Abstract
Alzheimer’s is a neurodegenerative disease with a complex and progressive pathological phenotype characterized first by hypometabolism and impaired mitochondrial bioenergetics followed by pathological burden. Increasing evidence indicates an antecedent and potentially causal role of mitochondrial bioenergetic deficits and brain hypometabolism coupled with increased mitochondrial oxidative stress in AD pathogenesis. Compromised mitochondrial bioenergetics lead to over-production and mitochondrial accumulation of β-amyloid coupled with oxidative stress to cause a shift in brain metabolic profile from glucose-driven bioenergetics towards a compensatory, but less efficient, ketogenic pathway. We propose that the compensatory shift from a primarily aerobic glycolysis pathway to a ketogenic/fatty acid β-oxidation pathway eventually leads to white matter degeneration. The essential role of mitochondrial bioenergetics and the unique trajectory of compensatory metabolic adaptations in brain enable a bioenergetic-centric strategy for development of biomarkers to detect the bioenergetic shift in brain to enable early identification of people at risk for developing AD. From a therapeutic perspective, this unique trajectory of alterations in brain metabolic capacity enable disease-stage specific strategies targeting brain metabolism for disease prevention and treatment. A combination of nutraceutical and pharmaceutical intervention that enhances glucose-driven metabolic activity and potentiates mitochondrial bioenergetic function could prevent the antecedent decline in brain glucose metabolism, promote healthy aging and prevent AD. Alternatively, during the prodromal incipient phase of AD, sustained activation of ketogenic metabolic pathways coupled with supplement of the alternative fuel source, ketone bodies, can sustain mitochondrial bioenergetic function to prevent or delay further progression of the disease.
Keywords: Mitochondria, Bioenergetics, Alzheimer’s disease, oxidative stress, white matter degeneration, biomarkers
1. Alzheimer’s Disease – Unlimited Cost but Limited Remedy
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disease and the leading cause of dementia among the aged population. It is estimated that 5.4 million people are currently living with AD in the United States, and this number is projected to at least double by the year 2050 (Association, 2011). Additionally, the prevalence of AD increases exponentially with age in people 65 or older (Hansson et al., 2006). The disease is symptomatically characterized by progressive memory deficits, cognitive impairments, and personality changes, which can be attributed to deteriorating synaptic function and the subsequent loss of neurons in vulnerable regions of the brain, including the neocortex, the limbic system, and the subcortical regions (Fassbender et al., 2001). From a histopathological view, AD is characterized by senile plaques and neurofibrillary tangles (NFT) in the medial temporal lobe and cortical areas of the brain (Hansson et al., 2006). AD has been categorized into two major forms: familial AD (FAD) and late onset AD (LOAD; also termed sporadic AD, or SAD) with the latter being the leading cause of dementia in the elderly. FAD is an autosomal dominant disorder with onset before 65 years of age. The majority of FAD cases have been attributed to mutations in three genes: amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) (Chen et al., 2007). In contrast, the complete etiology of LOAD has yet to be fully elucidated, although age has been recognized as the greatest risk factor.
Currently no treatment exists to prevent, modify, or halt the progression of AD(Schneider et al., 2011a; Schneider et al., 2011b). Available drugs approved by FDA only offer moderate and temporary symptom relief (Golde et al., 2011). Therapeutic developments for AD, particularly LOAD, have been largely impeded by our limited understanding of disease etiology. The prevailing “amyloid cascade” hypothesis, which was first introduced by Hardy and Higgins in 1992 and has been enriched over the past decade, emphasizes the neurotoxic characteristics of β-amyloid (Aβ) as the main contributor to disease progression. This hypothesis proposes that the deposition of Aβ initiates a cascade of events, including the formation of NFTs, prolonged inflammatory responses, increased oxidative stress and mitochondrial dysfunction, which eventually lead to cell death and dementia (Armstrong, 2011; Hardy, 2006; Hardy and Higgins, 1992; Sommer, 2002). While this “amyloid cascade” hypothesis proposes a unified etiopathogenic mechanism for both FAD and LOAD, findings from both basic research and clinical observations indicate that a far more complex mechanism underlies LOAD. Recent studies indicate that in LOAD both Aβ deposition and NFTs, rather than being the cause of the disease, may be reactive products that arise from increased vulnerability to genetic and environmental risk factors as a function of aging (Armstrong, 2011; Gibson and Shi, 2010; Pimplikar, 2009; Simon et al., 2010). Moreover, candidates that directly target amyloid pathways, either through passive immunotherapy against Aβ (Bapineuzumab) (Prins et al., 2010) or via inhibition of pathways involved in Aβ generation (Tarenflurbil, Semagacestat, or Flurizan) (Imbimbo and Giardina, 2011), failed to achieve efficacy in recent clinical trials, indicating the therapeutic limitation of amyloid-specific strategies. Increasing evidence suggests that Alzheimer’s disease, particularly LOAD, is a multifaceted disease that could at least be partially attributed to a decline in mitochondrial function and altered brain metabolic activity.
2. Mitochondrial Dysfunction and β-Amyloid
The fundamental role of mitochondria in cellular bioenergetics and survival is well established (Brinton, 2008; Magistretti, 2006; Wallace, 2005). In addition, the evidence for mitochondrial dysfunction as a pivotal factor in age-associated neurodegenerative diseases such as Alzheimer’s and Parkinson’s continues to mount (Brinton, 2008; Moreira et al., 2006; Moreira et al., 2010; Mosconi et al., 2011a; Mosconi et al., 2009b; Swerdlow and Khan, 2009). Perturbations in mitochondrial function have long been observed in samples derived from clinically confirmed AD patients, including altered mitochondrial morphology, compromised enzyme complexes in the tricarboxylic acid cycle, and reduced cytochrome c oxidase (COX) activity (Blass, 2000; Cardoso et al., 2004; Gibson et al., 1988; Parker, 1991; Perry et al., 1980; Sorbi et al., 1983). Later, the “cybrid model” of AD, generated by transferring mitochondrial DNA (mtDNA) from human AD patients into cell cultures that are devoid of endogenous mtDNA (ρ0 cells), exhibited characteristics that recapitulated previous findings from clinical AD specimens. These findings included decreased mitochondrial mobility, increased oxidative stress, decreased COX activity, decreased mitochondrial membrane potential, and increased Aβ production, thereby providing further evidence for involvement of mitochondria and mtDNA in AD etiopathogenesis (Khan et al., 2000; Swerdlow, 2007). Increasing evidence indicates that mitochondria are direct targets of Aβ. Aβ has been demonstrated to accumulate within mitochondria and interact with a mitochondrial protein, Aβ-binding-alcohol-dehydrogenase (ABAD), resulting in decreased COX activity and increased oxidative stress (Lustbader et al., 2004; Reddy and Beal, 2008; Takuma et al., 2005). Further, the Aβ-induced neurotoxicity requires functional mitochondrial respiratory chain enzyme complexes (Cardoso et al., 2001) and is exacerbated in synergy with mitochondrial dysfunction in AD cybrid models (Cardoso et al., 2004).
While the neurotoxic mechanisms of Aβ converge upon mitochondria, compromised mitochondrial function, particularly a decline in mitochondrial bioenergetics and an increase in oxidative stress, propagates the degenerative process by further increasing Aβ generation. This creates a vicious cycle in which excessive Aβ accumulation and sustained mitochondrial dysfunction synergize to activate a cascade of neurodegenerative pathways (Silva et al., 2011; Swerdlow et al., 2010; Swerdlow and Khan, 2004, 2009).
