that the downstream pathways requiring oxidative metabolism are elevated in addition to the oversupply of oxidative substrate (lipid). Congruent with this interpretation is an increase in mitochondrial anaplerosis with IHTG content (r = 0.53; p = 0.035) leading to a 30% higher rate of gluconeogenesis in individuals with elevated IHTG content. In this respect, lipid overload induces constitutive activation of mitochondrial activity and gluconeogenesis, a pathway with substantial endergonic requirements and a phenomenon that is well known in humans (Chen et al., 1999; Roden et al., 2000; Staehr et al., 2003). Nonetheless, elevated gluconeogenesis is unlikely to be sufficient to account for the 2-fold higher TCA cycle activity in individuals with NAFLD, suggesting that other endergonic processes may also be involved. In addition to activation of specific intermediary pathways during insulin resistance, ongoing liver damage during NAFLD and concomitant hepatocellular regeneration may be sufficient to increase the energy requirements of the liver, as occurs in other forms of liver damage (Schofield et al., 1987).
Alternatively, mitochondria may simply be less efficient during NAFLD, a possibility supported by mitochondrial damage (Caldwell et al., 2009; Pessayre and Fromenty, 2005) and uncoupling (Serviddio et al., 2008) in the liver of NASH patients. The cause of mitochondrial damage has been proposed to be the result of constitutive overactivation of oxidative metabolism during NAFLD, a view substantiated by the current findings. The oxidative stress associated with elevated hydride production in the TCA cycle may be sufficient to damage the electron transport chain during chronic steatosis, a condition functionally manifest by impaired ATP synthesis in people with NASH (Cortez-Pinto et al., 1999) or diabetes (Szendroedi et al., 2009). This would result in a degenerative spiral whereby damaged electron transport chain in turn requires elevated TCA cycle activity to produce sufficient reducing equivalents for normal ATP and cellular homeostasis.
In summary, the data provide direct evidence of elevated in vivo hepatic mitochondrial metabolism in human subjects with NAFLD. The strong association between IHTG content and hepatic oxidative and anaplerotic TCA cycle activity demonstrates the induction of mitochondrial fat metabolism in response to lipid overload during NAFLD. Simultaneous induction of pathways of lipid accretion, oxidation, and gluconeogenesis heralds oxidative stress, loss of glycemic control, and potential damage to the liver during chronic hepatic steatosis.
Experimental Procedures
Human Subjects
Sixteen subjects were recruited from health fairs and physician referrals. The inclusion criteria were African American or Hispanic subjects overweight or obese with elevated liver enzymes (ALT >30, AST >30) and/or characteristics of the metabolic syndrome (Grundy et al., 2004), including elevated waist circumference (>102 cm men, 88 cm in women), fasting glucose (≥110 mg/dL), blood pressure (>130/>85), elevated TG (≥150 mg/dL, low HDLc (<40 mg/dL for men, <50 for women). Subjects were nondiabetic (fasting glucose <125 mg/dl and/or HbA1c <6.5), aged 20–67 years, with stable body weight and maintenance of preenrollment physical activity. The intensive nature of the tracer studies precluded larger population studies. To ensure a wide range of IHTG content, Hispanics (n = 9; 2 males and 7 females) and African Americans (n =7; 2 males and 5 females) were recruited because these two ethnicities have disparate predispositions for NAFLD (Browning et al., 2004). Each subject participated in two metabolic tests. The first admission included an assessment of insulin sensitivity using an insulin-modified intravenous glucose tolerance test (IVGTT) (Boston et al., 2003) and stable isotope administration to profile hepatic glucose and mitochondrial metabolism by NMR (Browning et al., 2008; Jones et al., 2001). During the second admission, liver TG content was assessed by 1H MRS and whole-body fatty acid flux via stable isotope infusion and GC/MS (Barrows and Parks, 2006). All subjects gave written informed consent prior to inclusion in this study, which was approved by the Institutional Research Board (IRB) at UT Southwestern Medical Center (approval #062007-025).
