Abstract
Objective
Obsessive and compulsive symptoms (OCS) are more prevalent in patients with diagnosed Huntington’s disease (HD) than in the general population. Although psychiatric symptoms have been reported in individuals with the HD gene expansion prior to clinical diagnosis (pre-HD), little is known about OCS in this phase of disease.
Method
The goal of this study was to assess OCS in 300 pre-HD individuals and 108 non–gene-expanded controls from the Neurobiological Predictors of Huntington’s Disease (PREDICTHD)study (enrolled between November 2002 and April 2007) using a multidimensional, self-report measure of OCS, the Schedule of Compulsions, Obsessions, and Pathologic Impulses (SCOPI). Additionally, pre-HD individuals were classified into 3 prognostic groups on the basis of age and CAG repeat length as “near-to-onset” (< 9 estimated years to onset), “mid-to-onset” (9–15 years to onset), and “far-to-onset” (> 15 years to onset). We compared the 3 pre-HD groups to the controls on SCOPI total score and 5 subscales (checking, cleanliness, compulsive rituals, hoarding, and pathologic impulses), controlling for age and gender.
Results
All models showed a significant (p < .05) group effect except for hoarding, with an inverted-U pattern of increasing symptoms: controls < far-to-onset < mid-to-onset, with the near-to-onset group being similar to controls. Although the mid-to-onset group showed the most pathology, mean scores were below those of patients with diagnosed obsessive-compulsive disorder. SCOPI items that separated pre-HD individuals from controls were focused on perceived cognitive errors and obsessive worrying.
Conclusion
Subclinical OCS were present in pre-HD participants compared to controls. The OCS phenotype in pre-HD may present with obsessive worrying and checking related to cognitive errors and may be a useful target for clinical screening as it could contribute to functional status.
Introduction
Obsessive and compulsive symptoms (OCS) are more common than diagnosed obsessive-compulsive disorder (OCD) and have an estimated weighted lifetime prevalence of 5.5% in the general population.1 Obsessive and compulsive symptoms are associated with pathology of the frontal cortex and its related subcortical loops.2–4 The heterogeneity of OCS clusters has led to research efforts to determine the OCS “phenotypes” associated with different types of neuropathology or genetic loading.5,6 For example, patients with Tourette’s syndrome have been shown to exhibit a constellation of symptoms related to symmetry, hoarding, touching rituals, and aggressive obsessions, but not contamination or cleaning.7,8 Another neuropsychiatric disorder associated with elevated rates of OCS is Huntington’s disease (HD), with prevalence rates ranging between 15% and 50%.9–14 Huntington’s disease is an autosomal-dominant neurodegenerative disease caused by an unstable expansion of CAG repeats and is characterized by a triad of symptoms: cognitive impairment, psychiatric/behavioral disturbance, and motor abnormalities.15 The psychiatric manifestation of OCS is not surprising given that HD is a disease of the frontal-striatal circuitry,16,17 like OCD. Recent studies18,19 have indicated that symptoms of HD can occur years before clinical diagnosis. For example, Paulsen et al.19 found evidence of motor abnormalities, cognitive dysfunction, and mild psychiatric symptoms in a large cohort of individuals with the gene expansion but without a clinical diagnosis of HD.
Because HD is a relatively rare disease, there is a paucity of publications describing the neuropsychiatric sequelae of this disease. Literature is even more scant with regard to the earliest changes that occur in these patients. For the past 15 years, presymptomatic patients have been able to receive definitive genetic testing, which has increased the number of patients seeking care before diagnosis and the number of research studies targeting the earliest changes associated with HD. Not coincidentally, the field is moving toward a more dimensional understanding and a possible overhaul of diagnostic criteria for HD. This carries with it a number of important implications. First, it will become increasingly common for patients to seek care before diagnosis. Because psychiatric symptoms are associated with functional disability, even in the prediagnosed phase,20 they often facilitate contact with clinicians. Further, psychiatric symptoms may be more successfully ameliorated with currently available medications than symptoms in the other 2 domains (i.e., cognitive dysfunction and chorea). It is, therefore, important to understand the clinical presentation and course of psychiatric symptoms. A second and related point is that clinical trials are soon to begin in prediagnosed patients, making accurate understanding of early symptoms critical for therapeutic target selection and trial design. This may be particularly true for psychiatric symptoms, for which there are many psychotropics already FDA-approved that could be tested immediately. Finally, there has been longstanding speculation about whether the psychiatric symptoms in early and prediagnosed HD are related to the stress of living with the knowledge of having a fatal illness and caring for family members (i.e., reactive) or whether it is associated with neurologic changes that we now know occur decades before diagnosis.19 The previous research on OCS in HD has almost exclusively focused on patients with manifest HD (i.e., symptoms resulting in a diagnosis by a neurologist). Neuropsychiatric symptoms in diagnosed patients are specifically associated with decrements in everyday functioning9 and are thought to impact quality of life.21 Paulsen and colleagues22 found that psychiatric symptoms were prevalent in diagnosed HD and were independent of dementia. They argued for the importance of dimensional assessment in a comprehensive evaluation of psychiatric symptoms across all stages of HD. Clinical experience and recent research from our laboratory in individuals with the HD gene expansion prior to clinical diagnosis (pre-HD) indicate that psychiatric symptoms are present many years before formal diagnosis.19,20 In a large study of pre-HD participants, Duff and colleagues20 found significantly more psychiatric symptoms than in those negative for the HD gene mutation, with OCS reported at the highest level (followed by depression and anxiety), and those symptoms were associated with a measure of functional capacity suggesting that, even in prediagnosed patients, they may impact function. In that study, however, measurement of OCS was limited to a handful of items on a subscale of the Symptom Checklist-90-Revised, which is used as a screening instrument. Therefore, specific, comprehensive information about OCS that may characterize a phenotype in this early phase of HD is still lacking. Such information will be helpful in better screening and potentially treating the psychiatric symptoms associated with HD. Therefore, the current study was conducted to examine OCS in pre-HD by examining baseline data from a large cohort from the Neurobiological Predictors of Huntington’s Disease (PREDICT-HD)18 study. It was hypothesized that pre-HD expansion-positive individuals would endorse higher levels of OCS compared to expansion-negative individuals. We also sought to analyze individual items on a multidimensional measure of OCS to determine which items are most characteristic of the syndrome in pre-HD individuals.
