Abstract
Purpose
Adoptive transfer of natural killer (NK) cells combined with tumor-specific monoclonal antibodies (mAbs) has therapeutic potential for malignancies. We determined if large numbers of activated NK (aNK) cells can be grown ex vivo from peripheral blood mononuclear cells (PBMC) of children with high-risk neuroblastoma using artificial antigen-presenting cells (aAPC).
Experimental Design
Irradiated K562-derived Clone 9.mbIL21 aAPC were co-cultured with PBMC, and propagated NK cells were characterized with flow cytometry, cytotoxicity assays, Luminex® multi-cytokine assays, and a NOD/SCID mouse model of disseminated neuroblastoma.
Results
Co-culturing patient PBMC with aAPC for 14 days induced 2,363±443-fold expansion of CD56+CD3−CD14− NK cells with 83±4% purity (n=10). Results were similar with PBMC from normal donors (n=5). Expression of DNAM-1, NKG2D, FcγRIII/CD16 and CD56 increased 6±3, 10±2, 21±20, and 18±3-fold respectively on day 14 compared to day 0, demonstrating activation of NK cells. In vitro, aNK cells were highly cytotoxic against neuroblastoma cell lines, and killing was enhanced with GD2-specific monoclonal antibody ch14.18. When mediating cytotoxicity with ch14.18, release of TNFα, GM-CSF, IFNγ, sCD40L, CCL2/MCP-1, CXCL9/MIG, and CXCL11/I-TAC by aNK cells increased 4-, 5- 6-, 15-, 265-, 917- and 363-fold (151 to 9,121 pg/mL), respectively, compared to aNK cells alone. Survival of NOD/SCID mice bearing disseminated neuroblastoma improved when treated with thawed and immediately intravenously infused cryopreserved aNK cells compared to un-treated mice and was further improved when ch14.18 was added.
Conclusion
Propagation of large numbers of aNK cells that maintain potent anti-neuroblastoma activities when cryopreserved supports clinical testing of adoptive cell therapy with ch14.18.
Keywords: Immunotherapy, Neuroblastoma, Natural killer cells, Antibody, Artificial antigen-presenting cells
INTRODUCTION
Although outcome has steadily improved over the past 20 years for patients with high-risk, metastatic neuroblastoma (NBL), long-term event-free survival (EFS) is still only 45% (1–3). Addition of immunotherapy with anti-tumor cell disialoganglioside (GD2) monoclonal antibody ch14.18 along with IL-2 and GM-CSF to 13-cis retinoic acid improves EFS and overall survival for children who have a clinical response after induction chemotherapy and myeloablative consolidation therapy (3). However, 40% of patients still develop disease progression or relapse during or after immunotherapy (3).
Monoclonal antibody (mAb) immunotherapy of residual disease may be further enhanced by improving the ability of natural killer (NK) cells to function in antibody dependent cell-mediated cytotoxicity (ADCC) and to secrete anti-tumor cytokines and chemokines. Strategies include modifying monoclonal antibodies to have high affinity interaction with NK cell FcγRIII/CD16 or to deliver cytokines via mAb-cytokine fusion proteins to the tumor microenvironment (4), enhancing activation of NK cells in vivo using immune modulating drugs such as lenalidomide (5, 6), or growing and activating NK cells ex vivo for adoptive cell therapy. Some of these approaches also may be combined with cytotoxic chemotherapy or targeted therapy for more effective treatment of measurable disease.
Adoptive cell therapy with NK cells alone or combined with mAbs has therapeutic potential for a wide variety of human malignancies, including neuroblastoma (7). One approach for obtaining NK cells has been to harvest large numbers of peripheral blood lymphocytes by leukapheresis, deplete allogeneic T cells, and activate the remaining NK cells with IL-2 before re-infusion. In this manner, haploidentical NK cell therapy for acute myelogenous leukemia attained remission in poor-prognosis adults (8) and maintained remission in children (9). A second method is to grow NK cells ex vivo (10–14), but clinical testing of such NK cells has been limited due to the inability to obtain large numbers of pure NK cells that do not senesce after replication (15, 16).
We recently genetically engineered K562 cells that co-express CD64/FcγRI, CD86/B7-2, CD137L/4-1BBL, truncated CD19, and membrane-bound IL-21 (K562 Clone 9.mbIL21) to serve as artificial antigen-presenting cells (aAPC) promoting sustained ex vivo proliferation of human NK cells (17, 18). The responding NK cells have a significant increase in telomere length compared to freshly isolated NK cells, which may explain their sustained proliferation (18). With this method, large numbers of activated NK cells (aNK) can be generated from normal adult donors with high purity and functionality.
In this study, we show that K562 Clone 9.mbIL21 cells allow the generation of large numbers of NK cells exhibiting activation characteristics from Peripheral Blood Mononuclear Cells (PBMC) of children with high-risk neuroblastoma. These aNK cells are highly cytotoxic alone or with mAb ch14.18 against multi-drug sensitive and resistant neuroblastoma cell lines in vitro and secrete an array of cytokines and chemokines with anti-tumor potential while mediating ADCC. These aNK cells maintain their functional activities after viable cryopreservation, and, most importantly, retain potent anti-tumor activity with ch14.18 when intravenously infused immediately after thawing into NOD/SCID mice with disseminated human neuroblastoma.
MATERIALS AND METHODS
Cell lines
NBL cell lines CHLA-255 and CHLA-136 were maintained in Iscove's Modified Dulbecco's Media (IMDM) with 20% fetal bovine serum (FBS, Invitrogen), and LA-N-1 was maintained in RPMI 1640 (Mediatech) with 10% FBS. CHLA-255-Fluc cells were transduced with the firefly luciferase (Fluc) gene (CHLA-255-Fluc) using a lenti-virus vector (19). CHLA-255-Fluc is sensitive to etoposide and melphalan in vitro whereas CHLA-136 and LA-N-1 are resistant to etoposide and melphalan (resistance: IC90 >1,000 ng/mL and >10,000 ng/mL for etoposide and melphalan, respectively) [Dr. Nino Keshelava, personal communication and (20–22)]. The K562 Clone 9.mbIL21 cell line was grown in RPMI 1640 with 10% FBS (17, 18).
Preparation of peripheral blood mononuclear cells (PBMC)
Peripheral blood was obtained from 10 patients with high-risk neuroblastoma and 5 healthy adults, and PBMC were isolated by density separation using Histopaque®-1077 (Sigma-Aldrich) (23). Written informed consent was obtained from healthy donors in accordance with a protocol approved by the Committee on Clinical Investigation at Children’s Hospital Los Angeles for the use of cells for cancer and/or blood research. Anonymous specimens from patients with high-risk, stage 4 (metastatic) neuroblastoma were obtained from patients enrolled and consented in therapeutic and biology protocols of the Children’s Oncology Group (COG).
NK cell propagation and activation
K562 Clone 9.mbIL21 cells (clinical-grade master cell bank designated CJLCKT64.86.41BBL.CD19. mbIL21) were derived from Clone 9 cells (generated with Dr. Carl June, University of Pennsylvania) at MD Anderson Cancer Center using the Sleeping Beauty transposon/transposase system to express a membrane-bound variant of IL-21 (18). Before initiating co-cultures of K562 Clone 9.mbIL-21 aAPC and PBMC on day 0, the aAPC were irradiated with 100 Gy using a gamma irradiator, washed with phosphate buffered saline (PBS), and re-suspended in NK cell expansion medium (NKEM) containing RPMI 1640 and 10% FBS with 50 IU/mL recombinant human IL-2 (PeproTech) (addition of at least 20 IU/mL IL-2 to the medium was necessary to induce robust NK cell growth). PBMC (5×106) from normal donors were incubated with aAPC (2.5×106) in T25 flasks (Corning, 25 cm2), while PBMC (106) from neuroblastoma patients were incubated with aAPC (0.5×106) in 6-well tissue culture plates (Corning, 9.5 cm2), both in NKEM at a total cell concentration of 0.5×106/mL. An equal-volume of fresh NKEM was added on day 3. At day 7 of co-culture, cells were counted, new irradiated aAPC were added (total cell:aAPC ratio = 2:1), and cells were seeded into T75 or T150 flasks with additional NKEM (total cell concentration ≤0.5×106/mL). An equal-volume of fresh NKEM was added on day 11. Cells were grown for 14 days, at which time they were phenotyped by flow cytometry and tested for cytotoxicity. The remainder were aliquoted and viably frozen in a mixture of 50% Cryoprotective Medium (Lonza), 25% RPMI-1640, and 25% FBS.