3. Mitochondrial Bioenergetic Deficits in AD
Multiple levels of analysis and experimental paradigms, ranging from in vitro cell model systems and genomic analyses in animal models to postmortem autopsy of human brain and human brain imaging, indicate that dysfunction in glucose metabolism, bioenergetics and mitochondrial function are consistent antecedents to development of Alzheimer pathology (Gibson and Shi, 2010; Hauptmann et al., 2009; Nicholson et al., 2010; Yao et al., 2009). A decline in brain glucose metabolism and mitochondrial function can appear decades prior to the onset of histopathological and/or clinical features and thus may serve as a biomarker of AD risk as well as a therapeutic target (Mosconi et al., 2008; Mosconi et al., 2009a; Mosconi et al., 2009b; Mosconi et al., 2011b; Reiman et al., 2004). Studies using multiple preclinical in vitro and in vivo AD models have demonstrated a decline in mitochondrial function prior to the development of Alzheimer’s pathology, including decreased mitochondrial respiration, decreased metabolic enzyme expression and activity, decreased cerebral glucose metabolism, increased oxidative stress, and increased mitochondrial A β load and ABAD expression (Chou et al., 2011; Diana et al., 2011; Du et al., 2010; Hauptmann et al., 2009; Nicholson et al., 2010; Yao et al., 2009). The decline in mitochondrial function deteriorates with AD progression (Lustbader et al., 2004; Takuma et al., 2005). Consistent with basic science findings, multiple positron emission tomography (PET) studies also report antecedent abnormality in cerebral glucose utilization decades prior to the onset of AD, particularly in the hippocampal and entorhinal cortical regions(de Leon et al., 2001; Ishii et al., 1997; Mosconi et al., 2008; Mosconi et al., 2009a; Reiman et al., 2004; Rosenbloom et al., 2011; Spulber et al., 2008). This distinct pattern of brain hypometabolism predicted the cognitive decline in normal aging (Mosconi et al., 2008) or the progression of patients from mild cognitive impairment (MCI) to AD (Chetelat et al., 2003) with high accuracy. Recent clinical studies revealed a significant overlap between brain regions that exhibited abnormal glucose metabolism and regions that are most vulnerable to development of AD pathology (Bero et al., 2011; Vaishnavi et al., 2010; Vlassenko et al., 2010), providing further evidence of the association between disrupted glucose metabolism and AD pathogenesis.
4. Bioenergetic Deficits and Oxidative Stress
Impairment of mitochondrial bioenergetics and oxidative phosphorylation oxidative phosphorylation is often closely associated with increased free radical production and consequent oxidative damage. As the major source for cellular reactive oxygen species, mitochondria generate free radicals (superoxide anion, O2.−) and hydrogen peroxide (H2O2) as by-products of oxidative phosphorylation (Dumont et al., 2010; Lin and Beal, 2006). It is well documented that oxidative damage to mitochondrial membranes and proteins impairs mitochondrial oxidative phosphorylation efficiency and results in increased electron leak,, increased H2O2 levels and higher oxidative stress (Beal, 2005; Reddy and Beal, 2008). Key enzymes involved in mitochondrial bioenergetics, such as pyruvate dehydrogenase (PDH) and α-ketoglutarate dehydrogenase (αKGDH), are often the targets of oxidative modifications. This leads to deceased enzyme activity, decreased efficiency of mitochondrial electron transport, and increased production of free radicals (Park et al., 1999; Starkov et al., 2004).
Higher oxidative stress is characteristic of AD brains (Atamna and Frey, 2007; Gibson and Shi, 2010): in AD patients, significant increases in lipid peroxides, 8-oxoguanine, and oxidized amino acids, have been identified in vulnerable brain regions (Nunomura et al., 2004; Reddy, 2006). In preclinical AD animal models, increased generation of H2O2 and elevated oxidative damage to cellular components has also been shown to precede the development of AD pathology (Nunomura et al., 2009; Pratico et al., 2001; Rhein et al., 2009; Trushina and McMurray, 2007; Wang et al., 2005; Yao et al., 2009). Interestingly, an increase in oxidative stress has been demonstrated to increase β-amyloid production in vitro and in vivo (Li et al., 2004; Moreira et al., 2007; Nunomura et al., 2001).
5. Alternative Fuel Sources and White Matter Degeneration in AD
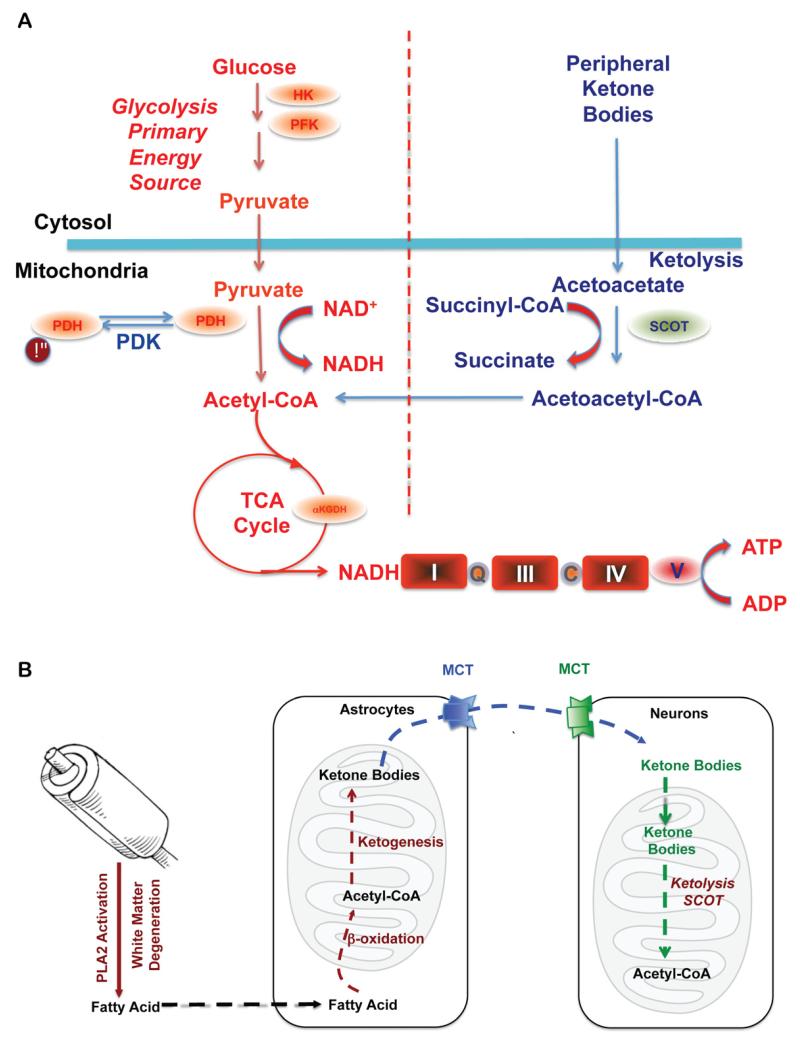
In parallel to the decline in brain glucose metabolism, white matter hyperintensities are also an early hallmark of AD (Kuczynski et al., 2010; Zhang et al., 2007). Defined as an alteration in white matter integrity, these hyperintensities are first seen in the cingulum bundle, uncinate fasciculus and superior longitudinal fasciculus of MCI patients (O’Dwyer et al., 2011). These regions are integral structures in the brain’s default mode network, a system that is active when an individual is not engaged in goal oriented activities or is at a resting state while awake. In addition to a loss in white matter integrity, patients with MCI have characteristic hypometabolism of the prefrontal and posterior cingulate cortices, also major components of the default mode network (O’Dwyer et al., 2011; Villain et al., 2010; Vlassenko et al., 2010). Interestingly, the connectivity between these cortices is provided by the superior longitudinal fasiculus, a region where hyperintesity positively correlates with the observed hypometabolism (Kuczynski et al., 2010). This loss in white matter integrity could be a direct result of the bioenergetic shift in these two cortices, indicating a switch from the use of ketone bodies supplied from the peripheral ketogenic organ, the liver, to ketone bodies resulting from local myelin breakdown via fatty acid oxidation by astroglia (Morris, 2005) (Fig. 1). Alternately, lesions in white matter integrity may be caused by inadequate lipid synthesis due to competition between consumption of ketones/acetyl-CoA for bioenergetics and lipid synthesis (Morris, 2005).