METHOD
Participants
Four hundred eight individuals enrolled in the PREDICT-HD study between November 2002 and April 2007 served as participants. Briefly, this multicenter, longitudinal project recruited individuals who were at risk for HD and who had decided to voluntarily undergo genetic testing and received results prior to, and independent of, participation in PREDICT-HD. Participants were separated into the following groups: “expansion-positive” but not yet diagnosed with HD (i.e., not showing significant motor signs to warrant a diagnosis) (N = 300) and “expansion-negative” (i.e., having a parent with HD but confirmed not to carry the expanded HD gene) (N = 108). Additional details about the recruitment and characterization of the sample can be found in Paulsen et al.18
Procedures
After giving informed consent, all participants were evaluated with a standardized clinical rating scale for HD, neuropsychological testing, psychiatric assessments, and magnetic resonance imaging. These procedures have all been previously described.18 Included in the psychiatric assessments was the Schedule of Compulsions, Obsessions, and Pathologic Impulses (SCOPI),5 which was completed by participants and their companions. The first available SCOPI data were used. In most cases, this occurred at the baseline or year 2 visit because the SCOPI was added to the neuropsychiatric battery after study initiation. Companions are persons well known to the participant, usually spouses or siblings, who have committed to accompany the participant to every visit and provide psychiatric and functional ratings. Each companion was instructed to complete the SCOPI on the basis of his or her view of the participant’s functioning at that time.
Measures
The SCOPI is a validated, multidimensional self-report measure of OCS. It is composed of 47 items that are rated on a 5-point scale (1 = strongly disagree, 2 = disagree, 3 = neutral or cannot decide, 4 = agree, 5 = strongly agree). There are 5 subscales: checking, cleanliness, compulsive rituals, hoarding, and pathologic impulses. The obsessive checking subscale includes items assessing recurring and intrusive thoughts (e.g., “I sometimes find that I cannot get rid of unpleasant thoughts that have popped into my mind”), obsessions of doubt, checking, and counting. The obsessive cleanliness subscale includes items about germs and contamination (e.g., “I worry a lot about germs”). The compulsive rituals subscale assesses individual differences in the need to perform common tasks in a fixed, ritualistic manner (e.g., “I have little rituals that I follow even though I know they are silly”; “If I don’t do certain tasks in a particular order, I feel uncomfortable”). The hoarding subscale includes items such as “I like to collect things” and “I find it difficult to throw things away, even when I know I don’t need them.” Finally, the pathologic impulses subscale contains content related to impulse-control disorders (e.g., “Occasionally I will have a sudden urge to steal something”), as well as other types of dysfunctional impulses (e.g., “While driving, I sometimes have the impulse to do something crazy”).
A total score is calculated by summing the first 3 subscales, as these are most reflective of classic OCS according to the authors of the SCOPI. Higher scores indicate a greater level of symptom endorsement. Normative data are available for the SCOPI in Watson and Wu5 for 4 samples (adults, college students, outpatients, patients with OCD). The SCOPI has excellent internal consistency (19/20 coefficients are 0.80 or higher), test-retest reliability (0.79–0.82), and convergent validity in a sample of over 2000 college students with the Obsessive Compulsive Inventory-Revised and the Yale-Brown Obsessive Compulsive Scale (see Watson and Wu5 for further psychometric details). Additionally, it has been shown to have convergent validity between ratings made by participants and spouses.5 The authors note that the SCOPI is the first published obsessive-compulsive rating scale to establish self-other agreement. In the current study, Pearson correlations showed that participants’ and companions’ ratings were moderately correlated for all subscales and the total score (all p < .0001, r = 0.34–0.50). The companions, however, consistently provided lower ratings of participants’ symptoms than the participants did, although a similar pattern of results emerged. Thus, participant ratings were used in the remaining analyses.