Flow cytometry
Surface marker staining was performed as previously described (24). Briefly, cells were washed twice in FACS buffer (PBS with 0.1% NaN3 and 0.1% bovine serum albumin) and centrifuged for 10 minutes at 400×g. Antibodies listed in Supplemental Table 1 were added in the dark at 4 °C using concentrations previously determined by titration. Isotype-matched irrelevant mAbs were used to define non-specific staining. Cells were incubated at 4 °C for 90 minutes and washed twice in FACS buffer. Flow cytometry analysis was performed using a BD LSR II flow cytometer, DIVA software (BD Biosciences) and FlowJo analysis software (Tree Star). The mean fluorescence intensity (MFI) ratio was calculated as follows: MFI of viable cells stained with specific antibody / MFI of viable cells stained with an isotype-matched irrelevant antibody.
In vitro cytotoxicity assays
NK cells that had been grown for 14 days were seeded into 96-well Costar black tissue culture plates (Corning) in 0.1 mL RPMI 1640 with 50 IU/mL IL-2 and 2% FBS. Three neuroblastoma cell lines (LA-N-1, CHLA-136 and CHLA-255-Fluc) were labeled with calcein-AM (5 µg/106 cells) for 30 minutes (25). Neuroblastoma cells were added to aNK cells at various effector-to-target (E:T) ratios in IMDM for CHLA-255-Fluc and CHLA-136 or RPMI 1640 for LA-N-1 with 2% FBS and without or with anti-GD2 mAb ch14.18 (0.1 μg/mL; provided by the National Cancer Institute-Frederick). Cells were then co-incubated for 6 hours at 37°C, and live cells, which contain calcein-AM, were quantified with digital imaging microscopy system (DIMSCAN) as previously described (25).
Cytometric bead array (CBA), and Luminex® assays
NK cells were grown for 14 days, cryopreserved, thawed and cultured in NKEM for 72 hours prior to generating conditioned media by co-culturing aNK cells with CHLA-255-Fluc or CHLA-136 tumor cell lines (aNK:tumor cells = 1:1) without or with ch14.18 (0.1 μg/mL) for 24 hours. Conditioned media were examined for granzymes A and B using the CBA assay from BD Biosciences and for cytokines and chemokines using the 39-, 23- and 9-plex Human Cytokine/Chemokine Panels from Millipore according to the manufacturer’s protocols. For granzyme analyses, data were collected on an LSRII flow cytometer using DIVA software (BD Biosciences). FCAP software (BD Biosciences) was used to fit standard curves to the data obtained from the analyte standards and to calculate absolute concentration values for each analyte from its respective standard curve. In the Luminex® assay, data were acquired with a Luminex®-200 instrument (Luminex® Corporation). Cytokine concentrations were determined by referring to a standard curve and expressed as pg/mL using xPonent software (Luminex® Corporation).
Murine model of disseminated neuroblastoma
Non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice were purchased from the Jackson Laboratory. Rat anti-mouse CD122 (200 µg/mouse) was injected intra-peritoneal one day prior to tumor cell injection and then every other week to eliminate residual murine NK cells. CHLA-255-Fluc cells were injected intravenously on day 0. Multiple intravenous injections of expanded aNK cells were given together with intravenous IL-2 and without or with ch14.18 as described in Results and Figure Legends. Tumor growth was assessed weekly by bioluminescence imaging 15 minutes after intra-peritoneal injection of a D-luciferin potassium salt solution (1.5 mg/mouse) using a Xenogen IVIS-200 system (Caliper Life Sciences). Photons emitted were quantified with the Living Image 3.0 software (Caliper Life Sciences). Animal experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Usage Committee of Children’s Hospital Los Angeles.
Statistical analysis
Data were analyzed using the statistical software Stata (version 11) and are represented as means ± standard deviation unless otherwise stated. ANOVA was performed to determine the significance of observed differences. Mouse survival time was defined as the length of time (in days) from the tumor injection date until the end of the study or time of sacrifice due to disease progression. Censored normal regression was utilized to examine whether any difference in survival time existed due to varying treatments. The censored Wilcoxon test was used to examine the difference in the survival curves among the different treatment groups. A P value of < 0.05 was considered statistically significant.
RESULTS
Propagation of NK cells
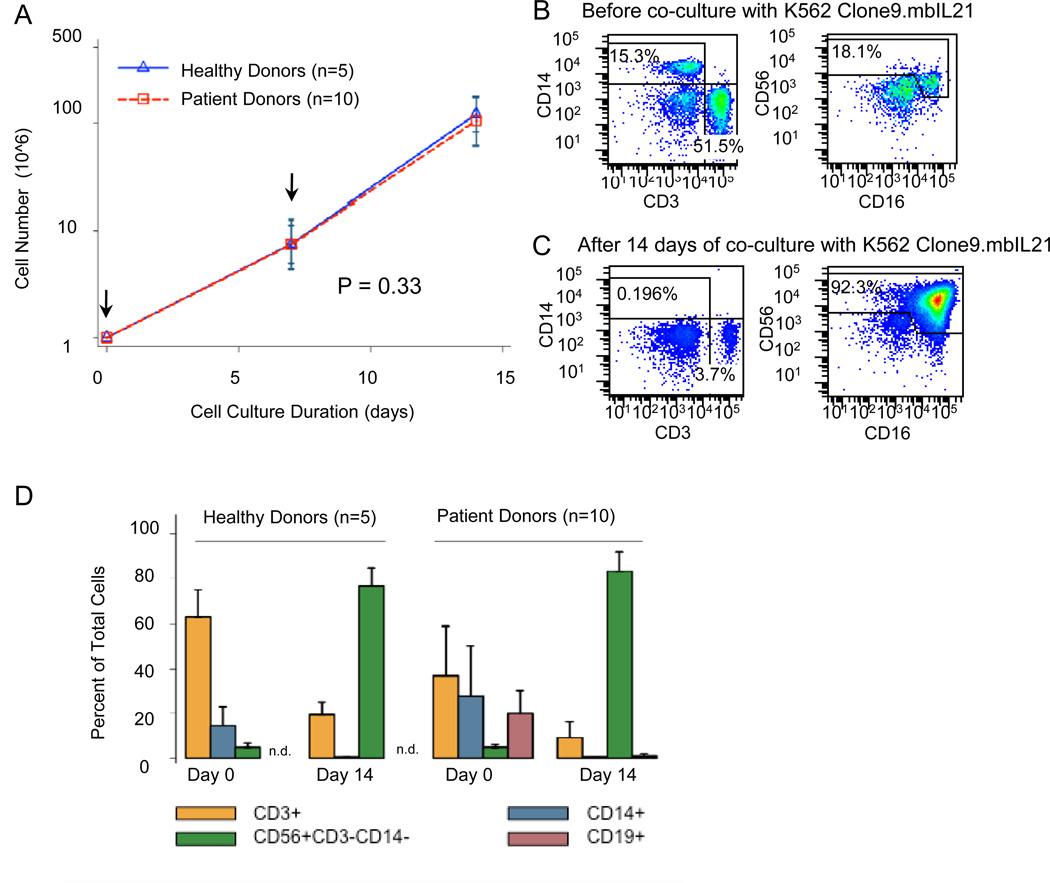
PBMC from 10 children with high-risk neuroblastoma and from 5 normal adults were cultured with K562-derived aAPC and IL-2 (Figure 1). Total cell number in cultures of PBMC from 10 neuroblastoma patients increased by a mean of 116-fold (range, 41–200 fold), and CD56+CD3− NK cells increased by a mean of 2,363-fold (range, 600–6,362 fold) by day 14 of co-culture. This growth was similar in cultures from 5 healthy adult donors, with a mean of 126-fold (range, 77–175) increase in total cells and 2,593-fold (range, 1051–5,606) increase in NK cells (Figure 1A). The doubling time for NK cells from patients was 1.24 days and from normal donors was 1.25 days. Propagation of the effector cells could be prolonged for at least 28 days with an additional 40-fold increase in total cell number compared to day 14 (data not shown). Final cultures from patients had an average of 83.2 ± 2.8% CD56+CD3− NK cells and 9.1 ± 2.2% CD3+ T cells of which 6.3 ± 2.1% were TCRγδ+ T cells. For normal donors, the final product had an average of 76.6 ± 3.4% CD56+CD3− NK cells and 19.4% ± 2.5% CD3+ T cells (TCRγδ+ T cells were not evaluated) (Figure 1B-D).
Figure 1.