Figure 1. Bioenergetic substrate and catalytic compensatory adaptations to sustain metabolic demand of the brain.
A. Compensatory bioenergetic adaptation I: Glucose, the primary fuel source of brain metabolism, is converted via glycolysis to pyruvate which is further converted into acetyl-CoA to initiate and sustain the TCA cycle. Under metabolically challenging conditions (i.e. starvation, aging and neurodegeneration) neurons can utilize peripheral ketone bodies (β-hydroxybutyrate and acetoacetate generated by the liver) through ketolysis to generate acetyl-CoA. B. Compensatory bioenergetic adaptation II: local consumption of white matter for bioenergetics. With disease progression, peripheral ketone bodies are exhausted and the brain has to consume local white matter for energy production. Degradation of white matter via activation of PLA2 generates fatty acids that are further metabolized into acetyl-CoA through β-oxidation in the astrocytes. Acetyl-CoA is further converted into ketone bodies and transported into neurons by monocarboxylate transporters (MCTs) where ketone bodies are converted back into acetyl-CoA by SCOT and other important enzymes in ketolysis and further utilized towards ATP generation.
Considering the role of the cingulum bundle in connecting the hippocampal formation to both the prefrontal cortex and the posterior cingulate cortex, the degeneration of this white matter tract in addition to the hypometabolism of the prefrontal and posterior cingulate cortices results in the early atrophy of the hippocampus (Risacher et al., 2009; Whitwell et al., 2007) as well as impaired memory symptomatic of AD (Villain et al., 2010).
The default mode network’s heavy reliance on glucose to perform aerobic glycolysis makes synaptic transmission especially susceptible to bioenergetic deficits (Vaishnavi et al., 2010; Vlassenko et al., 2010). Recently, it has been found that amyloid beta deposition and abnormal aerobic glycolysis are present in AD in a strikingly similar pattern, specifically in the default mode network (Vlassenko et al., 2010). Mitochondrial dysfunction results in a series of changes that contribute to Aβ accumulation in mitochondria, including impaired oxidative phosphorylation, uncoupled electron transport chain, compromised ATP synthase, and COX inhibition (Manczak et al., 2006; Readnower et al., 2011; Young and Bennett, 2010). The fact that white matter degeneration is also selectively localized to the default mode network converges on a mechanistic pathway that links Aβ localization and activation of phospholipase A2 (PLA2). PLA2 subsequently activates sphingomyelinase, which in turn breaks down the myelin sheath to generate fatty acids that can be used in ketogenic energy production (Kanno and Nishizaki, 2011). The region specific association between white matter degeneration, brain hypometabolism and Aβ accumulation provides compelling evidence in support of a bioenergetic mechanism that unifies both compromised glucose metabolism and white matter catabolism in AD pathogenesis.
In parallel with the decline in glucose metabolism in AD, there is a generalized shift away from glucose-derived energy production, which is associated with a decrease in the expression of glycolytic enzymes coupled to a decrease in the activity of the pyruvate dehydrogenase (PDH) complex (Blass, 2000). Alterations in the brain metabolic profile in AD are further evidenced by concomitant activation of compensatory pathways that promote the usage of alternative substrates, such as ketone bodies, to compensate for the decline in glucose-driven ATP generation. We have previously reported that in the female 3xTgAD mouse model, pre-pathological decreases in PDH expression and mitochondrial bioenergetics were paralleled by increased expression of succinyl-CoA:3-ketoacid coenzyme A transferase (SCOT) and hydroxyacyl-Coenzyme A dehydrogenase(HADHA) at a young age (3 months). HADHA is a subunit of the mitochondrial trifunctional protein, which catalyzes the last three steps of mitochondrial β-oxidation of long chain fatty acids to generate acetyl-CoA, whereas SCOT is the key enzyme that converts ketone bodies into acetyl-CoA. The increase in HADHA and SCOT expression indicates early activation of ketolytic and/or fatty acid oxidation (FAO) pathways to compensate for compromised PDH capacity, and to provide alternative sources of acetyl-CoA, to sustain ATP generation (Yao et al., 2010). Consistent with these mechanistic analyses, clinical observations have also reported a substrate switch that parallels AD progression. While there is a 100:0 ratio of glucose to other substrates utilization in young controls, there is a 2:1 ratio in AD patients compared to a ratio of 29:1 in healthy elderly controls (Hoyer et al., 1991).
6. A Bioenergetic-Centric Hypothesis of AD
We have discussed four different but important pathogenic factors of AD, including decreased mitochondrial bioenergetics, increased oxidative stress, compromised white matter, and elevated Aβ generation. While each individual aspect focuses on a specific perspective of AD pathogenesis, the unique temporal-spatial association between these components indicates a common mitochondrial-centric mechanism that unifies these aspects into a bioenergetic compensatory network. In healthy aging, the brain exhibits a glucose-driven metabolic phenotype. The energy-redox axis is tightly coupled and physiological concentrations of H2O2 are maintained by the coordinated activity of endogenous antioxidant systems. In contrast, in prodromal AD brains, glucose metabolism is compromised early in the disease process and creates a bioenergetic crisis, switching the brain from efficient glucose-driven energy production to less efficient ketone body-driven energy production. The compromised bioenergetic state is accompanied and further exacerbated by elevated oxidative stress, which is associated with increased expression of enzymes involved in ketogenesis and fatty acid oxidation, such as SCOT and HADHA, as well as mitochondrial Aβ accumulation (Young and Bennett, 2010). Further aiding this switch towards inefficiency is the elevated H2O2 production that results from decreased mitochondrial efficiency and increased oxidative stress. H2O2 leads to activation of PLA2, which degrades the myelin sheath so that it may be used as an additional source of fatty acids in ketogenesis. Consequently, the release and enrichment of free cholesterol resulting from white matter degeneration leads to impairment of the lipid-protein bilayer and contributes to hyper-activation of γ-secretase and Aβ over production (Burns et al., 2003; Petanceska et al., 2002; Vetrivel and Thinakaran, 2010). Cleavage of APP by γ-secretase leads to intraneuronal Aβ production and translocation of Aβ to mitochondria (Manczak et al., 2006; Readnower et al., 2011; Young and Bennett, 2010).
While we posit a stepwise progression of bioenergetic compensatory adaptations, it is more likely that there is a vicious cycle of exacerbating interactions. Mitochondrial accumulation of Aβ would exacerbate the bioenergetic deficits by contributing to decline in the energy-transducing efficiency. Mitochondrial accumulation of Aβ would also induce an increase in ABAD expression, perpetuating the activation of the fatty acid oxidation pathway and the degeneration of myelin, there by propagating the transition of brain metabolism into a ketogenic/ fatty acid oxidation phenotype.
7. Development of Bioenergetic-Centric Biomarkers for AD
Recently the clinical phases of AD have been expanded to include presymptomatic AD, during which time an individual appears cognitively normal but is beginning to exhibit some of the pathological changes of AD (Sperling et al., 2011). Defining this presymptomatic phase was particularly important for explaining why some individuals have no cognitive deficits but, upon autopsy, amyloid plaques and neurofibrillary tangles are present in the brain (De Meyer et al., 2010; Jack et al., 2008; Knopman et al., 2003; Mintun et al., 2006; Price and Morris, 1999). This provides further confirmation that a successful therapeutic intervention for AD will require very early identification of prodromal AD patients. Therefore, an area of concentrated focus within the Alzheimer’s research community is the identification and validation of biomarkers - biospecimen or neuroimaging variables that can be used to reliably predict individuals at risk of developing AD. Development of a biomarker profile of AD would be of great benefit both to clinicians and the drug development community; clinicians so that accurate diagnoses could be made ante-mortem, and pharmaceutical companies so that the efficacy of new drug formulations could be tested (Williams, 2011).