Participants were also evaluated on other markers of disease progression as part of the PREDICT-HD study. Probability of onset of HD in the next 5 years was estimated with current age and CAG repeat length using the published method of Langbehn et al.23 All participants were evaluated with the Unified Huntington’s Disease Rating Scale.24 A neurologist examined the participant’s individual motor signs (e.g., finger tapping, chorea, dysarthria) and then determined an overall confidence level that the participant had HD: 0 (normal), 1 (soft signs), 2 (possible HD), 3 (probable HD), and 4 (definite HD). The sum of these individual signs was the total motor score, which ranges from 0 to 124, with higher scores indicating more impaired motor functioning. The total functional capacity score (Shoulson and Fahn15), which is derived from reports of the participant and his or her companion, quantifies a participant’s ability to perform both basic and instrumental activities of daily living. This scale ranges from 0 to 13, with higher scores indicating more intact functioning. As part of the neuropsychological evaluation, an estimate of premorbid IQ was made by administering the American National Adult Reading Test25 (the greater the number of errors, the lower the estimated IQ).
Data Analyses
The expansion-positive and expansion-negative groups were compared on demographic and HD-related variables using independent t tests. The total score and 5 subscale scores of the SCOPI were also compared with independent t tests for participants and companions. Next, to evaluate which individual SCOPI items differentiated the expansion-positive from the expansion-negative group, t tests were calculated for each item. Finally, to determine whether OCS are higher in participants who are closest to estimated disease onset, we divided the expansion positive group into 3 prognostic levels—“near-to-onset” (i.e., less than 9 years until expected onset), “mid-toonset” (i.e., 9–15 years until expected onset), and “farto-onset” (i.e., greater than 15 years until expected onset)—according to the prediction equation published by Langbehn et al.,23 which uses the participant’s current age and CAG repeat length (note that this information was not available in some cases).19,23 These predicted groupings have shown excellent agreement with actual cases of diagnosis in a survival analysis.26 More specifically, using a Cox proportional hazards survival model, these groupings are significant (p < .0001) differentiators of diagnostic risk (χ2 = 55.92, df = 2 for the likelihood ratio test). (See Paulsen et al.19 for additional details about the prediction equation.) We then conducted 6 separate analyses of covariance (ANCOVAs). For these analyses, scores on the SCOPI (total score, checking, cleanliness, compulsive rituals, hoarding, and pathologic impulses) served as the dependent variable, prognostic groups (controls, far-to-onset, mid-to-onset, and near-to-onset) served as the independent variables, and age and gender were entered as covariates. Tukey-Kramer corrections were used for post hoc comparisons of prognostic group differences.
RESULTS
Participant Characteristics
Participant characteristics are shown in Table 1 for the expansion-positive and expansion-negative groups. The two groups were not significantly different with regard to education, gender, or estimated premorbid IQ (p > .05). However, the expansion-positive group was significantly younger than the expansion-negative group (t = −3.74, p < .001), and the 2 groups differed on the markers that are consistent with signs of developing HD, including total motor score (t = 5.07, p < .0001), CAG length (t = 54.59, p < .0001), and total functional capacity (t = −3.24, p < .005) in the expected direction. Expansion-Positive Compared to Expansion-Negative Participants on the SCOPI Total score on the SCOPI was not significantly different between the 2 groups (p = .20), but 2 of the 5 subscales were significantly different (checking, t = 2.35, p = .02; and pathologic impulses, t = 4.06, p < .0001), with expansion-positive participants endorsing higher levels of symptoms than expansion-negative participants. Scores on the SCOPI are presented in Table 2.
Table 1.
Denographic and Clinical Information by Group (total N = 408)a
| Variable | Expansion-Negative Groupb (N = 108) | Expansion-Positive Groupc (N = 300) |
|---|---|---|
| Age, y+ | 44.92 (11.69) | 40.18 (9.47) |
| Female gener, % | 62.67 | 72.64 |
| Education, y | 14.58 (2.77) | 14.13 (2.76) |
| CAG length+++ | 19.99 (3.60) | 42.56 (2.67) |
| Porability of onset in 5 years | NA | 0.20 (0.19) |
| Total motor score+++ | 2.27 (2.76) | 4.23 (4.89) |
| Total functional capacity score+ | 12.97 (0.17) | 12.85 (0.59) |
| ANART errors | 17.13 (7.78) | 17.65 (8.11) |
All data are shone as mean (SD) escept where indicated otherwise.
Control group with no Huntington’s disease gene expansion.
Individuals with Huntington’s disease gene expansion
p<.005
p<.001
p<.0001
Abreviations: ANART = American National Adult Reading Test, NA = Not Applicable.
Table 2.