Propagation of NK cells from PBMC of patient and normal donors by co-culture with gamma-irradiated K562 Clone 9.mbIL21 aAPC (total cells: aAPC ratio = 2:1) and 50 IU/mL IL-2 (A) Total cell growth curves for 5 normal donors and 10 neuroblastoma patients (see Materials and Methods for cell culture details; arrows indicate the days when aAPC were added to cultures). Recovery of viable cells after 7 and 14 days of co-culture with aAPC was determined by trypan blue exclusion. The slopes of the total viable cell growth curves for patients and healthy donors were identical (p=0.33). (B) and (C) Representative immunophenotyping results for a patient’s PBMC before (B) and after 14 days (C) co-culture with aAPC supplemented with 50 IU/mL IL-2. Aliquots of day 0 and 14 cells were viably frozen and then thawed for analysis on the same day. (D) Mean and standard deviation of immunophenotyping data from normal donors (n=5) and patients (n=10). CD19 was not included in the analysis of specimens from normal donors on days 0 and 14 (n.d., not done).
The ability of K562-derived aAPC to selectively propagate NK cells was demonstrated by analyses of the hematopoietic cell subpopulations on day 0 and then on day 14 of co-culture. NK-cell frequency in PBMC from patients and normal donors on day 0 was similar at 4.7 ± 0.5% and 4.7 ± 0.9%, respectively. Differences between groups were observed for CD3+ T cells (36.9 ± 6.9% for patients and 63.4 ± 5.2% for normal donors) and CD14+ monocytes (27.6 ± 7.2% for patients and 14.5 ± 3. 7% for normal donors) (P<0.0001; Figure 1D). Specimens from patients were anonymous, and so it is not possible to correlate clinical variables such as disease status and treatment with PBMC subsets. At day 14, large decreases in CD3+ and CD14+ cells were observed in both groups (Figure 1D). Additional analyses performed only on cells from patients demonstrated a decrease in CD4+CD3+ T cells, CD8+CD3+ T cells, CD4+CD25+CD3+ T cells, 6B11+CD3+ invariant NKT cells, CD14+ monocytes, and CD19+ B lymphocytes at day 14 (0.2 ± 0.2%, 2.4 ± 0.4%, 0.05 ± 0.02%, 0.07 ± 0.01%, 0.2 ± 0.1%, and 0.6 ± 0.4%, respectively) compared to day 0 (24.0 ± 5.3%, 12.1 ± 3.1%, 0.2 ± 0.1%, 0.2 ± 0.1%, 27.6 ± 7.2%, and 19.9 ± 3.2% respectively). The difference in the cell frequency of all cell types over time was significant (P<0.01).
Expression of cell surface immune-function markers by aNK cells
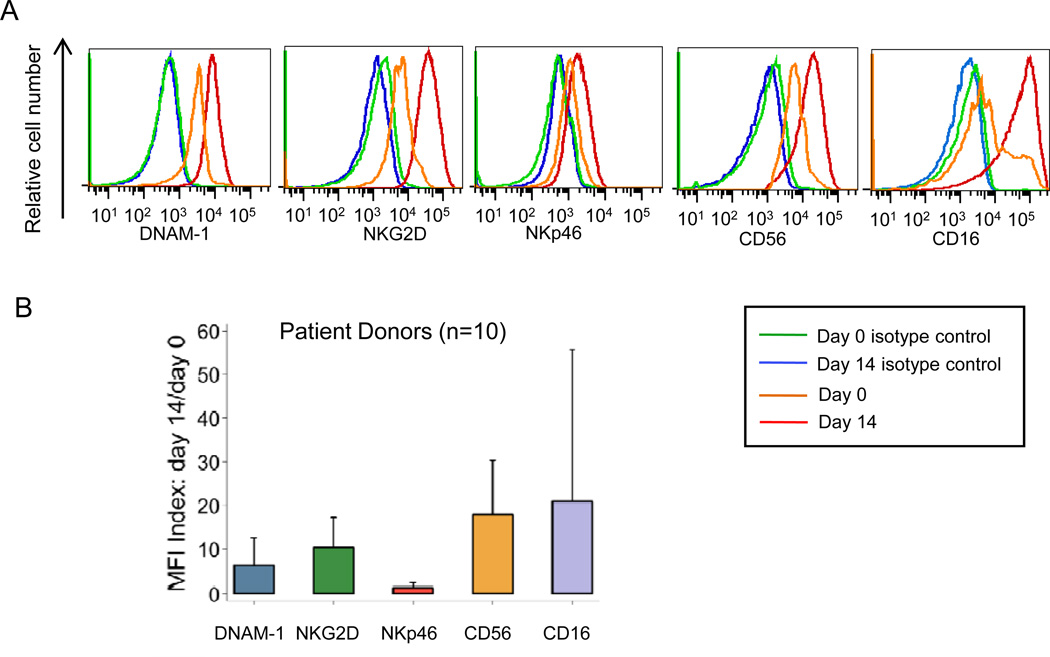
Expression of natural cytotoxicity receptors DNAM-1, NKG2D, and NKp46, the degranulation marker CD107a/LAMP1, the adhesion molecule CD56, the chemokine receptor CXCR4, and Fc receptors CD16, CD32, and CD64 was quantified by flow cytometry for NK cells from 10 patients before (day 0) and after K562-aAPC-stimulated expansion (day 14) (Figure 2). The MFI ratio for DNAM-1, NKG2D, CD16, and CD56 increased by 6.2 ± 3.2-, 10.3 ± 2.4-, 20.9 ±19.7-, and 17.8 ± 2.9-fold, respectively. On average, there was little or no difference in NKp46, CD107a, CXCR4, CD32, and CD64 expression between NK cells at days 0 and 14.
Figure 2.
Expression of activation markers and receptors on NK cells (CD56medCD16+CD3−CD14− and CD56highCD16−CD3−CD14−) from neuroblastoma patients before and after 14 days of co-culture with aAPC plus 50 IU/mL IL-2. (A) Histogram overlays show expression of natural cytotoxicity receptors (DNAM-1, NKG2D, and NKp46), adhesion molecule (CD56), and FcγRIII receptor (CD16). Results are representative of experiments testing PBMCs from ten patients. CD3+CD14+and CD19+ cells were excluded from the analysis electronically. (B) Relative expression levels of markers and receptors on NK cells before and after 14 days of co-culture. Mean and standard deviation of ratios of mean fluorescence intensity (MFI) ratios of NK cells on day 14/day 0 from ten NBL patients are shown.
Direct cytotoxicity and ADCC by expanded aNK cells
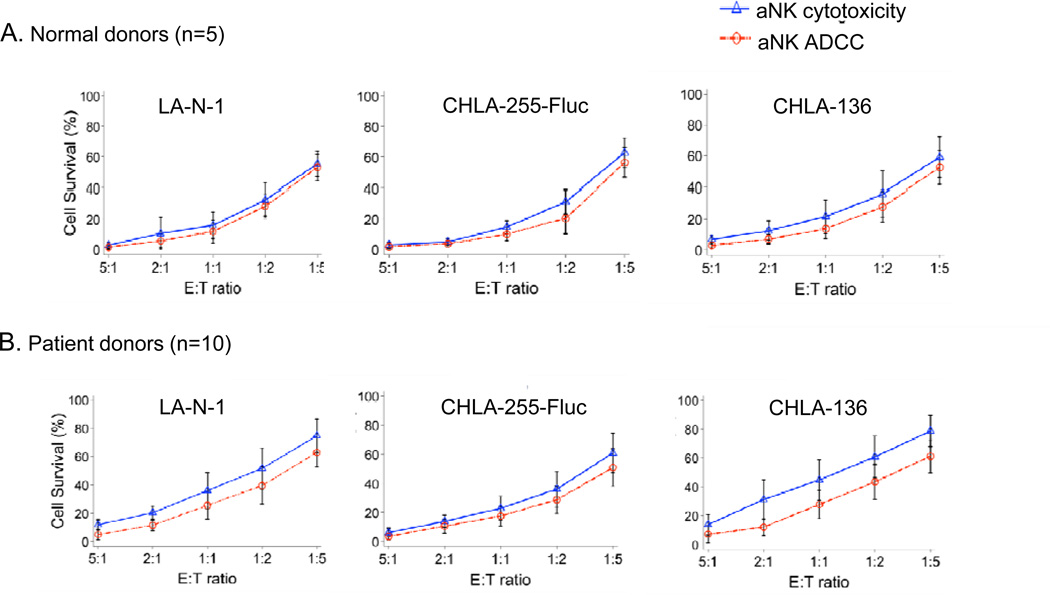
The cytotoxicity of aNK cells from 10 patients with neuroblastoma and 5 normal donors was tested against the neuroblastoma cell lines CHLA-255-Fluc (drug sensitive), LA-N-1 (mulit-drug resistant), and CHLA-136 (multi-drug resistant) (20–22) after a 6 hour-incubation using the calcein-AM assay (Figure 3) (25). Both multi-drug sensitive and resistant cell lines were sensitive to aNK cell direct cytotoxicity and to ch14.18-mediated ADCC with significant killing occurring at 1:1, 1:2, and 1:5 aNK:neuroblastoma cell ratios (P<0.001). aNK cell cytotoxicity was greater against all three tumor cell lines when mediating ADCC with ch14.18 (P<0.05 for all). Cytotoxicity mediated by aNK cells from patients and normal donors was similar.