The criteria for a biomarker of AD were proposed in 1998 by the Working Group on Molecular and Biochemical Markers of Alzheimer’s Disease, and have since become standards for the field. The Working Group specified: “the ideal biomarker for AD should detect a fundamental feature of neuropathology and be validated in neuropathologically-confirmed cases; it should have a diagnostic sensitivity >80% for detecting AD and a specificity of >80% for distinguishing other dementias; it should be reliable, reproducible, non-invasive, simple to perform, and inexpensive”(1998). The challenge of developing biomarkers that measure preclinical AD is that they must be able to discriminate between individuals who have AD pathology and those who do not, but all while the individuals are still at a cognitively intact stage so there is adequate time for prevention. As the prodromal stage of AD is known to exist decades prior to the manifestation of clinical symptoms, this would imply that preventative measures will require a method for routine screening of all patients in the 50-65 year age range. Thus, a useful biomarker would need to be not only specific and sensitive but cost-effective, so it would be broadly accessible.
Currently, the most thoroughly studied biomarkers of AD are the levels of three proteins measured in the cerebrospinal fluid (CSF): amyloid beta1-42 (Aβ42), total tau protein, and p-tau, a phosphorylated form of tau protein. CSF levels of Aβ42 are decreased in AD, which is predicted to be due to the incorporation of Aβ42 into amyloid plaques (Blennow et al., 2001). CSF levels of both total tau and p-tau are increased, likely due to degenerating neurons releasing these proteins into the CSF (Jack et al., 2010). All three of these biomarkers have been validated, but changes in CSF levels of these proteins are likely occurring far down stream of the initial mitochondrial bioenergetic crisis; this implies that by the time they are measurable, the window for disease prevention may have already passed.
A recent development in the biomarker field has been the use of neuroimaging. Magnetic resonance imaging (MRI) can be used to visualize changes in brain structure that are associated with AD. Longitudinal MRI studies conducted by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) showed a pattern of temporal lobe atrophy that was significantly greater in patients who converted from MCI to AD than those who did not convert (Trojanowski et al., 2010). Additionally, loss of hippocampal volume proved indicative of AD pathology and correlated with the APOε4 allele in FAD (Trojanowski et al., 2010). Unfortunately, by the time volumetric changes are quantifiable, substantial loss of grey and white matter has already occurred. Thus, while MRI detection of atrophy is useful as a diagnostic tool, its utility for prevention of AD is limited.
Positron emission tomography (PET) scanning is another neuroimaging method that has been used to study the development of Alzheimer’s disease. One type of PET imaging uses radiolabeled molecules which bind and label amyloid in the living brain. The most thoroughly studied examples of these compounds are Pittsburgh Compound B (PiB) (Klunk et al., 2004) and 18F-AV-45 (florbetapir) (Wong et al., 2010). Studies conducted using brain tissue from autopsy-confirmed AD patients show that PiB binds only to fibrillar amyloid, particularly plaques that are immunoreactive for Aβ40 or Aβ42 (Ikonomovic et al., 2008). 18F-AV-45 has also been shown to bind selectively to Aβ plaques in the postmortem AD brain (Choi et al., 2009). Unfortunately, amyloid neuroimaging techniques suffer from the same limitations as measurements of CSF Aβ42 and tau: by the time Aβ42 is aggregated into plaques, the pathogenesis of AD is established in brain. Additionally, PiB binding is not a 100% reliable biomarker, as there are cases of autopsy-confirmed AD which failed to show PiB labeling in the brain (Leinonen et al., 2008) (Rosen et al., 2010).
Using fluorodeoxyglucose PET (FDG-PET), a significant body of research indicates that abnormalities in cerebral glucose utilization appear decades prior to the onset of clinical AD (de Leon et al., 2001; Mosconi et al., 2009b; Reiman et al., 2004). Further, the decrease in brain metabolism precedes the atrophy detected by MRI (De Santi et al., 2001) and predicts a decline in cognitive function (de Leon et al., 2001; Jagust et al., 2006; Mosconi et al., 2008). A decline in glucose metabolism could be simply due to decrease in brain mass; however, deficits in brain metabolism exceeded the magnitude of cortical atrophy (Ibanez et al., 1998). Based on a bioenergetic perspective of the etiology of AD, brain hypometabolism represents a response to an antecedent shift from utilizing glucose to requiring the alternative fuels of fatty acids and derived ketone bodies. Thus hypometabolism still may be too late in the etiological cascade of events to be used as a biomarker for AD prevention, but could be applicable to identifying prodromal AD. Indeed, hypometabolism measured by FDG-PET has been identified as a “gold standard” for early-stage diagnosis of AD, although this method is hampered by high cost and relative inaccessibility of the scanning equipment (Hampel et al., 2008).
Considering the central and antecedent role of mitochondrial bioenergetics in AD pathogenesis, a biomarker which reliably detects a shift to inefficient mitochondrial bioenergetics in the brain could provide the earliest indication that an individual is at risk for AD. Based on the Working Group recommendations that a biomarker be simple to measure and inexpensive, and our requirement that it be broadly accessible, the most desirable biomarker would be measurable in blood samples. One such marker could be plasma levels of ketone bodies. In preclinical models, e.g. the 3xTgAD mouse, brain mitochondrial levels of enzymes involved in ketone body catabolism are upregulated very early in the disease process (Chou et al., 2011), suggesting a compensatory response which may be indicative of a shift towards the use of an alternate fuel source due to ineffective glucose metabolism in brain. Elevated levels of ketone bodies in the plasma would be then expected to indicate increased ketone generation by the liver in response to the disrupted brain glucose metabolism (Figure 1).
Mitochondrial enzyme activity also holds promise as a potential biomarker of preclinical AD. It is well established that complex IV activity is decreased in platelet mitochondria isolated from individuals with AD (Bosetti et al., 2002; Cardoso et al., 2004; Parker et al., 1990; Parker et al., 1994). Additionally, Valla et al reported a decrease in platelet mitochondrial complex IV that was present in MCI patients as well as patients with AD, suggesting that changes in mitochondrial complex IV activity may be occurring early in AD pathogenesis (Valla et al., 2006). Interestingly, it was recently reported that platelet mitochondrial complex IV activity is reduced in young, cognitively normal individuals who have a maternal history of LOAD (Mosconi et al., 2011a). Mitochondrial DNA is maternally inherited and codes for the proteins which make up complex IV, suggesting that some forms of LOAD may result from a maternally-transmitted mitochondrial deficit (Edland et al., 1996). Reduced platelet mitochondrial COX activity has potential as an early marker for individuals with a maternally-inherited risk of LOAD.
Activity of mitochondrial enzyme complexes has also been investigated in lymphocytes, with varying degrees of success. Some studies have found no effect of AD status on lymphocyte mitochondrial activity (Molina et al., 1997; Valla et al., 2006), whereas a recent study showed increased activity of mitochondrial respiratory chain complexes II and IV in lymphocytes isolated from patients with AD when compared with controls (Feldhaus et al., 2011). Another study showed that although there was no baseline difference in lymphocyte mitochondrial enzyme activities between controls and AD patients, those patients who were treated with the cholinesterase inhibitor rivastigmine showed increased activity of complexes II, III and IV (Casademont et al., 2003), indicating that increased mitochondrial efficiency might be associated with better disease outcome.