Means and Standar Deviations of the Ratings Made by Participants on the SCOPI Subscales and Total Score in the Expansion-Negative and Expansion-Positive Groups (Total N = 408)
| Expantion-Negative | Expantion-Positive | Far-to-Onseta | Mid-to-Onsetb | Near-to-Onsetc | |
|---|---|---|---|---|---|
| SCOPI Scale | Group (N = 108) | Group (N = 300) | (N = 105) | (N = 83) | (N = 70) |
| Obsessive checking | 28.09 (9.61)d | 30.76 (11.38) | 30.46 (11.11) | 33.2 (11.69)d | 28.76 (11.10) |
| Obsessive cleanliness | 28.70 (5.90) | 28.35 (7.29) | 28.76 (6.43) | 30.18 (8.48)c | 26.51 (6.42)c |
| Compulsive rituals | 16.52 (6.64) | 16.91 (7.17) | 17.76 (6.78) | 18.59 (7.89)c | 14.96 (6.25)c |
| Hoarding | 11.87 (4.98) | 10.92 (4.62) | 10.74 (4.27) | 11.92 (5.04) | 10.54 (4.70) |
| Pathologic impulses | 9.88 (2.72)d | 11.31 (4.07) | 11.11 (3.94) | 12.30 (4.96)d | 10.96 (3.53) |
| Total | 73.31 (17.69)d | 76.02 (21.95) | 76.98 (20.5) | 82.04 (24.30)d,e | 70.23 (19.64)c |
> 15 years to expected onset of Huntington’s disease.
9–15 years to expected onset of Huntington’s disease.
< 9 years to expected onset of Huntington’s disease.
Means with the same superscripts are significantly different from each other (all p < .05).
Abbreviation: SCOPI = Schedule of Compulsions, Obsessions, and Pathologic Impulses.
To learn more about the “phenotype” of pre-HD OCS, we calculated t tests for each item on the SCOPI for the expansion-positive versus expansion-negative group to identify items that might be critical in differentiating the 2 groups. According to the participants’ ratings, 12 of the 47 SCOPI items were significantly higher (i.e., greater symptom endorsement) in the expansion-positive individuals than the expansion-negative individuals at the p < .05 level. If a Bonferroni correction for multiple comparisons is applied, a more stringent cutoff would be p < .001, in which case only 4 items reach this level. We have presented all items with p < .05 in Table 3 for review.
Table 3.
Individual SCOPI Items Rated Higher by Expansion-Positive Than by Expansion-Negative Participants (total N = 408)
| SCOPI Itema | Expansion-Negative Groupb (N= 108) SD | Expansion-Positive Groupc (N= 300) SD | t Statistic | p Value |
|---|---|---|---|---|
| Obsessive Checking | ||||
| 1. Even when I do something very carefully, I worry that it is not quite right | 2.37 (1.23) | 2.74 (1.32) | 2.55 | <0.5 |
| 4. I sometimes am troubled by unpleasant thoughts that occur over and over again | 1.54 (0.88) | 1.90 (1.14) | 3.42 | <0.001 |
| 8. I spend a lot of time checking things over and over again | 1.74 (0.93) | 1.98 (1.10) | 2.15 | <.05 |
| 28. I am often plagued by the nagging doubt that I’ve failed to do something important | 2.02 (0.97) | 2.34 (1.16) | 2.8 | <0.01 |
| 31. I sometimes find that I cannot get rid of unpleasant thoughts that have popped into my mind | 1.61 (0.85) | 1.96 (1.18) | 3.28 | <.005 |
| 45. No matter how many times I check things over, I can’t help wondering whether I have done everything correctly | 1.81 (0.91) | 2.09 (1.13) | 2.6 | <.01 |
| Obsessive cleanliness | ||||
| 2. I worry a lot about germs | 1.64 (0.89) | 1.88 (1.12) | 2.28 | <.05 |
| Pathological impulses | ||||
| 5. I occasionally get a sudden impulse to do something violent or destructive | 1.17 (0.48) | 1.49 (0.91) | 4.65 | <.0001 |
| 30. I sometimes feel the need to break things for no reason | 1.18 (0.47) | 1.46 (0.77) | 4.57 | <.0001 |
| 37. I have wondered what it would be like to tear my clothes off in public | 1.17 (0.50) | 1.32 (0.67) | 2.47 | <.05 |
| 47. I sometimes feel the sudden urge to play with fire | 1.07 (0.26) | 1.30 (0.61) | 5.12 | <.0001 |
| Compulsive rituals | ||||
| 17. I have a number of different rituals that I follow in my everyday life | 1.49 (0.72) | 1.69 (1.00) | 2.18 | <.05 |
Adapted from Watson and Wu.5 The SCOPI is in the public domain.
Control group with no Huntington’s disease gene expansion.
Individuals with Huntington’s disease gene expansion.
Abbreviation: SCOPI = Schedule of Compulsions, Obsessions, and Pathologic Impulses.