Figure 3.
Anti-neuroblastoma cytotoxicity of effector cells expanded from PBMC after 14-day co-culture with K562 Clone 9.mbIL21 aAPC. Cytotoxicity of aNK alone (aNK cytotoxicity) or combined with anti-GD2 antibody ch14.18 (aNK ADCC) against neuroblastoma cell lines was determined using a 6-hour calcein-AM assay. aNK from 5 healthy donors (A) and 10 neuroblastoma patients (B) were tested at different effector:target (E:T) cell ratios against neuroblastoma cell lines LA-N-1, CHLA-255-Fluc and CHLA-136. Target cell survival (mean ± SD) at the indicated E:T ratios for all experiments are shown (6 replicate wells per condition per experiment). Linear regression was used to compare aNK cytotoxicity and aNK ADCC for the three tumor cell lines. Tumor cell survival for all cell lines was greater as the E:T ratio decreased (P<0.001) and was greater for aNK alone compared to aNK combined with ch14.18 (P=0.03, =0.009, and =0.007 for LA-N-1, CHLA-255-Fluc, and CHLA-136 respectively).
Cytokine secretion and release of granzymes A and B by aNK cells during direct cytotoxicity and ADCC
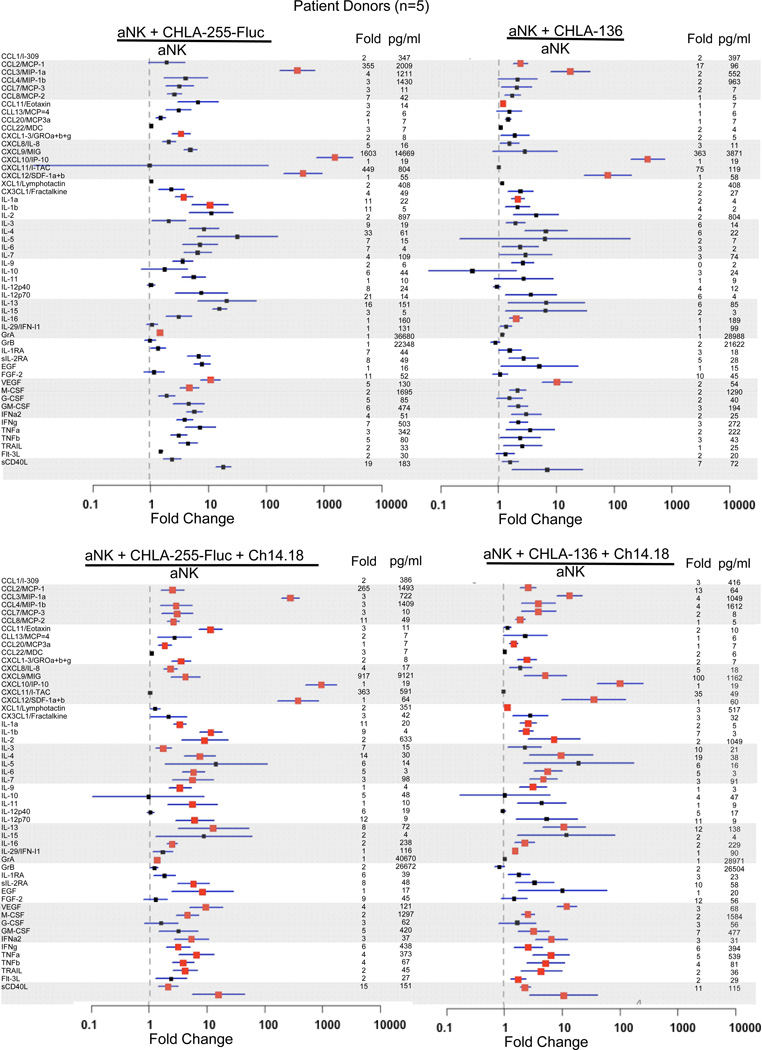
To further determine the potential anti-tumor effects of aNK cells, we evaluated the release of seventy-one cytokines and of granzymes A and B after co-culturing aNK cells from 5 patients with CHLA-255-Fluc (drug sensitive) or CHLA-136 (multi-drug resistant) neuroblastoma cells without or with ch14.18 for 24 hours (Figure 4). In the absence of ch14.18, co-culture of aNK cells and CHLA-255-Fluc or CHLA-136 cells significantly increased 9 and 8 cytokines in the culture media, respectively, compared to aNK cells alone. In the presence of ch14.18, co-culture of aNK cells and CHLA-255-Fluc or CHLA-136 cells significantly increased 36 and 32 cytokines in the culture media, respectively, compared to aNK cells alone (Figure 4). Notably, patient aNK cells co-cultured with CHLA-255-Fluc or CHLA-136 with ch14.18 increased the release of TNFα (4- and 5-fold), GM-CSF (5- and 7-fold), IFNγ (6-fold for each cell line), CCL2/MCP-1 (265- and 13-fold), CXCL9/MIG (917- and 100-fold), CXCL11/I-TAC (363- and 35-fold), FGF2 (9- and 12-fold), and sCD40L (15- and 11-fold) compared to aNK cells alone (Figure 4). In similar experiments, co-culture of aNK cells from 5 normal donors with CHLA-255-Fluc cells without or with ch14.18 significantly increased release of 19 and 11 cytokines, respectively, compared to aNK cells alone (Supplemental Figure 1). Cytokines and chemokines for which at least one-half of the tests were below the level of detection were excluded from these analyses (see listing in Supplemental Table 2). Tumor cells alone or combined with ch14.18 produced a background cytokine/chemokine level of less than 10 pg/ml for each analyte. High levels of granzyme A and B were released by aNK cells from patients and normal donors but were not different for aNK cells alone versus aNK cells combined with tumor cells without or with ch14.18 (Figure 4 and Supplemental Figure 1). Thus, the interaction of aNK cells with neuroblastoma cells significantly affected the release of potential anti-tumor cytokines.
Figure 4.
Cytokine and chemokine release from K562 Clone 9.mbIL21 aAPC-expanded aNK cells after a 24-hour incubation with neuroblastoma cells lines CHLA-255-Fluc and CHLA-136 alone or with anti-GD2 antibody ch14.18. Day 14 expanded aNK cells from 5 neuroblastoma patient donors were thawed and cultured for 72 hours with 50 IU/mL IL-2 prior to co-culture with CHLA255-Fluc or CHLA-136 cells (1:1 E:T ratio, 24 hours) without or with ch14.18, and then supernatants were collected for both Luminex® and the CBA assays. Means and standard deviation of fold changes are shown in dots and lines, respectively; red square dots indicate significant P values (P<0.05) for each comparison versus aNK cells alone. Fold changes and concentration (pg/ml) of cytokines or chemokines secreted by aNK cells exposed to different conditions (numerator in each function) are shown on the right-hand side of each data point.
Anti-tumor activity of cryopreserved aNK cells in a NOD/SCID mouse model of disseminated neuroblastoma
The anti-tumor activity of cryopreserved aNK cells grown with K562-mbIL21 aAPC was tested in vivo using a model of disseminated neuroblastoma in which CHLA-255-Fluc cells are injected intravenously into NOD/SCID mice. Bioluminescent imaging of untreated mice does not detect disease at 7 days but does so in at least 50% at 21 days and 100% at 28 days, and so treatments were begun at 7 or 21 days to model different levels of tumor burden.