8. Therapeutics Targeting Mitochondria and Bioenergetics for AD Treatment and Prevention
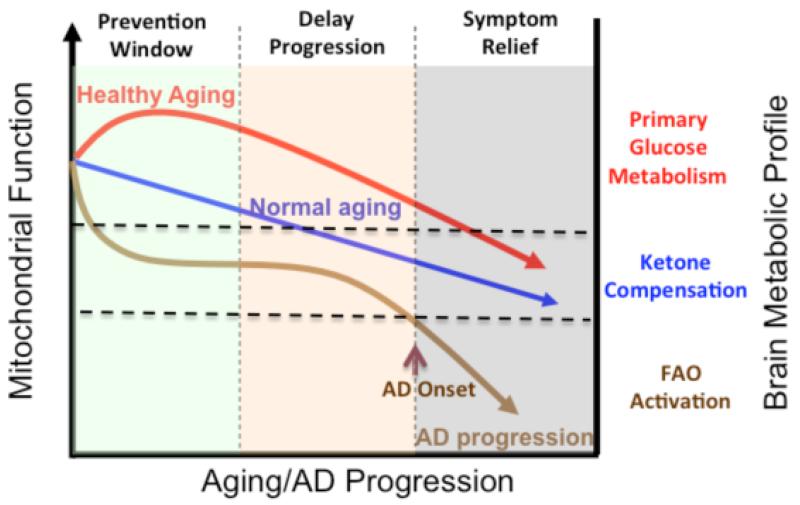
Alzheimer’s is a neurodegenerative disease with a complex and progressive pathological phenotype characterized first by hypometabolism and impaired mitochondrial bioenergetics followed by pathological burden. The progressive and multifaceted degenerative phenotype of Alzheimer’s suggests that successful treatment strategies need to be equally multi-faceted and stage specific. Increasing evidence indicates an antecedent and potentially causal role of mitochondrial bioenergetic deficits and brain hypometabolism coupled with increased mitochondrial oxidative stress in AD pathogenesis. Mitochondrial deficits have been demonstrated to activate a cassette of neurotoxic events that all contribute to synaptic dysfunction, pathology development and eventually neuronal loss and cognitive impairment (Beal, 2005; Reddy and Beal, 2008). Further, deficits in mitochondrial bioenergetics and brain metabolism exhibit a stage-specific trajectory with disease progression, which was first evidenced by the decline in glucose uptake and utilization that takes place decades prior to AD onset, followed by parallel activation of pathways to use alternative fuel substrates, ketone bodies, to compensate for the decline in glucose metabolism (Yao et al., 2010; Yao et al., 2009). As disease progresses, exacerbated decline in glucose utilization and exhaustion of available ketone reservoir leads to further disturbance of mitochondrial function and activation of fatty acid oxidation (FAO) pathway that eventually results in white matter degeneration and neuronal death observed in AD (Bartzokis et al., 2004; Brinton, 2008; Carmichael et al., 2010; Kuczynski et al., 2010). This unique trajectory of glucose-ketone-FAO progression of brain mitochondrial metabolic alteration provides an ideal therapeutic target that is both disease modifying and stage specific (Fig. 2).
Figure 2. Trajectory of mitochondrial function, substrate utilization during AD progression and therapeutic strategy.
At young age or in healthy aging, brain metabolic activity is supported by glucose, the primary fuel source, whereas in prodromal and incipient AD the antecedent decline in glucose metabolism is first paralleled by compensatory activation of ketogenic pathways, which later diminishes and progresses into local fatty acid oxidation and white matter degeneration. The prevention strategy aims to enhance the glucose driven mitochondrial bioenergetics to promote healthy aging and prevent AD. Alternatively, in prodromal and incipient AD, sustained activation of ketogenesis provides prolonged supplement of the alternative fuel source, ketone bodies, and therefore sustains mitochondrial bioenergetic function and prevents/delays further progression of the disease. At the middle to late stage of AD, rather than modifying disease progression, treatments merely offer symptom relief.
Candidates that potentiate mitochondrial bioenergetics and enhance brain glucose metabolism are expected to prevent the antecedent decline in brain glucose metabolism, promote healthy aging and therefore prevent AD. Interestingly, many candidates within this category are naturally occurring herbals and small co-factors, which often are on the GRAS (generally considered as safe) list. R-α-lipoic acid, an important co-factor for key mitochondrial metabolic enzymes, including PDH, KGDH, and branched chain α-ketoacid dehydrogenase (BCKDH), has been demonstrated to up-regulate mitochondrial bioenergetics, promote glucose metabolism, and suppress oxidative stress due to its potent antioxidant capacity (Packer and Cadenas, 2011). Resveratrol, a redox active ingredient in grapes and wine, improves brain energy metabolism and reduces amyloid accumulation in preclinical animal models (Karuppagounder et al., 2009; Marambaud et al., 2005; O’Dwyer et al., 2011; Vingtdeux et al., 2008). Both R-α-lipoic acid and resveratrol are currently under clinical trials for their efficacy in AD prevention and treatment (Packer and Cadenas, 2011; Wollen, 2010). Other important regulators of mitochondrial metabolic activity include B-vitamins which are also co-factors of key metabolic/mitochondrial enzymes. These naturally occurring compounds often possess antioxidant properties. All together, these compounds exhibit potential to promote brain glucose utilization, potentiate brain metabolic activity, and simultaneously suppress oxidative damage with relatively low toxicity, which make them ideal candidates for development of nutraceutical cocktails to promote brain metabolism during healthy aging and therefore prevent AD.
While the preventive strategy focuses heavily on the enhancement of brain glucose metabolism, the shift towards an alternative fuel source, ketone bodies, observed in both preclinical AD models and in AD patients provides a second therapeutic window that targets the specific glucose – ketone transition stage to sustain brain metabolic activity and therefore prevent or delay further exacerbation in brain bioenergetic deficits. Ketone bodies are mainly synthesized in the liver through fatty acid oxidation (FAO) and are well documented to serve as alternative energy substrates for the heart, muscle, and brain. Ketogenic pathways have been demonstrated to exist in astrocytes (Auestad et al., 1991; Guzman and Blazquez, 2004). Epidemiological analyses indicate a positive association between dietary intake of ketones/consumption of ketogenic diets and reduced risk for AD (Henderson, 2008; Morris, 2005). The switch from glucose as the primary fuel to the alternative of ketone bodies in the AD brain was the basis for Accera to develop Ketasyn, which is converted to ketone bodies in the liver for subsequent use by the brain. This approach capitalizes on the brain’s relative inability to utilize glucose and its dependency on ketone bodies. Phase II clinical trial in Alzheimer’s patients and in individuals suffering from age-associated memory impairment has been completed and both groups showed improvement in memory function using the ketone body alternative fuel source (http://www.accerapharma.com).
While increasing ketone body supply provides more substrate to the brain to utilize as an alternative fuel, the therapeutic efficacy could be limited due to a diminished brain capacity to utilize ketone bodies. To address the issue of deficits in the ketogenic metabolic pathway, our group investigated the efficacy of the ketogenic modulator, 2-deoxy-d-glucose (2-DG) to increase brain capacity to utilize ketone bodies as fuel. Results of these analyses demonstrated that dietary 2-DG intake induced ketogenesis, sustained mitochondrial bioenergetics, and reduced pathology in the triple transgenic Alzheimer’s (3xTgAD) mouse model (Jia Yao, 2011). Based on these clinical and preclinical findings, a combination of nutraceutical and pharmaceutical modulators that simultaneously enhance mitochondrial bioenergetics while sustaining availability and utilization of an alternative fuel substrate (ketone bodies), could prevent further decline in brain metabolism and to delay progression of AD.