Additionally, 2 items were higher in the expansion negative group (“People should wash their hands frequently to eliminate contamination from germs” [t = −3.77, p < .0005] and “I collect items that others would consider junk” [t = −2.17, p < .05]). Near-to-Onset, Mid-to-Onset, Far-to-Onset, and Expansion-Negative Comparisons on the SCOPI Separate ANCOVAs were run to examine group effects (controls, far-to-onset, mid-to-onset, and near-to-onset) on the 6 SCOPI variables (i.e., 5 subscales and total score), controlling for age and gender. Five of the 6 SCOPI variables showed significant group effects. As can be seen in Table 2 and Figure 1, there was a general pattern of increasing symptom endorsement; expansion negative controls and near-to-onset participants endorsed the fewest symptoms and far-from-onset and mid-to-onset endorsed the most symptoms. Total score showed an overall group effect (F = 4.71, p = .003), and post hoc tests corrected for multiple comparisons (Tukey-Kramer) revealed that the mid-to-onset group scored higher than the expansion-negative group (Tukey-Kramer adjusted p = .02) and the near-to-onset group (p = .003). Similar results were found for the checking subscale (F = 4.0, p = .008), with the mid-to-onset group scoring higher than the controls (Tukey-Kramer adjusted p = .006) and trending toward higher than the near-to-onset group (p = .055). The cleanliness subscale showed a group effect (F = 3.34, p = .02), with the mid-to-onset group scoring higher than the near-to-onset group (p = .009). The compulsive rituals subscale was similar (F = 3.86, p = .010; p = .007 for mid > near); on this subscale, the mid-to-onset group was also higher than controls on the uncorrected t test comparison (p = .05), but this did not hold with the adjusted p (Tukey-Kramer p = .21). The pathologic impulses subscale showed an overall effect (F = 6.20, p = .0004), with mid-to-onset higher than controls (Tukey-Kramer adjusted p < .0001). Again, with this subscale, there was a significant difference between controls and far-to-onset (t = 2.07, p = .04) that did not hold with the more stringent post hoc test. There were no significant differences on the hoarding subscale.
Figure 1. SCOPI Subscale Means and Standard Deviations for the 4 Diagnostic Groups Showing an Inverted U Pattern of Symptom Endorsement, With Mid-to-Onset Participants Showing the Highest Level of Symptom Endorsement.
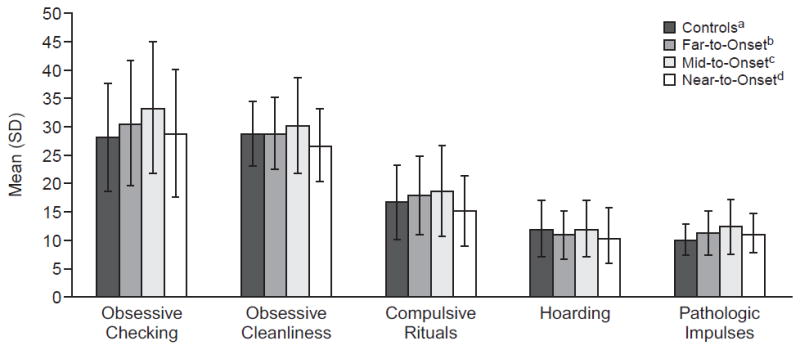
aIndividuals with no Huntington’s disease gene expansion.
b> 15 years to expected onset of Huntington’s disease.
c9–15 years to expected onset of Huntington’s disease.
d< 9 years to expected onset of Huntington’s disease.
Abbreviation: SCOPI = Schedule of Compulsions, Obsessions, and Pathologic Impulses.
DISCUSSION
The results of the current study provide information about OCS in a large sample of individuals who have not yet met criteria for an HD diagnosis but have the genetic mutation. These results support and extend our prior work showing increased OCS in pre-HD on a screening measure of general psychiatric symptoms.20 In the present study, these pre-HD participants endorsed a higher level of symptoms than the comparison group of at-risk, expansion-negative individuals. Elevations were seen on scales assessing obsessive checking and pathologic impulses when the expansion-positive group was considered as a whole. When the expansion-positive group was divided into 3 groups on the basis of estimated nearness to disease onset, significant elevations were seen compared to controls on the pathologic impulses, compulsive rituals, and obsessive checking subscales of the SCOPI. In addition, there were elevations in symptoms of those midway from onset compared to those near onset for the obsessive checking, obsessive cleanliness, and compulsive rituals subscales. It is important to note, however, that even in the group with the highest symptom endorsement compared to controls (the “mid” group who are expected to be 9–15 years from disease onset), symptoms remained below the level endorsed by patients with diagnosed OCD (on the 5 subscales, z scores ranged from −0.19 to −0.96), indicating that these are subclinical elevations.5
In this study, we also asked the participants’ companions to complete the SCOPI by rating their perceptions of OCS for the participants. Although participants’ and companions’ ratings were highly correlated, the companions consistently underreported OCS compared to the participants. This finding is interesting in light of other recent research that has shown mixed convergence of patient and companion ratings. Anosagnosia is a common feature of HD,27 and underreporting of psychiatric symptoms has been suggested by past research.28 Recent research has shown that reduced insight/lack of symptom awareness may also be present in pre-HD. Duff and colleagues20 found only small to moderate correlations between ratings made by pre-HD participants and companions on the Symptom Checklist-90-Revised, a measure of general psychiatric symptoms, possibly suggesting a lack of awareness of symptoms. Additionally, Duff et al. (K.D., J.S.P., L.J.B., et al., manuscript submitted) found that ratings diverged between participants and companions only in the near-to-onset group on the Frontal System Behavioral Scale (FrSBe), a self-report measure of “frontal” symptoms, including executive dysfunction, apathy, and disinhibition. Further, the companions’ ratings were most correlated with other measures of disease progression, such as cognitive dysfunction, which suggests that subjects near diagnosis had lost a self-appreciation for these types of neuropsychiatric symptoms. Interestingly, Hoth and colleagues29 found reduced insight for only certain types of symptoms in diagnosed patients, indicating that awareness may vary as a function of symptom domain.