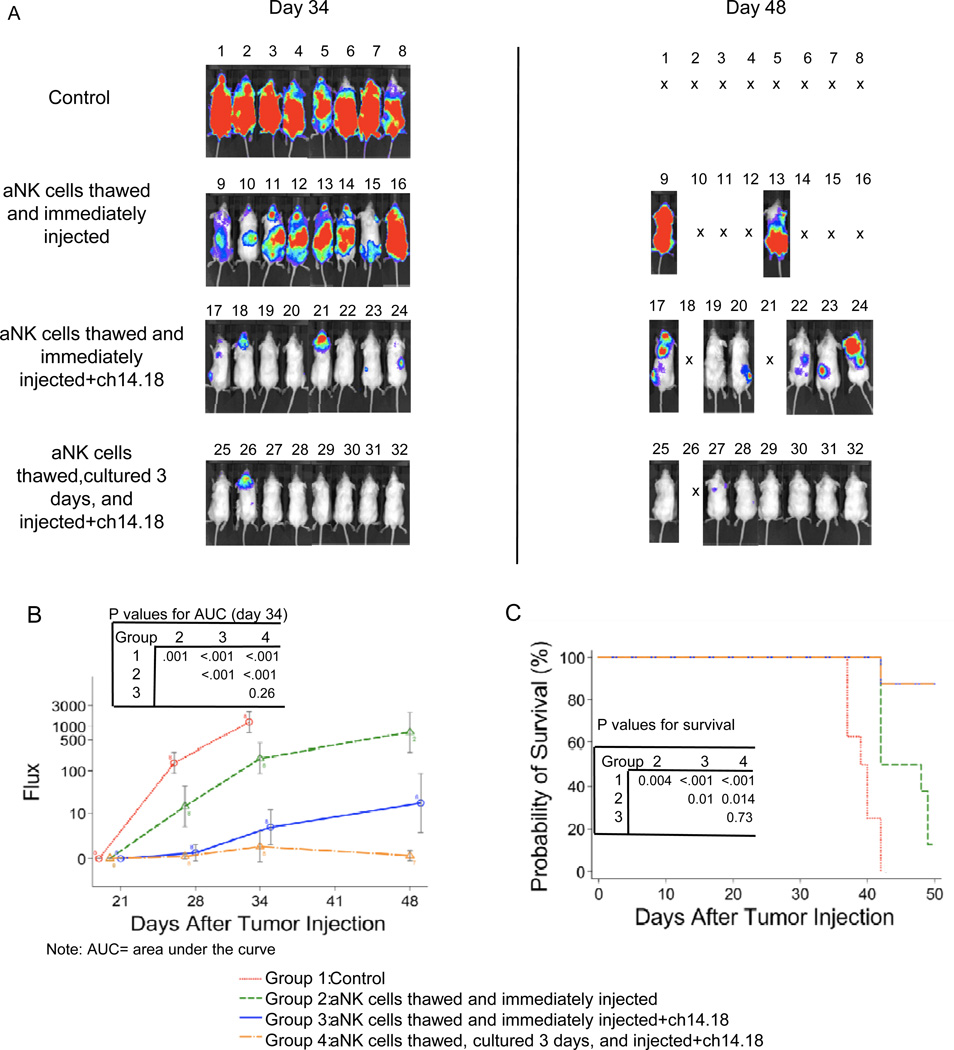
Initial experiments compared aNK cells from single normal donors that were cryopreserved, thawed and either cultured for 3 days before injection or thawed and immediately injected intravenously (Figure 5). Beginning at 7 days, mice were treated weekly for 4 weeks with aNK cells alone or in combination with ch14.18 (107 aNK cells/mouse 1x/week, 3µg IL-2/mouse 2x/week, and 15 µg ch14.18/mouse 2x/week). Tumor growth was reduced and mouse survival was longer among mice receiving any treatment compared to untreated mice. Mice receiving treatment that included ch14.18 had an increased survival time compared to those receiving aNK cells alone (P=0.01). Mice receiving thawed and immediately injected or thawed and cultured aNK cells with ch14.18 had similar tumor growth (P=0.26) and survival (P=0.73). A second experiment using aNK cells from another normal donor with the same schedules and doses of aNK cells, IL-2, and ch14.18 confirmed no difference in efficacy between thawed and cultured vs. thawed and immediately injected aNK cells (Supplemental Figure 2). These results demonstrate that cryopreserved aNK cells infused immediately after thawing retain their anti-tumor functions.
Figure 5.
Anti-tumor activity of K562 Clone 9.mbIL21 aAPC-expanded and cryopreserved aNK cells that were thawed and immediately infused or thawed, cultured, and then infused into NOD/SCID mice with disseminated neuroblastoma. .Cryopreserved effector cells (80% CD56+CD3−CD14− NK) derived from normal donor PBMC were thawed and cultured for 3 days or thawed and immediately injected intravenously into NOD/SCID mice beginning 7 days after they received 106 CHLA-255-Fluc neuroblastoma cells intravenously. All mice receiving effector cells also received IL-2 (3 µg/mouse intravenously, 2x/week). Anti-GD2 mAb ch14.18 (15 µg/mouse intravenously, 2x/week) was given to the indicated groups. (A) Neuroblastoma tumor growth was visualized 34 and 48 days after tumor cell injection using bioluminescence imaging. Neuroblastoma progressed in all 8 untreated mice (control group) who died or were euthanized from days 38 to 43. (B) Signal intensities (total Flux) were detected at the time points shown in control and treated mice and plotted as mean ± SD. Comparison of Area Under the Curve (AUC) for the groups showed significant differences, with the exception of thawed and immediately injected aNK + ch14.18 versus thawed, cultured and injected aNK + ch14.18. (C) Overall survival curves for all treatment groups were generated by Kaplan-Meier analysis. Comparison of survival for the three groups showed significant difference, except for thawed and immediately injected aNK + ch14.18 versus thawed, cultured and injected aNK + ch14.18. Note that PBMC were isolated and cryopreserved at Children’s Hospital Los Angeles and shipped frozen to MD Anderson Cancer Center for NK cell growth, cryopreservation, and shipment back to Children’s Hospital for the experiment.
The next experiment compared the impact of frequency and duration of aNK treatment using cells from a single patient donor that were cryopreserved, thawed, and then immediately injected intravenously (Supplemental Figure 3). aNK cells (107) were injected twice weekly×3 weeks (group 2, aNK alone and group 3, aNK with ch14.18) or once weekly×6 weeks (group 4, aNK with ch14.18) beginning at day 7. IL-2 (3µg/mouse, 4x or 2x/week) and ch14.18 (15 µg/mouse, 4x or 2x/week) were given in the same weeks as aNK cell infusions. Tumor growth in untreated mice was significantly greater than that of all three treated groups (P< 0.001) (Supplemental Figure 3B). Tumor growth of treatment groups with or without ch14.18 also were significantly different (P< 0.001). Tumor growth of treatment groups receiving 2x/week or 1x/week aNK with ch14.18 was significantly different up to day 62 after tumor cell injection (P=0.006) but not afterwards (P=0.49), and so overall there was no difference (P=0.10). For mice treated with aNK and ch14.18, 3 of 10 in the 2x/week group and 1 of 10 in the 1x/week group had no detectable tumor by imaging at day 83 (55 and 34 days after the last treatment). With respect to survival, the untreated group was significantly worse than all treatment groups (P< 0.001). Survival of the two groups receiving aNK and ch14.18 was significantly better than that of the group receiving aNK alone (P<0.0001 and P=0.0002) (Supplemental Figure 3C). Survival of mice receiving aNK cells and ch14.18 2x/week was better than that of mice receiving aNK and ch14.18 1x/week (P=0.038). These results confirm in vivo the anti-neuroblastoma activity of aNK cells that were cryopreserved, thawed, and then immediately infused with ch14.18 and suggest a modest effect of treatment schedule.