Study Design and Procedures
Before admission to the Clinical and Translational Research Center (CTRC), subjects consumed weight-maintaining diets formulated by comparison to the previous 3 day dietary record. This diet was prepared by the CTRC metabolic kitchen and delivered to the subjects for consumption on an outpatient basis for 3 days before admission #1 and for 7 days before admission #2. Once admitted to the CTRC, identical evening meals were provided the evening before admissions #1 and #2. The energy content of the meal represented 40% of the subject's daily energy needs, and the composition of these meals averaged (mean ±SD) 790.4 ± 118.7 kcal, 15.8 ± 8.2 g protein, 90.1 ± 22.7 g carbohydrate, 4.22 ± 2.11 g fiber, and 83.3 ± 11.9 mg cholesterol.
Admission #1: Measurements of Insulin Sensitivity and Hepatic Metabolism
As shown in Figure S1, available with this article online, admission #1 included an overnight CTRC stay with the IVGTT, DEXA (Hologic DiscoveryW, QDR series), and administration of oral [U-13C]propionate and deuterated water and intravenous infusion of [3,4-13C2]glucose, [1,2-13C2]β-hydroxybutyrate, [3,4-13C2]acetoacetate stable isotopes to profile hepatic glucose and mitochondrial metabolism as previously reported (Browning et al., 2008; Satapati et al., 2008) and detailed in the Supplemental Information. The IVGTT was performed, and glucose and insulin responses were analyzed using the minimal model technique and the MINMOD Millenium software (Boston et al., 2003). Hepatic insulin sensitivity index was calculated as 100/(EGP × fasting insulin) (DeFronzo, 1988).
Admission #2: Measurement of IHTG and Fatty Acid Turnover
Within 2 weeks of admission #1, the subject underwent a 1H-MRS to assess IHTG as previously described and detailed in the Supplemental Information. Immediately following 1H-MRS, subjects were admitted to the CTRC for measurement of the rate of appearance of FFA (RaFFA). After consumption of the evening meal, a constant infusion (0000–1200 the next day) of [13C4]palmitate (7 μg/kg/min bound to albumin) was performed as described previously (Barrows and Parks, 2006) and detailed in the Supplemental Information.
Metabolic Flux in the Liver
Ketones were isolated from 10 ml of plasma, and apparent ketone turnover was assessed from their 13C tracer enrichment indicated by 13C NMR as we previously described (Satapati et al., 2008) and detailed in the Supplemental Information. Deuterium enrichment of glucose was used to determine fractional gluconeogenesis as originally described by Landau (Landau et al., 1995) and adapted for 2H NMR analysis (Jones et al., 2001). Carbon-13 isotopomers of glucose imparted by the oral [U-13C]propionate (Landau et al., 1993) were evaluated by 13C NMR spectroscopy (Browning et al., 2008; Jones et al., 2001) and used to determine fractional TCA cycle and anaplerotic fluxes (Magnusson et al., 1991) as detailed in the Supplemental Information. Fractional fluxes were converted to absolute fluxes by normalization with endogenous glucose production (EGP) determined by [3,4-13C2]glucose turnover (Browning et al., 2008; Jones et al., 2001).
Metabolites and Hormone Measurements
Blood samples were collected in tubes containing sodium fluoride for plasma glucose and tubes containing EDTA for plasma insulin and FFA. Plasma from the EDTA tubes was separated immediately by centrifugation at 2,850 RPM for 10 min at 5°C and stored at −20°C. For measurement of plasma metabolite concentrations, enzymatic kits were used for glucose (WAKO, #439-90901) and FFA (WAKO, #999-34691, #991-34891); insulin was measured by ELISA (Millipore, Billerica, MA, #EZHI-14L).
Reagents and Materials
[3,4-13C2]glucose (98%) was purchased from Omicron Biochemicals (South Bend, IN). [1,2- 13C]sodium β-hydroxybutyrate (98%), [3,4-13C2]acetoacetate, and [13C4]palmitate were purchased from Isotec (St. Louis, MO); [U-13C] propionate and deuterium oxide (99%) were purchased from Cambridge Isotopes (Andover, MA). Other common chemicals were obtained from Sigma (St. Louis, MO).