Specifically, patients overestimated their level of behavioral control and activities of daily living; there was significant disagreement in their ratings of emotional control, with both over reporting and underreporting compared to their companions. In the Hoth study,29 unawareness was related to memory and executive deficits. A possible explanation for the lower agreement on psychiatric symptom ratings is that awareness declines as patients become more impaired. Further, the internalized and less observable nature of psychiatric symptoms may make them more difficult to reach agreement on. It is, therefore, an important finding, with both research and treatment implications, that pre-HD participants may be better judges of their OCS than their companions, at least at some stages. This warrants further investigation. By separating the expansion-positive participants into 3 groups on the basis of nearness to onset, it is possible to determine whether OCS track with disease progression in a cross-sectional sample. When the group means on the SCOPI scales across the 4 groups (controls, far-to-onset, mid-to-onset, and near-to-onset) are compared, an inverted U pattern of symptom endorsement is seen: controls report the lowest levels of symptoms, followed by participants far from onset, and then by those mid-toonset, who have the highest symptom endorsement. The near-to-onset group was similar to controls—and in some cases lower. Although it may seem counterintuitive that those participants closest to onset (i.e., those with the most HD pathology) would have the lowest ratings of symptoms, this pattern has been found in previous work.28,30 In these past studies, smaller sample sizes in the most severe group may have confounded results. This is an unlikely explanation in the current study because the mid-to-onset and near-to-onset groups were roughly comparable in sample size (83 and 70, respectively). There are 3 other possible explanations. First, perhaps early neuroanatomical changes produce psychiatric manifestations of the disease that present at different stages of illness depending on the structures involved. As an example, frank hallucinations are typically a later-occurring phenomenon.
The longitudinal design of PREDICT-HD, which repeatedly assesses psychiatric functioning and utilizes neuroimaging, is well suited to address this hypothesis in the future. A second explanation is that there may be a loss of insight or ability to acknowledge symptoms in the near-to-onset group. Although the participants in this study made higher ratings of OCS than did their companions, perhaps the companions’ ratings are less helpful for these subjective and less-observable symptoms. It is possible that the participants are also underreporting in the near-to-onset group due to cognitive changes in awareness. As noted above, Duff et al. (K.D., J.S.P., L.J.B., et al., manuscript submitted) found companions’ ratings on the FrSBe to be more associated with cognitive dysfunction than the participants’ own ratings, suggesting diminished insight in relation to cognitive decline.
Paulsen and colleagues19 have shown that cognitive changes occur 1 to 2 decades before a diagnosis is traditionally made. Finally, and less likely, participants may show an increase in symptoms as they begin to notice other subtle symptoms of disease (i.e., reactive symptoms) when they are still several years from onset. This may then be followed by a decline in psychiatric symptoms as they approach diagnosis. Some participants have described receiving increased support once they begin showing symptoms, and they become more connected to the HD community such that their sense of well-being increases. One of the strengths of this study was the comprehensive, multidimensional assessment of OCS that will inform us about a potential phenotype in HD, which might not have been elucidated with other OCD screening measures (e.g., the Yale-Brown Obsessive Compulsive Scale).
In contrast to patients with Tourette’s syndrome, who tend to display symptoms related to symmetry, hoarding, and aggressive obsessions, the pre-HD participants in this study endorsed symptoms related to intrusive thoughts, worry, and aggressive impulses. More specifically, the 5 SCOPI items that differentiated expansion-positive from expansion-negative participants and had the highest group mean ratings in the pre-HD group had to do with perceived cognitive errors and obsessive worrying. Given the projections of the frontal-striatal circuits31 and the cortical-subcortical atrophy in HD associated with those loops,16,17,32,33 both cognitive and psychiatric impairments are expected early manifestations of HD. For example, impairments in attention and executive functions can be localized to the dorsolateral-subcortical circuit, while the orbitofrontal circuit is associated with emotional regulation. Thus, our finding suggesting a cognitive and impulsive phenotype of OCS is not surprising. Pre-HD participants do experience subtle cognitive changes that may impact their quality of work or school performance a decade or more before they receive a diagnosis34; thus, worry about cognitive failures may be justified and anatomically based.