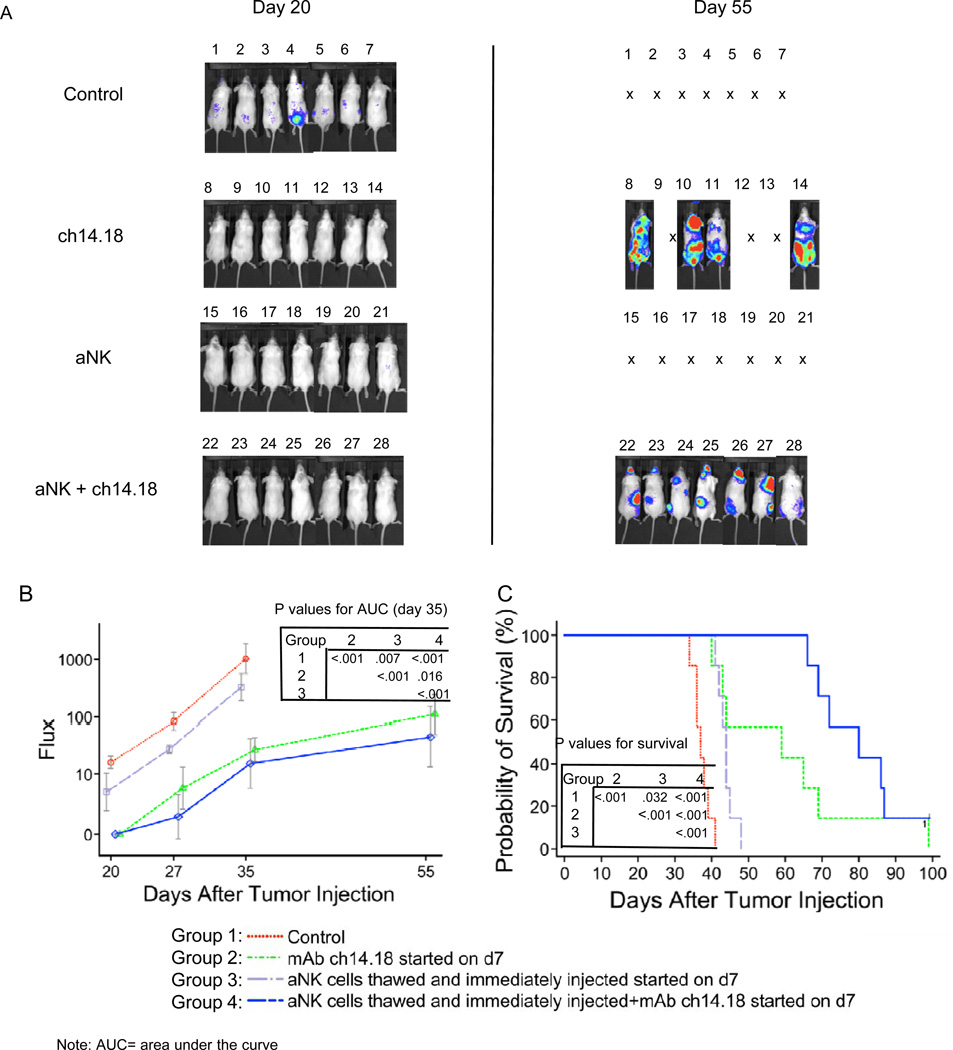
The last experiment compared different treatments (aNK alone, ch14.18 alone, and aNK combined with ch14.18) beginning at day 7 or 21 when disease was not or was detectible by imaging (Figure 6 and Supplemental Figure 4). Groups of mice received cryopreserved aNK cells (107) from a single normal donor twice weekly×4 weeks with IL-2 (3µg/mouse, 2x/week) and with or without ch14.18 (15 µg/mouse, 2x/week). Two other groups received ch14.18 alone in the same schedule and dose. Beginning treatment at day 7 with NK cells alone was associated with decreased tumor growth (p=0.007) and increased survival (p=0.032) when compared to untreated control mice (Figure 6). Treatment with ch14.18 alone caused a further decrease in tumor growth (p< 0.001) and increase in survival (p< 0.001). Tumor growth was most reduced and survival most increased when treatment included both ch14.18 and NK cells, and this combination was more effective than either NK cells (p< 0.001) or ch14.18 (p< 0.001) alone. Even when treatment was begun 21 days after tumor cell injection (Supplemental Figure 4), when disseminated disease was visualized in all mice, tumor growth was less after two weeks of treatment with NK and ch14.18 together than after no treatment (p<0.001), NK cells alone (p=0.003), or ch14.18 alone (p=0.039). Survival was greater after treatment with NK and ch14.18 than no treatment (p=0.004), marginally better than NK cells alone (p=0.086), and equivalent to ch14.18 alone (p=0.297). All treatments were more effective when begun on day 7 compared to day 21 (2-way ANOVA, P=0.009), and tumor growth was inhibited by the combination of aNK with ch14.18 regardless of when treatment was initiated.
Figure 6.
Anti-tumor activity of K562 Clone 9.mbIL21 aAPC-expanded and cryopreserved aNK cells when treatment was begun 7 days (early treatment) after tumor cell injection. Effector cells (77% CD56+CD3−CD14− NK) derived from a normal donor PBMC after 21 days of culture were cryopreserved and then thawed and immediately injected through the tail vein into NOD/SCID mice that had received 106 CHLA-255-Fluc neuroblastoma cells intravenously 7 days previously. All mice receiving effector cells also received IL-2 (3 µg /mouse intravenously, 2x/week) with each aNK injection. Anti-GD2 mAb ch14.18 (15 µg/mouse intravenously, 2x/week) for each aNK injection was given to indicated groups. (A) Neuroblastoma cell growth was visualized 20 and 55 days after tumor cell injection using bioluminescence imaging. Neuroblastoma progressed in all 7 untreated control mice who died or were euthanized from days 35 to 42. (B) Signal intensities (total Flux) were detected at the time-points shown in control and treated mice and plotted as mean ± SD. Comparison of AUCs demonstrated that all treatments had an anti-tumor effect with aNK cells combined with ch14.18 having the greatest effect as shown in the see inset table. (C) Survival curves for all groups were generated by Kaplan-Meier analysis. All treatments significantly improved survival with aNK cells combined with ch14.18 being the most effective as shown in the inset table. The surviving mouse in this group did not have detectible disease at day 100 but did so at days 35 and 55.
DISCUSSION
Repeated infusions of activated NK cells and anti-tumor antibodies may provide an effective strategy for treating minimal residual disease and possibly measureable disease when combined with cytotoxic therapy. Somanchi et al. and Denman et al. reported a new method to efficiently grow large numbers of aNK cells ex vivo using K562 Clone 9.mbIL21 cells as aAPC (14, 18). In their study, the number of NK cells from PBMC of normal donors increased by a mean of 47,967-fold in 21 days, had a marked increase in telomere length after stimulation, and did not senesce, even after six weeks of culture (17, 18). Using the same method, we show for the first time that large numbers of aNK cells can be grown from PBMC of patients with high-risk neuroblastoma. These aNK cells alone and with anti-GD2 mAb ch14.18 are highly cytotoxic and secrete multiple cytokines with anti-tumor potential when cultured with multi-drug sensitive and resistant neuroblastoma cell lines. Importantly, these aNK cells retain anti-tumor function(s) in vivo after cryopreservation. These results provide a model for clinical testing of adoptive cell therapy with activated autologous NK cells and anti-GD2 mAb ch14.18.
While a number of strategies are possible for generating human NK cells for adoptive cell therapy, the low cell number available for adoptive transfer has limited clinical testing of this immunotherapeutic strategy (7). Leukapheresis, T cell depletion, and short-term culture in IL-2 can provide haploidentical or autologous NK cells, but rarely in sufficient quantity for more than a single infusion (8, 9, 26, 27). Culture of T cell depleted products from patients with melanoma for approximately 21 days in IL-2 resulted in 278- to 1097-fold expansion of autologous NK cells, and re-infused NK cells were demonstrated to circulate for at least one week without causing tumor regression (14). In another clinical study, allogeneic NK cells from related donors were expanded ex vivo 3- to 131-fold with hydrocortisone and IL-15 and infused to treat patients with advanced non-small cell lung cancer (28). Other methods for ex vivo expansion have been reported from pre-clinical studies. NK cells from normal donors cultured in defined medium with IL-2 expanded a median of 193-fold (range, 21- to 277-fold) (29) and from patients with multiple myeloma a mean of 1625-fold (range, 502- to 2658-fold) (30). A mixture of IL-7, SCF, IL-2, and IL-15 in defined medium stimulated a 3-log increase in NK cells from cord blood (11). aAPC have been genetically engineered and used with or without additional cytokines to stimulate expansion. Addition of IL-2 to K562 cells that were modified to express membrane-bound IL-15 and 41BB ligand stimulated a mean of 277-fold increase (range, 201- to 1459-fold) in NK cells from normal donors and a similar level from patients with acute leukemia (12). KT64.41BBL.A2 cells, which naturally express IL15Ra and MICA/B, stimulated a 1000-fold increase of NK cells from blood of normal donors (13). K562-MICA-41BBL-IL-15 cells stimulated the expansion of NK cells by a mean of 550-fold (range, 201–880) (31).
