Abstract
In this issue of Immunity, Hong et al. (2012) report the first structural analysis of the C-terminal fragment of an NLR [nucleotide-binding domain (NBD) andleucine-rich repeat (LRR) containing] protein, NLRX1. This fragment forms a hexamer and binds RNA.
The NLRs (nucleotide-binding domain (NBD) and leucine-rich repeat (LRR) containing proteins) are a large protein family that plays important roles in a wide-range of biological processes,a prime function of whichis the detection of pathogen associated molecular patterns (PAMPs). NLRX1 is the first NLR protein shown to be localized at the mitochondria. It has been associated with multiple functions, including negatively impacting anti-viral inflammatory response via the MAVS-RIG-Isensing pathway(Moore et al., 2008; Xia et al., 2011), or lipopolisaccharide-elicited response via the TRAF6-IKK signaling pathway(Allen et al., 2011; Xia et al., 2011), and positively controlling NF-κB and JNKsignaling pathwayto activatereactive oxygen species (ROS) production in response to TNF-α, poly(I:C) and pathogens(Abdul-Sater et al., 2010; Tattoli et al., 2008). Gene deletion of exons 4 and 5 within the Nlrx1gene resulted in elevated type I interferonand IL-6 response to viral infection incells and whole animals, and a modest reduction of basal ROS in innate immune cells(Allen et al., 2011). A different gene targeted strain showed reduced ROS production to Chlamydia, although the deleted genetic sequence was not described(Abdul-Sater et al., 2010).A third group studied a strain that has a deletion of the first four exons of Nlrx1(Rebsamen et al., 2011). These authors did not detect an effect of this deletion on either IFN expression or TNF-α amplification of NF-κB and JNK signaling. In addition to possibly mediating different functions, the precise site of cellular localization varies in different reports. Althoughone group has found endogenous NLRX1 localization as a mitochondrial outer membrane (MOM) protein(Moore et al., 2008), another group has shown its localization in the mitochondrial matrix(Arnoult et al., 2009). Different serologic reagentswere used in these localization studies, which might impact the outcome. Additionally,one has to entertain that these varied localizationsmay be relevant to the multiple functions NLRX1 reported to date.
Hong et al. (2012) in this issue of Immunitypresents the first crystallographic analysis of the C-terminal fragment of NLRX1 that includes two α helical domains flanking a middle LRR section. Several important and surprising features of the NLRX1 are revealed in this study. A key finding is that the NLRX1 LRR and its associated helical domains form a hexameric platform composed of a trimer of three dimers, through extensive intramolecular and intermolecular interactions (Figure 1, insert). These higher order oligomers may promote cooperative association between NLRX1 and its target proteins, thus ensure stringent regulation of innate immune responses. Althoughthe NLR nucleotide-binding domain (NBD) is typically considered an oligomerization motif, an early report that first described oligomerization of the founding NLR protein, CIITA, revealed that both NBD and LRR domains undergo homotypic (NBD-NBD, LRR-LRR) and heterotypic association (NBD-LRR) (Linhoff et al., 2001). In agreement with this, Hong et al. shows that LRR-LRR interaction occurs through theC-terminal α-helical domain (referred to as LRRCT by these authors) mediated intra- and intermolecular interactions at the dimer interface, and the N-terminal α-helical domain (referred to as LRRNT) mediated intermolecular interactions at the trimer interface. Whether the NLRX1 NBD domain promotes further oligomerization of NLRX1 is unknown. Interestingly, the NLRX1 NBD has a classic Walker A motif that binds ATPbut a poor Walker B motif. It is possible that an uncommon ATP binding motif endows different oligomerization function for the NLRX1 NBD.
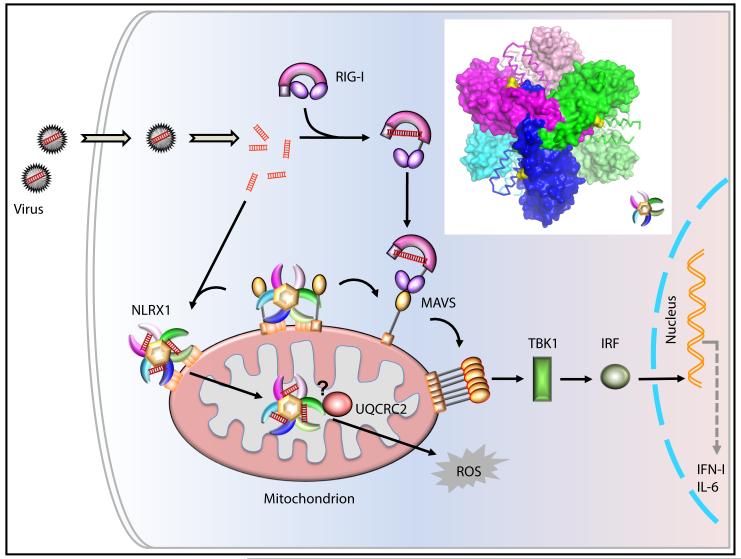
Figure 1. A Working Model of NLRX1 Function.
The C-terminal fragment of NLRX1 is comprised of the LRR flanked by N- and C-terminal α-helices to form a hexamer, with the trimericleucine-rich repeats resembling a tri-blade boomerang (magenta, green and blue as one trimer and pink, lime, cyan as the other; see insert). The identified RNA binding sites are marked in yellow. This oligomerization platform may promote cooperative association between NLRX1 and its downstream target. In this model, NLRX1 constitutively binds to MAVS to inhibit its function (Moore et al., 2008; Allen, et al., 2011; Xia et al., 2011), while RNA binding to NLRX1 releases MAVS. RNA-bound NLRX1then activates ROS. The model proposes a possible NLRX1 translocation from MOM to the matrix via an unknown modification or mechanism, which allows interaction with potential downstream target to activate ROS. The functional connection of NLRX1 and UQCRC2 is proposed and not experimentally demonstrated.