9. Summary
Alzheimer’s disease is a complex disease with a prolonged trajectory of etiopathogenic changes in brain bioenergetics decades prior to the clinical onset of the disease. Although it remains to be clinically confirmed, the unique trajectory of alterations in brain metabolic profile provides the foundation upon which to develop an array of bioenergetic-centric biomarkers to predict AD risk at the preclinical stage and therefore provide the best opportunity to prevent and/or delay the onset of AD. From a therapeutic perspective, this unique trajectory of alterations in brain metabolic capacity enable a bioenergetic-centric strategy that targets disease-stage specific profile of brain metabolism for disease prevention and treatment. A combination of nutraceutical and pharmaceutical intervention that enhances glucose-driven metabolic activity and potentiates mitochondrial bioenergetic function could prevent the antecedent decline in brain glucose metabolism, promote healthy aging and prevent AD. Alternatively, during the prodromal incipient phase of AD, sustained activation of ketogenic metabolic pathways coupled with supplement of the alternative fuel source, ketone bodies, could sustain mitochondrial bioenergetic function to prevent or delay further progression of the disease.
Acknowledgment
This study was supported by National Institute on Aging Grant 2R01AG032236 (to RDB) National Institute on Aging Grant 5P01AG026572 (to RDB; Project 1 to EC and RDB) and Eileen L. Norris Foundation (to RDB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Consensus report of the Working Group “Molecular and Biochemical Markers of Alzheimer’s Disease”. The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group. Neurobiol Aging. 1998;19(2):109–116. [PubMed] [Google Scholar]
- Armstrong RA. The pathogenesis of Alzheimer’s disease: a reevaluation of the “amyloid cascade hypothesis”. Int J Alzheimers Dis 2011. 2011:630865. doi: 10.4061/2011/630865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association A.s. 2011 Alzheimer’s Disease. 2011 Facts and Figures 12. [Google Scholar]
- Atamna H, Frey WH. Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer’s disease. Mitochondrion. (2nd) 2007 doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Auestad N, Korsak RA, Morrow JW, Edmond J. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J Neurochem. 1991;56(4):1376–1386. doi: 10.1111/j.1471-4159.1991.tb11435.x. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol Aging. 2004;25(7):843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58(4):495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-beta deposition. Nat Neurosci. 2011 doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass JP. The mitochondrial spiral. An adequate cause of dementia in the Alzheimer’s syndrome. Ann N Y Acad Sci. 2000;924:170–183. doi: 10.1111/j.1749-6632.2000.tb05576.x. [DOI] [PubMed] [Google Scholar]
- Blennow K, Vanmechelen E, Hampel H. CSF total tau, Abeta 42 and phosphorylated tau protein as biomarkers for Alzheimer’s disease. Mol Neurobiol. 2001;24(1-3):87–97. doi: 10.1385/MN:24:1-3:087. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiol Aging. 2002;23(3):371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer’s disease. Adv Drug Deliv Rev. 2008;60(13-14):1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns M, Gaynor K, Olm V, Mercken M, LaFrancois J, Wang L, Mathews PM, Noble W, Matsuoka Y, Duff K. Presenilin redistribution associated with aberrant cholesterol transport enhances beta-amyloid production in vivo. J Neurosci. 2003;23(13):5645–5649. doi: 10.1523/JNEUROSCI.23-13-05645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso SM, Proenca MT, Santos S, Santana I, Oliveira CR. Cytochrome c oxidase is decreased in Alzheimer’s disease platelets. Neurobiol Aging. 2004;25(1):105–110. doi: 10.1016/s0197-4580(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Santos S, Swerdlow RH, Oliveira CR. Functional mitochondria are required for amyloid beta-mediated neurotoxicity. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15(8):1439–1441. doi: 10.1096/fj.00-0561fje. [DOI] [PubMed] [Google Scholar]
- Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR, Jr., Weiner M, DeCarli C. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67(11):1370–1378. doi: 10.1001/archneurol.2010.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casademont J, Miro O, Rodriguez-Santiago B, Viedma P, Blesa R, Cardellach F. Cholinesterase inhibitor rivastigmine enhance the mitochondrial electron transport chain in lymphocytes of patients with Alzheimer’s disease. Journal of the neurological sciences. 2003;206(1):23–26. doi: 10.1016/s0022-510x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- Chen K, Reiman EM, Alexander GE, Caselli RJ, Gerkin R, Bandy D, Domb A, Osborne D, Fox N, Crum WR, Saunders AM, Hardy J. Correlations between apolipoprotein E epsilon4 gene dose and whole brain atrophy rates. Am J Psychiatry. 2007;164(6):916–921. doi: 10.1176/ajp.2007.164.6.916. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60(8):1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- Choi SR, Golding G, Zhuang Z, Zhang W, Lim N, Hefti F, Benedum TE, Kilbourn MR, Skovronsky D, Kung HF. Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50(11):1887–1894. doi: 10.2967/jnumed.109.065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JL, Shenoy DV, Thomas N, Choudhary PK, Laferla FM, Goodman SR, Breen GA. Early dysregulation of the mitochondrial proteome in a mouse model of Alzheimer’s disease. J Proteomics. 2011;74(4):466–479. doi: 10.1016/j.jprot.2010.12.012. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proceedings of the National Academy of Sciences of the United States of America. 2001;98(19):10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H, Blennow K, Shaw L, Trojanowski JQ. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 2010;67(8):949–956. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, Tsui WH, Kandil E, Boppana M, Daisley K, Wang GJ, Schlyer D, Fowler J. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22(4):529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Diana FF, Silva Esteves AR, Oliveira CR, Cardoso SM. Mitochondria: The Common Upstream Driver of Abeta and Tau Pathology in Alzheimer s Disease. Curr Alzheimer Res. 2011 doi: 10.2174/156720511796391872. [DOI] [PubMed] [Google Scholar]
- Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M, Lin MT, Beal MF. Mitochondria and antioxidant targeted therapeutic strategies for Alzheimer’s disease. J Alzheimers Dis. 2010;20(Suppl 2):S633–643. doi: 10.3233/JAD-2010-100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edland SD, Silverman JM, Peskind ER, Tsuang D, Wijsman E, Morris JC. Increased risk of dementia in mothers of Alzheimer’s disease cases: evidence for maternal inheritance. Neurology. 1996;47(1):254–256. doi: 10.1212/wnl.47.1.254. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Masters C, Beyreuther K. Alzheimer’s disease: molecular concepts and therapeutic targets. Naturwissenschaften. 2001;88(6):261–267. doi: 10.1007/s001140100237. [DOI] [PubMed] [Google Scholar]
- Feldhaus P, Fraga DB, Ghedim FV, De Luca RD, Bruna TD, Heluany M, Matos MP, Ferreira GK, Jeremias IC, Heluany C, Streck EL, Zugno AI. Evaluation of respiratory chain activity in lymphocytes of patients with Alzheimer disease. Metab Brain Dis. 2011 doi: 10.1007/s11011-011-9253-y. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, Perrino P. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch Neurol. 1988;45(8):836–840. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- Gibson GE, Shi Q. A mitocentric view of Alzheimer’s disease suggests multi-faceted treatments. J Alzheimers Dis. 2010;20(Suppl 2):S591–607. doi: 10.3233/JAD-2010-100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron. 2011;69(2):203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman M, Blazquez C. Ketone body synthesis in the brain: possible neuroprotective effects. Prostaglandins Leukot Essent Fatty Acids. 2004;70(3):287–292. doi: 10.1016/j.plefa.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Hampel H, Burger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimers Dement. 2008;4(1):38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- Hardy J. Alzheimer’s disease: the amyloid cascade hypothesis: an update and reappraisal. J Alzheimers Dis. 2006;9(3 Suppl):151–153. doi: 10.3233/jad-2006-9s317. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hauptmann S, Scherping I, Drose S, Brandt U, Schulz KL, Jendrach M, Leuner K, Eckert A, Muller WE. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging. 2009;30(10):1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Henderson ST. Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics. 2008;5(3):470–480. doi: 10.1016/j.nurt.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect. 1991;3(1):1–14. doi: 10.1007/BF02251132. [DOI] [PubMed] [Google Scholar]