Some limitations of the current study should be noted. First, although OCS were assessed with a thorough, multidimensional tool, we did not complete a formal psychiatric diagnostic interview to assess for OCD. Thus, our findings are limited to self-report and companion report of symptoms. Future research should further clarify whether there is a common OCS presentation in HD (i.e., an obsessive-compulsive phenotype) and what percentage of patients has a diagnosable level of symptoms. In order to meet diagnostic criteria, patients would have to have functional impairment secondary to their symptoms. A final limitation is that these data are cross-sectional. Future research should examine the course of OCS extending into the symptomatic period in a longitudinal design to determine whether OCS track with disease progression. Information about the onset and course may be useful in optimizing the identification and treatment of patients. In future studies, data from PREDICT-HD will allow for the full examination of more regionally specific neuropsychological, motor, and imaging relationships with OCS, specifically those associated with frontal-striatal loops (e.g., executive functions and orbitofrontal volume).
Acknowledgments
This research is supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NS440068) and National Institute of Mental Health (MH01579); The Roy J. and Lucille Carver Trust; the Howard Hughes Medical Institute; the Huntington’s Disease Society of America; the Huntington’s Society of Canada; the Hereditary Disease Foundation; and the High Q Foundation.
Footnotes
Preliminary results of this study were presented in a poster session at the 3rd World Congress on Huntington’s Disease; Sept 8–11, 2007; Dresden, Germany.
The list of the investigators, coordinators, and cognitive raters in the PREDICT-HD study of the Huntington Study Group was published in Brain 2007;130:1732–1744.
The authors report no additional financial affiliations or other relationships relevant to the subject of this article.
References
- 1.Degonda M, Wyss M, Angst J. The Zurich Study, XVIII: obsessive compulsive disorders and syndromes in the general population. Eur Arch Psychiatry Clin Neurosci. 1993;243(1):16–22. doi: 10.1007/BF02191519. [DOI] [PubMed] [Google Scholar]
- 2.Salloway S, Cummings J. Subcortical disease and neuropsychiatric illness. J Neuropsychiatry Clin Neurosci. 1994;6(2):93–99. doi: 10.1176/jnp.6.2.93. [DOI] [PubMed] [Google Scholar]
- 3.Joel D. Open interconnected model of basal ganglia–thalamocortical circuitry and its relevance to the clinical syndrome of Huntington’s disease. Mov Disord. 2001;16(3):407–423. doi: 10.1002/mds.1096. [DOI] [PubMed] [Google Scholar]
- 4.Laplane D, Levasseur M, Pillon B, et al. Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions: a neuropsychological, magnetic resonance imaging and positron tomography study. Brain. 1989;112:699–725. doi: 10.1093/brain/112.3.699. [DOI] [PubMed] [Google Scholar]
- 5.Watson D, Wu KD. Development and validation of the Schedule of Compulsions, Obsessions, and Pathological Impulses (SCOPI) Assessment. 2005;12(1):50–65. doi: 10.1177/1073191104271483. [DOI] [PubMed] [Google Scholar]
- 6.Leckman JF, Grice DE, Boardman J, et al. Symptoms of obsessive compulsive disorder. Am J Psychiatry. 1997;154(7):911–917. doi: 10.1176/ajp.154.7.911. [DOI] [PubMed] [Google Scholar]
- 7.Baer L. Factor analysis of symptom subtypes of obsessive compulsive disorder and their relation to personality and tic disorders. J Clin Psychiatry. 1994 Mar;55(suppl):18–23. [PubMed] [Google Scholar]
- 8.Eapen V, Robertson MM, Alsobrook JP, II, et al. Obsessive-compulsive symptoms in Gilles de la Tourette’s syndrome and obsessive-compulsive disorder: differences by diagnosis and family history. Am J Med Genet. 1997;74(4):432–438. doi: 10.1002/(sici)1096-8628(19970725)74:4<432::aid-ajmg15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Marder K, Zhao H, Myers RH, et al. Rate of functional decline in Huntington’s disease. Huntington Study Group. Neurology. 2000 Jan;54(2):452–458. doi: 10.1212/wnl.54.2.452. [DOI] [PubMed] [Google Scholar]
- 10.De Marchi N, Mennella R. Huntington’s disease and its association with psychopathology. Harv Rev Psychiatry. 2000;7(5):278–289. doi: 10.1093/hrp/7.5.278. [DOI] [PubMed] [Google Scholar]
- 11.Anderson KE, Louis ED, Stern Y, et al. Cognitive correlates of obsessive and compulsive symptoms in Huntington’s disease. Am J Psychiatry. 2001;158(5):799–801. doi: 10.1176/appi.ajp.158.5.799. [DOI] [PubMed] [Google Scholar]