Using K562 Clone 9.mbIL21 cells and added IL-2 (17) (18), we show a mean of 2363-fold (range, 600- to 6362-fold) expansion of NK cells from patients with high-risk neuroblastoma at day 14, with 83% of the total cells being CD56+CD3neg NK cells. In contrast to other methods, telomere length in NK cells significantly increases compared to fresh NK cells with this method (18), which allows culturing for at least 28 days to obtain an even greater number of aNK cells. This positive effect on telomere length is likely due to IL-21 activation of STAT3 (32) that in turn regulates human telomerase reverse transcriptase (hTERT) expression.(33) Importantly, growth of NK cells from patients and normal donors was equivalent, which strongly supports the feasibility of preparing autologous aNK cells for therapy. Without T cell depletion, less than 20% of the total population at day 14 was T cells, and most of these were TCRγδ+ T cells. Because K562 Clone 9.mbIL21 cells are lethally irradiated before culture and are lysed by the expanding NK cells, the risk of infusing viable K562 Clone 9.mbIL21 cells to patients is negligible. Thus, we anticipate that it will be possible to generate billions of aNK cells from peripheral blood of children with neuroblastoma for multiple infusion treatments, without the need for apheresis.
Generation of activated CD56+CD3− NK cells from PBMC of both patients and normal donors with K562 Clone 9.mbIL21 cells and IL-2 is highly reproducible. More than 80% of cells are CD56+CD3− NK cells, and they display an activated phenotype as shown by more than a 6-fold increase in the MFI ratio for NKG2D, DNAM-1, CD16, and CD56 at day 14 compared to day 0. NK cells generated from PBMC of patient and normal donors are highly cytotoxic against chemotherapy sensitive and resistant neuroblastoma cell lines when alone and even more so with mAb ch14.18. Remarkably, only approximately 20% of tumor cells survived ADCC at a 1:1 aNK : tumor cell ratio after 6 hours. Our previous study showed increased expression of inhibitory KIR2DL2/3 but not of inhibitory KIR2DL1, NKG2A, or NKG2C on expanded compared to fresh NK cells.(18) NKG2A was expressed by 85% of expanded NK cells but the other receptors were expressed by only 15% to 30%. Expanded NK cells killed 721.221 cell line targets expressing HLA group C1 or Bw4, which are ligands for inhibitory KIR2DL2/3 or KIR3DL1 respectively, equally as well as the parent cell line without these ligands.(18) Altogether, our results are similar to earlier reports with IL-2 alone or in combination with other cytokines, except that a much larger number of NK cells are obtained with the K562 Clone 9.mbIL21 model (11, 12, 34).
We demonstrated that a complex array of cytokines and chemokines are released in vitro upon aNK cell interaction with drug sensitive and resistant tumor cell lines, especially during ADCC mediated by ch14.18. Although it is not possible to relate these data to what might occur in vivo, on balance, the pattern suggests an anti-tumor effect. Levels of TNFα, GM-CSF, IFNγ, sCD40L, CCL2/MCP-1, CXCL9/MIG, and CXCL11/I-TAC increased 4-, 5- 6-, 15-, 265-, 917- and 363-fold with concentrations ranging from 151–9121 pg/ml. However, cytokines that are important for NK cell proliferation and activation, IL-12p40, IL-12p70, and IL-15 were present at low levels (<25 pg/ml). IFNγ induces CXCL9 and CXCL11 that recruit T cells and NK cells and have angiostatic properties (35). CD40 ligand (CD40L) is essential for the initiation of antigen-specific T cell responses, and activation of the CD40 pathway by sCD40L may contribute to antitumor immune responses. sCD40L also may have direct effects upon tumor cell proliferation and survival (36, 37). Since the increased release of these cytokines was only achieved when aNK cells interacted with neuroblastoma cells, especially via mAb ch14.18, quantification of cytokines in blood may provide useful indicators of aNK-tumor cell interactions in vivo.
The use of autologous aNK cells for expansion will likely prevent host reactions against the adoptively-transferred cells that may occur with allogeneic aNK cells, potentially resulting in impaired survival, migration, and function. Although there is a possibility that autologous aNK anti-tumor function could be suppressed by KIR inhibitory receptor/HLA class I molecule interactions as has been suggested from reviews of patients undergoing myeloablative therapy and autologous hematopoietic stem cell transplantation (38) and of patients treated with an anti-GD2/IL-2 fusion protein (39), available in vitro data suggest that highly activated NK cells are not impacted by these interactions (13, 18). Furthermore, 60% of individuals have NK cells that express KIRs but do not express the cognate HLA class I ligands for the KIRs (missing KIR ligand) (40, 41), and these NK cells can kill neuroblastoma cells with anti-GD2 mAb (42). Finally, neuroblastoma cells often do not express surface HLA class I molecules, the ligands for inhibitory KIR receptors (43–47). To date, the impact of KIR/HLA class I interactions on outcome in patients with neuroblastoma treated with the ch14.18 mAb has not been evaluated (3).
aNK cells that are grown and activated with K562 clone 9.mbIL21 cells are active against disseminated human neuroblastoma cells growing in NOD/SCID mice. Viably cryopreserved aNK cells that were thawed and cultured for three days or thawed and immediately infused intravenously were equally effective when combined with mAb ch14.18 against neuroblastoma. Treatment with aNK cells alone, ch14.18 alone, or the combination of both was more effective when begun at seven days after tumor cell injection before disease could be imaged than when begun at 21 days when disseminated disease was readily imaged. In either setting, aNK combined with ch14.18 was more effective than aNK cells or ch14.18 alone.
The K562 Clone 9.mbIL21 NK cell growth and activation method provides an important advance for generating aNK cells in high numbers, purity, and functionality from PBMC of neuroblastoma patients for use in NK cell-based immunotherapy. Because viably cryopreserved aNK can be thawed and immediately infused into patients, it will be feasible to grow and cryopreserve aNK cells in a central laboratory for later shipment to institutions participating in multi-center treatment protocols to evaluate dose and toxicity as well as aNK cell survival, expansion, migration, and, within the context of such studies, anti-tumor activity. Adoptive cell therapy with aNK combined with ch14.18 may be effective against a relatively small amount of disease but likely will need to be combined with cytotoxic therapy to be effective against a large amount of disease.
Supplementary Material
TRANSLATIONAL RELEVANCE.
Adoptive cell therapy with natural killer (NK) cells has therapeutic potential for malignancies. We report highly efficient ex vivo numeric growth and activation of NK cells (aNK) from blood of patients with neuroblastoma. K562-derived artificial antigen-presenting cells (aAPC) designated as Clone 9.mbIL21 act as feeder cells to stimulate NK cells to proliferate >2,000-fold in 14 days and to become highly cytotoxic against multi-drug sensitive and resistant neuroblastoma cell lines when alone or when combined with anti-GD2 antibody ch14.18. Incubation of aNK cells and ch14.18 with neuroblastoma cells markedly increased secretion of TNFα, GM-CSF, IFNγ, sCD40L, CCL2/MCP-1, CXCL9/MIG, and CXCL11/I-TAC. Cryopreserved aNK cells that were infused intravenously immediately after thawing into NOD/SCID mice bearing disseminated neuroblastoma significantly decreased tumor growth and increased mouse survival, especially when combined with ch14.18. These results support clinical testing of ex vivo grown and activated autologous NK cells combined with ch14.18 as treatment for neuroblastoma.
Acknowledgements
The authors thank Ms. Jemily Malvar for assistance with statistical analyses and Dr. Martine Torres for editorial assistance.
Financial support: This work was supported by grants to R. C. Seeger from the National Cancer Institute (P01 CA81403-12), the Bogart Pediatric Cancer Research Program, the ThinkCure Foundation, the Al Sherman Foundation, and the Anna Bing Arnold endowment; to M.A. Sheard from BD Biosciences/Immunology; to D.A. Lee from the St. Baldrick’s Foundation, the Sunbeam Foundation, and the Farrah Fawcett Foundation; and to L.J.N. Cooper from the National Cancer Institute (CA141303), Hyundai Hope on Wheels, and the Pediatric Cancer Research Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare no conflicts of interest.
Authors’ contributions: R.C. Seeger, Y. Liu, H-W Wu, M.A. Sheard planned the experiments; Y. Liu, H-W Wu, S.S. Somanchi, and M.A. Sheard conducted experiments and analyzed data with R. Sposto. D.A. Lee, S.S. Somanchi, and L.J.N. Cooper developed the K562 Clone 9.mbIL21 cells and expansion method and made them available for this study; Y. Liu wrote the first draft of the manuscript, which was revised in cooperation with R.C. Seeger and all other authors.