Statistical Analysis
Differences between groups were determined using unpaired, two-tailed t tests. Data are presented as means ±SD. Spearman correlation analysis was used to determine the correlation between two variables of interest. Statistical significance was taken as p ≤ 0.05, and a p ≤ 0.1 was reported as a trend.
Supplementary Material
Acknowledgments
This research was supported by the Clinical and Translational Science Award (CTSA) at UT Southwestern (UL1RR024982), the Task Force for Obesity Research (TORS) at UT Southwestern (UL1DE019584), the TORS Research Training Grant (RL9DK081180), the TORS Human Biology Core (PL1DK081183), and the TORS Molecular and Metabolic Phenotyping Core (PL1DK081182). Individual investigators were supported as follows: 5RL1DK081187 (E.J.P.), K23DK074396 and R01DK087977 (J.D.B.), and R01DK078184, P01DK058398, and ADA7-09-BS-24 (S.C.B.). Special thanks to Dora Bradford, RN-C, WHCNP, and Jeannie Davis Baxter, RN, CCRC, for clinical trial coordination; to Maressa Valdez, RD, Maria Ramos-Roman, MD, M.Sc, Sonya Rios, RN, and Carol Parcel, RN, for subject recruitment, clinical data collection, and care; and to Janet Jerrow PhD, Joe Lee, BS, and Yelena Hovhannisyan, MS, for data generation and management.
Footnotes
Supplemental Information: Supplemental Information includes Supplemental Experimental Procedures, Figure S1, and Supplemental References and can be found with this article online at doi:10.1016/j.cmet.2011.11.004.
References
- Barrows BR, Parks EJ. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab. 2006;91:1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- Bickerton A, Roberts R, Fielding B, Tornqvist H, Blaak E, Wagenmakers A, Gilbert M, Humphreys S, Karpe F, Frayn K. Adipose tissue fatty acid metabolism in insulin-resistant men. Diabetologia. 2008;51:1466–1474. doi: 10.1007/s00125-008-1040-x. [DOI] [PubMed] [Google Scholar]
- Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Browning JD, Weis B, Davis J, Satapati S, Merritt M, Malloy CR, Burgess SC. Alterations in hepatic glucose and energy metabolism as a result of calorie and carbohydrate restriction. Hepatology. 2008;48:1487–1496. doi: 10.1002/hep.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell SH, de Freitas LAR, Park SH, Moreno MLV, Redick JA, Davis CA, Sisson BJ, Patrie JT, Cotrim H, Argo CK, et al. Intramitochondrial crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2009;49:1888–1895. doi: 10.1002/hep.22851. [DOI] [PubMed] [Google Scholar]
- Chen X, Iqbal N, Boden G. The effects of free fatty acids on gluconeogenesis and glycogenolysis in normal subjects. J Clin Invest. 1999;103:365–372. doi: 10.1172/JCI5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab. 2011;22:353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122:1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis. JAMA. 1999;282:1659–1664. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303–306. doi: 10.1136/gut.2003.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma nonesterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49:791–801. doi: 10.1002/hep.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo P, Bucci M, Roivainen A, Nagren K, Jarvisalo MJ, Kiss J, Guiducci L, Fielding B, Naum AG, Borra R, et al. Fatty acid metabolism in the liver, measured by positron emission tomography, is increased in obese individuals. Gastroenterology. 2010;139:846–856. doi: 10.1053/j.gastro.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Jones JG, Solomon MA, Cole SM, Sherry AD, Malloy CR. An integrated (2)H and (13)C NMR study of gluconeogenesis and TCA cycle flux in humans. Am J Physiol Endocrinol Metab. 