A second finding in this paper relates to how NLRs detect PAMPs, a central unresolved issue in the field. One of the most-studied NLRs is NLRP3, which mediates inflammasomeresponse to numerous PAMPs and DAMPs (damage-associated molecular patterns). An emerging consensus is that NLRP3 is unlikely to directly bind to such a wide variety of molecular structures. Rather it might“sense”through indirect detectionof PAMPs and DAMPs by an intermediate protein(s) that remains elusive. A recent large-scale analysis of plant NLRs has been illuminating regarding the intersection of NLRs and PAMPs. This work shows that only 2 of 30 NLRs directly interact with PAMPs (pathogen effectors)(Mukhtar et al., 2011). By contrast, nearly half of the host cell proteins that interact with NLRs are direct targets of pathogen effectors. These findings led to the conclusion that a majorityof pathogen effectors interact with host proteins that in turn activate plant NLRs, while a minority directly associate with NLRs. Support for the latter is emerging in mammalian cellsalthough direct binding between NLRs and their respective ligands have yet to be demonstrated in a purified recombinant system.
Hong et al., used a purified recombinant C-terminal fragment of NLRX1 to demonstrate its binding to ssRNA, dsRNA and poly(I:C), but not DNA. Based on the X-ray crystallographic analysis, they identified a large continuous positively-charged surface patch that might represent the RNA binding face. A single-residue mutation within this patch greatly reduced RNA binding by purified NLRX1 in a gel-shift assay. This mutant protein failed to induce ROS upon stimulation with poly(I:C).However, further characterization of NLRX1 co-localization with RNA within cells are important for physiological verification, and co-crystallization of NLRX1 with RNA would be a strong confirmation of this interaction. Finally exactly where NLRX1 binds RNA within the cell is important. As mentioned earlier, NLRX1 has been localized to the MOM and mitochondria matrix in separate studies. Specifically, NLRX1 interacts with a mitochondrial matrix protein, UQCRC2, which is a component of the respiratory chain complex III, thus providing a possible basis for ROS induction by NLRX1 (Arnoult et al., 2009; Rebsamen et al., 2011) (Figure 1). The current work used ROS induction upon poly(I:C) stimulation as a readout. While it is conceivable that viral RNA bind to NLRX1 at the MOM, it is more difficult to envision how NLRX1 can access viral RNA from the mitochondria matrix. However as revealed by this work, there is much that we do not understand about this fascinating protein, and only additional studies can clarify these issues.
Exactly how RNA binding elicits the biological function of NLRX1 is unclear.Does RNA-binding to NLRX1 influence NLRX1-UQCRC2 association to affect ROS production? One report showed that endogenous NLRX1 constitutively interacts with MAVS(Moore et al., 2008)Does RNA induce conformational changes or oligomerization states of NLRX1 so that NLRX1 releases MAVS to allow for RIG-I signaling? The hexameric full-length NLRX1 model shows close proximity of its RNA binding surface with the modeled NBD domains. It is conceivable that RNA-binding at LRR and MAVS binding at NBD are mutually exclusive, thus providing a mechanism to release the repression of the RIG-I pathway upon RNA exposure to both RIG-I and NLRX1. The above working models can now be tested with the information provided by Hong et al.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Sater AA, Said-Sadier N, Lam VM, Singh B, Pettengill MA, Soares F, Tattoli I, Lipinski S, Girardin SE, Rosenstiel P, Ojcius DM. Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1. The Journal of biological chemistry. 2010;285:41637–41645. doi: 10.1074/jbc.M110.137885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, Gris D, Roney KE, Zimmermann AG, Bowzard JB, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult D, Soares F, Tattoli I, Castanier C, Philpott DJ, Girardin SE. An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. J Cell Sci. 2009;122:3161–3168. doi: 10.1242/jcs.051193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, et al. This issue. Immunity. 2012 [Google Scholar]
- Linhoff MW, Harton JA, Cressman DE, Martin BK, Ting JP. Two distinct domains within CIITA mediate self-association: involvement of the GTP-binding and leucine-rich repeat domains. Mol Cell Biol. 2001;21:3001–3011. doi: 10.1128/MCB.21.9.3001-3011.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- Mukhtar MS, Carvunis AR, Dreze M, Epple P, Steinbrenner J, Moore J, Tasan M, Galli M, Hao T, Nishimura MT, et al. Independently evolved virulence effectors converge onto hubs in a plant immune system network. Science. 2011;333:596–601. doi: 10.1126/science.1203659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebsamen M, Vazquez J, Tardivel A, Guarda G, Curran J, Tschopp J. NLRX1/NOD5 deficiency does not affect MAVS signalling. Cell Death Differ. 2011;18:1387. doi: 10.1038/cdd.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattoli I, Carneiro LA, Jehanno M, Magalhaes JG, Shu Y, Philpott DJ, Arnoult D, Girardin SE. NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO Rep. 2008;9:293–300. doi: 10.1038/sj.embor.7401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Cui J, Wang HY, Zhu L, Matsueda S, Wang Q, Yang X, Hong J, Songyang Z, Chen ZJ, Wang RF. NLRX1 negatively regulates TLR-induced NF-kappaB signaling by targeting TRAF6 and IKK. Immunity. 2011;34:843–853. doi: 10.1016/j.immuni.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]