Abstract
The emergence of clinical drug resistance is still one of the most challenging factors in cancer treatment effectiveness. Until more recently, the assumption has been that random genetic lesions are sufficient to explain the progression of malignancy and escape from chemotherapy. Here we propose an additional perspective, one in which the tumor cells despite the malignant genome could find a microenvironment either within the tumor or as a dormant cell to remain polar and blend into an organized context. Targeting this dynamic interplay could be considered a new avenue to prevent therapeutic resistance, and may even provide a promising effective cancer treatment.
Keywords: Microenvironment, Context, Tumor–stroma interactions, Dormancy, Multidrug resistance
1. Introduction
Despite the large repertoire of therapies available and the continuing efforts to incorporate new drugs into clinical practice, it is generally realized that we still have a long way to go to control cancer. This is particularly evident in patients with metastatic solid cancers, frequently resistant to first-line chemotherapy, the approach thus merely palliative, most often leading to progression of the disease and ultimate demise. Many factors conspire to limit treatment effectiveness, including restrictions in drug distribution and penetration (Jain, 1989), and a certain degree of selectivity for the very cells drugs are designed to eradicate. One of the most challenging of these limiting factors is multidrug resistance (MDR), reflected in our lack of clear understanding of how cells evolve to ensure their survival and facilitate metastasis when challenged by therapeutic intervention.
The conventional assumption, based on single cell studies of drug-resistant clones selected after prolonged exposure to cytotoxic agents, has been that multiple mutations are sufficient to fuel both tumor growth and clinical MDR (Vogelstein and Kinzler, 2004). Although this may reflect dispersed tumor cell systems such as leukemia, there is substantial data indicating that such unicellular drug resistance mechanisms represent but one cause of the effective clinical resistance expressed by multicellular solid cancers in vivo. These tumors are more than just a clonal expansion of mutant cells; they are organ-like structures (Bissell and Radisky, 2001; Radisky et al., 2001) and as such exist in intimate relationship with other cells within the tumor and the surrounding microenvironment. It is thus reasonable to hypothesize that the dynamics of this rich and ever changing ecosystem encloses additional, but crucial information for mutated genes to exert their influence, and can itself determine the overall sensitivity to anti-cancer drugs. Here we briefly describe how the solid tumor microenvironment/architecture may in fact significantly contribute to the emergence of therapeutic resistance, and discuss the possibility of targeting and manipulating this complex symbiotic interplay to overcome MDR.
2. Cells and their microenvironment: the reciprocal communication that defines normal and malignant contexts
Maintaining the status quo in adult tissues requires that newly generated cells adopt the appropriate fate and contribute to the structure and function of the organ to which they belong. Two-way communication therefore has emerged as the organizing principle that enables “dynamic and reciprocal” exchanges of information between cells and their surroundings (Bissell et al., 1982, 2002). According to this model, tissues and organs are embedded in extracellular matrix (ECM)/basement membrane (BM) that provide them structural support and contextual information together with soluble factors. The model of Bissell et al. (1982) took the bidirectional cross talk between the ECM and the cell membrane (Bornstein et al., 1982), and extended it to the level of control of gene expression, by connecting ECM–ECM receptor interactions to the cytoskeleton and to the nuclear matrix and chromatin. Indeed Bissell and Hall (1987) argued that in the last analysis the organ (or indeed the organism) is the unit of function in all organisms. Cells respond then to cocktails of soluble and insoluble signaling molecules and, in turn, tune their microenvironment. It is the result of this harmonious combination that governs tissue dynamics and function.