2001;281:E848–E856. doi: 10.1152/ajpendo.2001.281.4.E848. [DOI] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Kucejova B, Sunny NE, Nguyen AD, Hallac R, Fu X, Pena-Llopis S, Mason RP, Deberardinis RJ, Xie XJ, Debose-Boyd R, et al. Uncoupling hypoxia signaling from oxygen sensing in the liver results in hypoketotic hypoglycemic death. Oncogene. 2011;30:2147–2160. doi: 10.1038/onc.2010.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TKT, Yoshii H, Haber CA, Bogdanovic E, Lam L, Fantus IG, Giacca A. Free fatty acid-induced hepatic insulin resistance: a potential role for protein kinase C-delta. Am J Physiol Endocrinol Metab. 2002;283:E682–E691. doi: 10.1152/ajpendo.00038.2002. [DOI] [PubMed] [Google Scholar]
- Landau BR, Schumann WC, Chandramouli V, Magnusson I, Kumaran K, Wahren J. 14C-labeled propionate metabolism in vivo and estimates of hepatic gluconeogenesis relative to Krebs cycle flux. Am J Physiol Endocrinol Metab. 1993;265:E636–E647. doi: 10.1152/ajpendo.1993.265.4.E636. [DOI] [PubMed] [Google Scholar]
- Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Use of 2H2O for estimating rates of gluconeogenesis. Application to the fasted state. J Clin Invest. 1995;95:172–178. doi: 10.1172/JCI117635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson I, Schumann WC, Bartsch GE, Chandramouli V, Kumaran K, Wahren J, Landau BR. Noninvasive tracing of Krebs cycle metabolism in liver. J Biol Chem. 1991;266:6975–6984. [PubMed] [Google Scholar]
- McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB. Molecular and metabolic mechanisms of insulin resistance and [beta]-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- Pérez-Carreras M, Hoyo PD, Martín MA, Rubio JC, Martín A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005;42:928–940. doi: 10.1016/j.jhep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden M, Stingl H, Chandramouli V, Schumann W, Hofer A, Landau B, Nowotny P, Waldhausl W, Shulman G. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes. 2000;49:701–707. doi: 10.2337/diabetes.49.5.701. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in nonalcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- Satapati S, He T, Inagaki T, Potthoff M, Merritt ME, Esser V, Mangelsdorf DJ, Kliewer SA, Browning JD, Burgess SC. Partial resistance to peroxisome proliferator-activated receptor-alpha agonists in ZDF rats is associated with defective hepatic mitochondrial metabolism. Diabetes. 2008;57:2012–2021. doi: 10.2337/db08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PS, Sugden MC, Corstorphine CG, Zammit VA. Altered interactions between lipogenesis and fatty acid oxidation in regenerating rat liver. Biochem J. 1987;241:469–474. doi: 10.1042/bj2410469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serviddio G, Bellanti F, Tamborra R, Rollo T, Capitanio N, Romano AD, Sastre J, Vendemiale G, Altomare E. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut. 2008;57:957–965. doi: 10.1136/gut.2007.147496. [DOI] [PubMed] [Google Scholar]
- Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- Staehr P, Hother-Nielsen O, Landau BR, Chandramouli V, Holst JJ, Beck-Nielsen H. Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis. Diabetes. 2003;52:260–267. doi: 10.2337/diabetes.52.2.260. [DOI] [PubMed] [Google Scholar]
- Szendroedi J, Chmelik M, Schmid AI, Nowotny P, Brehm A, Krssak M, Moser E, Roden M. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology. 2009;50:1079–1086. doi: 10.1002/hep.23093. [DOI] [PubMed] [Google Scholar]
- Walter P, Paetkau V, Lardy HA. Paths of carbon in gluconeogenesis and lipogenesis. J Biol Chem. 1966;241:2523–2532. [PubMed] [Google Scholar]
- Wei Y, Rector RS, Thyfault JP, Ibdah JA. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol. 2008;14:193–199. doi: 10.3748/wjg.14.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA. 2007;104:17075–17080. doi: 10.1073/pnas.0707060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.