- 12.De Marchi N, Morris M, Mennella R, et al. Association of obsessive compulsive disorder and pathological gambling with Huntington’s disease in an Italian pedigree: possible association with Huntington’s disease mutation. Acta Psychiatr Scand. 1998;97(1):62–65. doi: 10.1111/j.1600-0447.1998.tb09964.x. [DOI] [PubMed] [Google Scholar]
- 13.Dewhurst K, Oliver J, Trick KL, et al. Neuro-psychiatric aspects of Huntington’s disease. Confin Neurol. 1969;31(4):258–268. doi: 10.1159/000103486. [DOI] [PubMed] [Google Scholar]
- 14.Beglinger LJ, Langbehn DR, Duff K, et al. Probability of obsessive and compulsive symptoms in Huntington’s disease. Biol Psychiatry. 2007 Feb;61(3):415–418. doi: 10.1016/j.biopsych.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 15.Shoulson I, Fahn S. Huntington disease: clinical care and evaluation. Neurology. 1979;29(1):1–3. doi: 10.1212/wnl.29.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Aylward EH, Codori AM, Barta PE, et al. Basal ganglia volume and proximity to onset in presymptomatic Huntington’s disease. Arch Neurol. 1996;53(12):1293–1296. doi: 10.1001/archneur.1996.00550120105023. [DOI] [PubMed] [Google Scholar]
- 17.Rosas HD, Koroshetz WJ, Chen YI, et al. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60(10):1615–1620. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- 18.Paulsen JS, Hayden M, Stout JC, et al. Preparing for preventive clinical trials: the PREDICT-HD study. Arch Neurol. 2006;63(6):883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington’s disease decades before diagnosis: the PREDICT-HD study. J Neurol Neurosurg Psychiatry. 2008 Aug;79(8):874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duff K, Paulsen JS, Beglinger LJ, et al. Psychiatric symptoms in Huntington’s disease before diagnosis: the PREDICT-HD study. Biol Psychiatry. 2007 Dec;62(12):1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 21.Anderson KE, Marder KS. An overview of psychiatric symptoms in Huntington’s disease. Curr Psychiatry Rep. 2001;3(5):379–388. doi: 10.1007/s11920-996-0030-2. [DOI] [PubMed] [Google Scholar]
- 22.Paulsen JS, Ready RE, Hamilton JM, et al. Neuropsychiatric aspects of Huntington’s disease. J Neurol Neurosurg Psychiatry. 2001;71(3):310–314. doi: 10.1136/jnnp.71.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langbehn DR, Brinkman RR, Falush D, et al. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65(4):267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 24.Huntington Study Group. Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11(2):136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 25.Gladsjo JA, Heaton RK, Palmer BW, et al. Use of oral reading to estimate premorbid intellectual and neuropsychological functioning. J Int Neuropsychol Soc. 1999;5(3):247–254. doi: 10.1017/s1355617799533079. [DOI] [PubMed] [Google Scholar]
- 26.Langbehn DR, Paulsen JS the PREDICT-HD investigators of the Huntington Study Group. Prospective validation of CAG-based age of diagnosis in Huntington’s disease. Presented at the 3rd World Congress on Huntington’s Disease; Sept 8–11, 2007; Dresden, Germany. [Google Scholar]
- 27.Deckel AW, Morrison D. Evidence of a neurologically based “denial of illness” in patients with Huntington’s disease. Arch Clin Neuropsychol. 1996;11(4):295–302. [PubMed] [Google Scholar]
- 28.Paulsen JS, Nehl C, Hoth KF, et al. Depression and stages of Huntington’s disease. J Neuropsychiatry Clin Neurosci. 2005;17(4):496–502. doi: 10.1176/jnp.17.4.496. [DOI] [PubMed] [Google Scholar]
- 29.Hoth KF, Paulsen JS, Moser DJ, et al. Patients with Huntington’s disease have impaired awareness of cognitive, emotional, and functional abilities. J Clin Exp Neuropsychol. 2007;29(4):365–376. doi: 10.1080/13803390600718958. [DOI] [PubMed] [Google Scholar]
- 30.Paulsen JS, Hoth KF, Nehl C, et al. Critical periods of suicide risk in Huntington’s disease. Am J Psychiatry. 2005;162(4):725–731. doi: 10.1176/appi.ajp.162.4.725. [DOI] [PubMed] [Google Scholar]
- 31.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000 Mar;31(2–3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 32.Rosas HD, Goodman J, Chen YI, et al. Striatal volume loss in HD as measured by MRI and the influence of CAG repeat. Neurology. 2001;57(6):1025–1028. doi: 10.1212/wnl.57.6.1025. [DOI] [PubMed] [Google Scholar]
- 33.Aylward EH, Sparks BF, Field KM, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63(1):66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 34.Solomon AC, Stout JC, Johnson SA, et al. Verbal episodic memory declines prior to diagnosis in Huntington’s disease. Neuropsychologia. 2007;45(8):1767–1776. doi: 10.1016/j.neuropsychologia.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