- Ibanez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, Rapoport SI, Schapiro MB, Horwitz B. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology. 1998;50(6):1585–1593. doi: 10.1212/wnl.50.6.1585. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, Debnath ML, Hope CE, Isanski BA, Hamilton RL, DeKosky ST. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain : a journal of neurology. 2008;131(Pt 6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbimbo BP, Giardina GA. gamma-Secretase Inhibitors and Modulators for the Treatment of Alzheimer’s Disease: Disappointments and Hopes. Curr Top Med Chem. 2011 doi: 10.2174/156802611795860942. [DOI] [PubMed] [Google Scholar]
- Ishii K, Sasaki M, Kitagaki H, Yamaji S, Sakamoto S, Matsuda K, Mori E. Reduction of cerebellar glucose metabolism in advanced Alzheimer’s disease. J Nucl Med. 1997;38(6):925–928. [PubMed] [Google Scholar]
- Jack CR, Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet neurology. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr., Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain : a journal of neurology. 2008;131(Pt 3):665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann Neurol. 2006;59(4):673–681. doi: 10.1002/ana.20799. [DOI] [PubMed] [Google Scholar]
- Jia Yao SC, Zisu Mao, Enrique Cadenas, Roberta Diaz Brinton. 2-Deoxy-D-Glucose Treatment Induces Ketogenesis, Sustains Mitochondrial Function, and Reduces Pathology in Female Mouse Model of Alzheimer’s Disease. PLoS One. 2011;6(7):e21788. doi: 10.1371/journal.pone.0021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T, Nishizaki T. Sphingosine induces apoptosis in hippocampal neurons and astrocytes by activating caspase-3/-9 via a mitochondrial pathway linked to SDK/14-3-3 protein/Bax/cytochrome c. J Cell Physiol. 2011;226(9):2329–2337. doi: 10.1002/jcp.22571. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int. 2009;54(2):111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SM, Cassarino DS, Abramova NN, Keeney PM, Borland MK, Trimmer PA, Krebs CT, Bennett JC, Parks JK, Swerdlow RH, Parker WD, Jr., Bennett JP., Jr. Alzheimer’s disease cybrids replicate beta-amyloid abnormalities through cell death pathways. Ann Neurol. 2000;48(2):148–155. [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62(11):1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- Kuczynski B, Targan E, Madison C, Weiner M, Zhang Y, Reed B, Chui HC, Jagust W. White matter integrity and cortical metabolic associations in aging and dementia. Alzheimers & Dementia. 2010;6(1):54–62. doi: 10.1016/j.jalz.2009.04.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen V, Alafuzoff I, Aalto S, Suotunen T, Savolainen S, Nagren K, Tapiola T, Pirttila T, Rinne J, Jaaskelainen JE, Soininen H, Rinne JO. Assessment of beta-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11-labeled Pittsburgh Compound B. Arch Neurol. 2008;65(10):1304–1309. doi: 10.1001/archneur.65.10.noc80013. [DOI] [PubMed] [Google Scholar]
- Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, Takahashi RH, Carlson GA, Flint Beal M, Lin MT, Gouras GK. Increased plaque burden in brains of APP mutant Mn SOD heterozygous knockout mice. J Neurochem. 2004;89(5):1308–1312. doi: 10.1111/j.1471-4159.2004.02455.x. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304(5669):448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209(Pt 12):2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15(9):1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280(45):37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C] PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- Molina JA, de Bustos F, Jimenez-Jimenez FJ, Benito-Leon J, Gasalla T, Orti-Pareja M, Vela L, Bermejo F, Martin MA, Campos Y, Arenas J. Respiratory chain enzyme activities in isolated mitochondria of lymphocytes from patients with Alzheimer’s disease. Neurology. 1997;48(3):636–638. doi: 10.1212/wnl.48.3.636. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Cardoso SM, Santos MS, Oliveira CR. The key role of mitochondria in Alzheimer’s disease. J Alzheimers Dis. 2006;9(2):101–110. doi: 10.3233/jad-2006-9202. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Seica R, Oliveira CR. Brain mitochondrial dysfunction as a link between Alzheimer’s disease and diabetes. J Neurol Sci. 2007;257(1-2):206–214. doi: 10.1016/j.jns.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, Perry G, Smith MA. Mitochondria: a therapeutic target in neurodegeneration. Biochim Biophys Acta. 2010;1802(1):212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AA. Cerebral ketone body metabolism. J Inherit Metab Dis. 2005;28(2):109–121. doi: 10.1007/s10545-005-5518-0. [DOI] [PubMed] [Google Scholar]
- Mosconi L, de Leon M, Murray J, E L, Lu J, Javier E, McHugh P, Swerdlow RH. Reduced Mitochondria Cytochrome Oxidase Activity in Adult Children of Mothers with Alzheimer’s Disease. J Alzheimers Dis. 2011a doi: 10.3233/JAD-2011-110866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29(5):676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Brys M, Glodzik L, Rich K, Pirraglia E, Tsui W, De Santi S, de Leon MJ. Declining brain glucose metabolism in normal individuals with a maternal history of Alzheimer disease. Neurology. 2009a;72(6):513–520. doi: 10.1212/01.wnl.0000333247.51383.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, de Leon MJ. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009b;36(5):811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Tsui W, Murray J, McHugh P, Li Y, Williams S, Pirraglia E, Glodzik L, De Santi S, Vallabhajosula S, de Leon MJ. Maternal age affects brain metabolism in adult children of mothers affected by Alzheimer’s disease. Neurobiol Aging. 2011b doi: 10.1016/j.neurobiolaging.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson RM, Kusne Y, Nowak LA, LaFerla FM, Reiman EM, Valla J. Regional cerebral glucose uptake in the 3xTG model of Alzheimer’s disease highlights common regional vulnerability across AD mouse models. Brain Res. 2010;1347:179–185. doi: 10.1016/j.brainres.2010.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Chiba S, Lippa CF, Cras P, Kalaria RN, Takeda A, Honda K, Smith MA, Perry G. Neuronal RNA oxidation is a prominent feature of familial Alzheimer’s disease. Neurobiol Dis. 2004;17(1):108–113. doi: 10.1016/j.nbd.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, Perry G. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathol. 2009;118(1):151–166. doi: 10.1007/s00401-009-0508-1. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- O’Dwyer L, Lamberton F, Bokde AL, Ewers M, Faluyi YO, Tanner C, Mazoyer B, O’Neill D, Bartley M, Collins DR, Coughlan T, Prvulovic D, Hampel H. Multiple indices of diffusion identifies white matter damage in mild cognitive impairment and Alzheimer’s disease. PLoS One. 2011;6(6):e21745. doi: 10.1371/journal.pone.0021745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer L, Cadenas E. Lipoic acid: energy metabolism and redox regulation of transcription and cell signaling. J Clin Biochem Nutr. 2011;48(1):26–32. doi: 10.3164/jcbn.11-005FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park LC, Zhang H, Sheu KF, Calingasan NY, Kristal BS, Lindsay JG, Gibson GE. Metabolic impairment induces oxidative stress, compromises inflammatory responses, and inactivates a key mitochondrial enzyme in microglia. J Neurochem. 1999;72(5):1948–1958. doi: 10.1046/j.1471-4159.1999.0721948.x. [DOI] [PubMed] [Google Scholar]