Reference List
- 1.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 2.Matthay KK, Reynolds CP, Seeger RC, Shimada H, Adkins ES, Haas-Kogan D, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children's oncology group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alderson KL, Sondel PM. Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol. 2011;2011:379123. doi: 10.1155/2011/379123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128:192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- 7.Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med. 2009;266:154–181. doi: 10.1111/j.1365-2796.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 8.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 9.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spanholtz J, Preijers F, Tordoir M, Trilsbeek C, Paardekooper J, de Witte T, et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PloS one. 2011;6:e20740. doi: 10.1371/journal.pone.0020740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spanholtz J, Tordoir M, Eissens D, Preijers F, van der Meer A, Joosten I, et al. High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy. PloS one. 2010;5:e9221. doi: 10.1371/journal.pone.0009221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Cui Y, Voong N, Sabatino M, Stroncek DF, Morisot S, et al. Activating signals dominate inhibitory signals in CD137L/IL-15 activated natural killer cells. J Immunother. 2011;34:187–195. doi: 10.1097/CJI.0b013e31820d2a21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parkhurst MR, Riley JP, Dudley ME, Rosenberg SA. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-11-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujisaki H, Kakuda H, Imai C, Mullighan CG, Campana D. Replicative potential of human natural killer cells. Br J Haematol. 2009;145:606–613. doi: 10.1111/j.1365-2141.2009.07667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy. 2012;14:1131–1143. doi: 10.3109/14653249.2012.700767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Somanchi SS, Senyukov VV, Denman CJ, Lee DA. Expansion, purification, and functional assessment of human peripheral blood NK cells. J Vis Exp. 2011 doi: 10.3791/2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, et al. Membrane-Bound IL-21 Promotes Sustained Ex Vivo Proliferation of Human Natural Killer Cells. PloS one. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Rosol M, Ge S, Peterson D, McNamara G, Pollack H, et al. Dynamic tracking of human hematopoietic stem cell engraftment using in vivo bioluminescence imaging. Blood. 2003;102:3478–3482. doi: 10.1182/blood-2003-05-1432. [DOI] [PubMed] [Google Scholar]
- 20.Keshelava N, Davicioni E, Wan Z, Ji L, Sposto R, Triche TJ, et al. Histone deacetylase 1 gene expression and sensitization of multidrug-resistant neuroblastoma cell lines to cytotoxic agents by depsipeptide. J Natl Cancer Inst. 2007;99:1107–1119. doi: 10.1093/jnci/djm044. [DOI] [PubMed] [Google Scholar]
- 21.Keshelava N, Groshen S, Reynolds CP. Cross-resistance of topoisomerase I and II inhibitors in neuroblastoma cell lines. Cancer Chemother Pharmacol. 2000;45:1–8. doi: 10.1007/PL00006736. [DOI] [PubMed] [Google Scholar]
- 22.Keshelava N, Zuo JJ, Chen P, Waidyaratne SN, Luna MC, Gomer CJ, et al. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res. 2001;61:6185–6193. [PubMed] [Google Scholar]
- 23.Xu Y, Li J, Ferguson GD, Mercurio F, Khambatta G, Morrison L, et al. Immunomodulatory drugs reorganize cytoskeleton by modulating Rho GTPases. Blood. 2009;114:338–345. doi: 10.1182/blood-2009-02-200543. [DOI] [PubMed] [Google Scholar]
- 24.Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen RL, Reynolds CP, Seeger RC. Neutrophils are cytotoxic and growth-inhibiting for neuroblastoma cells with an anti-GD2 antibody but, without cytotoxicity, can be growth-stimulating. Cancer Immunol Immunother. 2000;48:603–612. doi: 10.1007/s002620050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, et al. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98–107. doi: 10.3109/14653249.2010.515582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother. 2010;59:1739–1744. doi: 10.1007/s00262-010-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer immunol immunother. 2010;59:1781–1789. doi: 10.1007/s00262-010-0904-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlens S, Gilljam M, Chambers BJ, Aschan J, Guven H, Ljunggren HG, et al. A new method for in vitro expansion of cytotoxic human CD3-CD56+ natural killer cells. Hum Immunol. 2001;62:1092–1098. doi: 10.1016/s0198-8859(01)00313-5. [DOI] [PubMed] [Google Scholar]
- 30.Alici E, Sutlu T, Bjorkstrand B, Gilljam M, Stellan B, Nahi H, et al. Autologous antitumor activity by NK cells expanded from myeloma patients using GMP-compliant components. Blood. 2008;111:3155–3162. doi: 10.1182/blood-2007-09-110312. [DOI] [PubMed] [Google Scholar]
- 31.Gong W, Xiao W, Hu M, Weng X, Qian L, Pan X, et al. Ex vivo expansion of natural killer cells with high cytotoxicity by K562 cells modified to co-express major histocompatibility complex class I chain-related protein A: 4-1BB ligand, and interleukin-15. Tissue antigens. 2010;76:467–475. doi: 10.1111/j.1399-0039.2010.01535.x. [DOI] [PubMed] [Google Scholar]
- 32.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 33.Konnikova L, Simeone MC, Kruger MM, Kotecki M, Cochran BH. Signal transducer and activator of transcription 3 (STAT3) regulates human telomerase reverse transcriptase (hTERT) expression in human cancer and primary cells. Cancer Res. 2005;65:6516–6520. doi: 10.1158/0008-5472.CAN-05-0924. [DOI] [PubMed] [Google Scholar]
- 34.Kao IT, Yao CL, Kong ZL, Wu ML, Chuang TL, Hwang SM. Generation of natural killer cells from serum-free, expanded human umbilical cord blood CD34+ cells. Stem Cells Dev. 2007;16:1043–1051. doi: 10.1089/scd.2007.0033. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 2010;21:27–39. doi: 10.1016/j.cytogfr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Loskog AS, Eliopoulos AG. The Janus faces of CD40 in cancer. Semin Immunol. 2009;21:301–307. doi: 10.1016/j.smim.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Vonderheide RH. Prospect of targeting the CD40 pathway for cancer therapy. Clin Cancer Res. 2007;13:1083–1088. doi: 10.1158/1078-0432.CCR-06-1893. [DOI] [PubMed] [Google Scholar]
- 38.Venstrom JM, Zheng J, Noor N, Danis KE, Yeh AW, Cheung IY, et al. KIR and HLA genotypes are associated with disease progression and survival following autologous hematopoietic stem cell transplantation for high-risk neuroblastoma. Clin Cancer Res. 2009;15:7330–7334. doi: 10.1158/1078-0432.CCR-09-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delgado DC, Hank JA, Kolesar J, Lorentzen D, Gan J, Seo S, et al. Genotypes of NK cell KIR receptors, their ligands, and Fcgamma receptors in the response of neuroblastoma patients to Hu14. 18-IL2 immunotherapy. Cancer Res. 2010;70:9554–9561. doi: 10.1158/0008-5472.CAN-10-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 42.Tarek N, Le Luduec JB, Gallagher MM, Zheng J, Venstrom JM, Chamberlain E, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J Clin Invest. 2012;122:3260–3270. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Main EK, Lampson LA, Hart MK, Kornbluth J, Wilson DB. Human neuroblastoma cell lines are susceptible to lysis by natural killer cells but not by cytotoxic T lymphocytes. J Immunol. 1985;135:242–246. [PubMed] [Google Scholar]
- 44.Handgretinger R, Kimmig A, Lang P, Daurer B, Kuci S, Bruchelt G, et al. Interferon-gamma upregulates the susceptibility of human neuroblastoma cells to interleukin-2-activated natural killer cells. Nat Immun Cell Growth Regul. 1989;8:189–196. [PubMed] [Google Scholar]
- 45.Foreman NK, Rill DR, Coustan-Smith E, Douglass EC, Brenner MK. Mechanisms of selective killing of neuroblastoma cells by natural killer cells and lymphokine activated killer cells. Potential for residual disease eradication. Br J Cancer. 1993;67:933–938. doi: 10.1038/bjc.1993.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossi AR, Pericle F, Rashleigh S, Janiec J, Djeu JY. Lysis of neuroblastoma cell lines by human natural killer cells activated by interleukin-2 and interleukin-12. Blood. 1994;83:1323–1328. [PubMed] [Google Scholar]
- 47.Reid GS, Shan X, Coughlin CM, Lassoued W, Pawel BR, Wexler LH, et al. Interferon-gamma-dependent infiltration of human T cells into neuroblastoma tumors in vivo. Clin Cancer Res. 2009;15:6602–6608. doi: 10.1158/1078-0432.CCR-09-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.