- Parker WD., Jr. Cytochrome oxidase deficiency in Alzheimer’s disease. Annals of the New York Academy of Sciences. 1991;640:59–64. doi: 10.1111/j.1749-6632.1991.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr., Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40(8):1302–1303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr., Mahr NJ, Filley CM, Parks JK, Hughes D, Young DA, Cullum CM. Reduced platelet cytochrome c oxidase activity in Alzheimer’s disease. Neurology. 1994;44(6):1086–1090. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, Tomlinson BE, Blessed G, Gibson PH. Coenzyme A-acetylating enzymes in Alzheimer’s disease: possible cholinergic ‘compartment’ of pyruvate dehydrogenase. Neurosci Lett. 1980;18(1):105–110. doi: 10.1016/0304-3940(80)90220-7. [DOI] [PubMed] [Google Scholar]
- Petanceska SS, DeRosa S, Olm V, Diaz N, Sharma A, Thomas-Bryant T, Duff K, Pappolla M, Refolo LM. Statin therapy for Alzheimer’s disease: will it work? J Mol Neurosci. 2002;19(1-2):155–161. doi: 10.1007/s12031-002-0026-2. [DOI] [PubMed] [Google Scholar]
- Pimplikar SW. Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. Int J Biochem Cell Biol. 2009;41(6):1261–1268. doi: 10.1016/j.biocel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21(12):4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Prins ND, Visser PJ, Scheltens P. Can novel therapeutics halt the amyloid cascade? Alzheimers Res Ther. 2010;2(2):5. doi: 10.1186/alzrt28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readnower RD, Sauerbeck AD, Sullivan PG. Mitochondria, Amyloid beta, and Alzheimer’s Disease. Int J Alzheimers Dis. 2011;2011:104545. doi: 10.4061/2011/104545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial oxidative damage in aging and Alzheimer’s disease: implications for mitochondrially targeted antioxidant therapeutics. J Biomed Biotechnol. 2006;2006(3):31372. doi: 10.1155/JBB/2006/31372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14(2):45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhein V, Song X, Wiesner A, Ittner LM, Baysang G, Meier F, Ozmen L, Bluethmann H, Drose S, Brandt U, Savaskan E, Czech C, Gotz J, Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher SL, Saykin AJ, West JD, Shen L, Firpi HA, McDonald BC. Baseline MRI predictors of conversion from MCI to probable AD in the ADNI cohort. Curr Alzheimer Res. 2009;6(4):347–361. doi: 10.2174/156720509788929273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Ciliax BJ, Wingo TS, Gearing M, Dooyema J, Lah JJ, Ghiso JA, LeVine H, 3rd, Walker LC. Deficient high-affinity binding of Pittsburgh compound B in a case of Alzheimer’s disease. Acta Neuropathol. 2010;119(2):221–233. doi: 10.1007/s00401-009-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MH, Alkalay A, Agarwal N, Baker SL, O’Neil JP, Janabi M, Yen IV, Growdon M, Jang J, Madison C, Mormino EC, Rosen HJ, Gorno-Tempini ML, Weiner MW, Miller BL, Jagust WJ, Rabinovici GD. Distinct clinical and metabolic deficits in PCA and AD are not related to amyloid distribution. Neurology. 2011 doi: 10.1212/WNL.0b013e31821cccad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Dagerman KS, Higgins JP, McShane R. Lack of evidence for the efficacy of memantine in mild Alzheimer disease. Arch Neurol. 2011a;68(8):991–998. doi: 10.1001/archneurol.2011.69. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Insel PS, Weiner MW. Treatment with cholinesterase inhibitors and memantine of patients in the Alzheimer’s Disease Neuroimaging Initiative. Arch Neurol. 2011b;68(1):58–66. doi: 10.1001/archneurol.2010.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva DF, Esteves AR, Arduino DM, Oliveira CR, Cardoso SM. Amyloid-beta-Induced Mitochondrial Dysfunction Impairs the Autophagic Lysosomal Pathway in a Tubulin Dependent Pathway. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-110423. [DOI] [PubMed] [Google Scholar]
- Simon AM, Frechilla D, del Rio J. [Perspectives on the amyloid cascade hypothesis of Alzheimer’s disease] Rev Neurol. 2010;50(11):667–675. [PubMed] [Google Scholar]
- Sommer B. Alzheimer’s disease and the amyloid cascade hypothesis: ten years on. Curr Opin Pharmacol. 2002;2(1):87–92. doi: 10.1016/s1471-4892(01)00126-6. [DOI] [PubMed] [Google Scholar]
- Sorbi S, Bird ED, Blass JP. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol. 1983;13(1):72–78. doi: 10.1002/ana.410130116. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr., Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spulber G, Niskanen E, Macdonald S, Smilovici O, Chen K, Reiman EM, Jauhiainen AM, Hallikainen M, Tervo S, Wahlund LO, Vanninen R, Kivipelto M, Soininen H. Whole brain atrophy rate predicts progression from MCI to Alzheimer’s disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci. 2004;24(36):7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J Neurosci Res. 2007;85(15):3416–3428. doi: 10.1002/jnr.21167. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20(Suppl 2):S265–279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63(1):8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218(2):308–315. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuma K, Yao J, Huang J, Xu H, Chen X, Luddy J, Trillat AC, Stern DM, Arancio O, Yan SS. ABAD enhances Abeta-induced cell stress via mitochondrial dysfunction. FASEB J. 2005;19(6):597–598. doi: 10.1096/fj.04-2582fje. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Vandeerstichele H, Korecka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter WZ, Weiner MW, Jack CR, Jr., Jagust W, Toga AW, Lee VM, Shaw LM. Update on the biomarker core of the Alzheimer’s Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6(3):230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushina E, McMurray CT. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145(4):1233–1248. doi: 10.1016/j.neuroscience.2006.10.056. [DOI] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(41):17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, Ahern GL, Baxter L, Alexander G, Walker DG, Reiman EM. Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion. 2006;6(6):323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrivel KS, Thinakaran G. Membrane rafts in Alzheimer’s disease beta-amyloid production. Biochim Biophys Acta. 2010;1801(8):860–867. doi: 10.1016/j.bbalip.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain N, Fouquet M, Baron JC, Mezenge F, Landeau B, de La Sayette V, Viader F, Eustache F, Desgranges B, Chetelat G. Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain. 2010;133(11):3301–3314. doi: 10.1093/brain/awq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, Dreses-Werringloer U, Zhao H, Davies P, Marambaud P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl 2):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta) deposition. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(41):17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem. 2005;93(4):953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007;130(Pt 7):1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. Biomarkers: warning signs. Nature. 2011;475(7355):S5–7. doi: 10.1038/475S5a. [DOI] [PubMed] [Google Scholar]
- Wollen KA. Alzheimer’s disease: the pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Altern Med Rev. 2010;15(3):223–244. [PubMed] [Google Scholar]
- Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, Dannals RF, Nandi A, Brasic JR, Ye W, Hilton J, Lyketsos C, Kung HF, Joshi AD, Skovronsky DM, Pontecorvo MJ. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18) Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51(6):913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Hamilton RT, Cadenas E, Brinton RD. Decline in mitochondrial bioenergetics and shift to ketogenic profile in brain during reproductive senescence. Biochim Biophys Acta. 2010;1800(10):1121–1126. doi: 10.1016/j.bbagen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106(34):14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KJ, Bennett JP. The mitochondrial secret(ase) of Alzheimer’s disease. J Alzheimers Dis. 2010;20(Suppl 2):S381–400. doi: 10.3233/JAD-2010-100360. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68(1):13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]