The importance of ‘tissue interaction’ to formation of organs was first hypothesized by Pander (1817). Over a century later, seminal work of early developmental biologists demonstrated that cells of distinct embryonic lineages engage in a highly organized cross talk that ensures proper cell sorting and directs tissue and organ morphogenesis and differentiation (reviewed by Nelson and Bissell, 2006). It is important to mention that phenotypic plasticity is implicit to this normal differentiation (Bissell, 1981), as within an individual, genotype does not specify a strictly defined phenotype, but instead a range of phenotypic manifestations within a norm of reaction. In an example of the dominance of the microenvironment over even a potent oncogene, Dolberg and Bissell injected Rous sarcoma virus (RSV), encoding the oncogene v-src, into the wings of chick embryos, and observed the initial normal development of the embryos, despite the presence of the active oncogene (Dolberg and Bissell, 1984; Howlett et al., 1988). However, when these same embryonic wings were removed from the greater context of the embryo, they quickly displayed a transformed phenotype in a tissue culture dish (Stoker et al., 1990), suggesting the embryonic environment or context was dominant over the pp60 Src. This suppression was not absolute, and a profound microenvironmental change, such as the one occurring when the embryos got closer to hatching, favors aberration and disintegration in blood vessels and other tissues as was seen also in experiments of Hochedlinger et al. (2004). Subsequent experiments showed that the wound-healing response is a critical event that creates a permissive environment also for RSV tumorigenesis in chickens (Dolberg et al., 1985). Together, these studies demonstrated that oncogene expression was compatible with an apparently normal tissue morphogenesis in the embryo presumably since the wound healing response is different in the embryo, and that the tumorigenic phenotype could be revealed after microenvironmental perturbations, such as those induced by culturing cells on plastic with serum or wounding in the adult chicken.
Evidence of the coexistence of normal and malignant cell populations within the same tissue, without resulting in a frank malignant tumor, has been reported also in human tissue specimens. Studies of large autopsy series have revealed that the majority of middle-aged and older people who die from causes other than cancer have frequent precancerous lesions throughout their bodies (Rich, 1979; Nielsen et al., 1987; Harach et al., 1985; Folkman and Kalluri, 2004). Analyses of ‘normal’ epithelial tissue adjacent to tumors have shown that similar patterns of mutations can be found in both, yet tumor growth is restrained by normal contextual cues (Deng et al., 1996; Washington et al., 2000). These and other related findings led Bissell and Hines recently to propose the microenvironment as the attenuator of both tumor onset and malignant progression, providing a rational framework to explain why the majority of people live cancer-free lives for decades, yet harboring a number of harmful mutations they accumulate over time (Bissell and Hines, 2011). Another example is that despite the fact that people with familial BRCA or APC mutations have these in all their cells yet they develop tumors only in a few of cells in specific organs.
If reciprocal communication between a normal context and ECM defines the normal tissue homeostasis, the opposite should also be true: abnormal context should lead to abnormal conversation allowing cells to disregard sorting rules and violate normal tissue boundaries, setting the stage for cancer progression. That this indeed is the case has long been obvious to pathologists, as judged by common reports of fibrotic tissue, ECM deposition, and immune and inflammatory infiltration, collectively called ‘reactive’ tumor stroma. As early as 1938, Orr observed that morphological changes in the microenvironment of the skin of carcinogen-treated mice appear long before neoplastic alterations in epithelial cells (Orr, 1938). Subsequently, Tarin showed that complex sequential changes occur at the epithelial–mesenchymal boundary during mammary tumor progression (Tarin, 1969), and insights into the nature of these reciprocal tumor–stromal interactions have gradually accumulated. The presence of cancer-associated fibroblasts (CAFs) has been reported in many cancer types, and bidirectional CAF–epithelial interactions were shown to precede invasion and stimulate tumor growth and progression (Picard et al., 1986; Camps et al., 1990; Hayashi et al., 1990; Skobe and Fusenig, 1998; Thomasset et al., 1998; Olumi et al., 1999; Cunha et al., 2003; Bhowmick et al., 2004). Concomitantly, cancer cells overproduce proteolytic enzymes, particularly metalloproteases (MMPs) (Chambers and Matrisian, 1997), which generate fragments with pro-migratory and pro-angiogenic functions (Folkman and Kalluri, 2002) as well as activate cell-surface and ECM-bound growth factors (Egeblad and Werb, 2002), reflecting the extensive crosstalk between the microenvironment and the malignant cells. Other examples include overexpression of an autoactivated form of MMP3 in mouse mammary gland epithelium where the MMP disrupts the integrity of the BM, leading to the development of a reactive stroma and eventually genomically unstable mammary tumors (Sympson et al., 1994; Thomasset et al., 1998; Sternlicht et al., 1999). Recently, adipocytes have been recognized as important mediators of normal context disruption as well, since they produce a host of biologically active molecules that promote the inflammatory process and angiogenesis (Iyengar et al., 2005; Motrescu and Rio, 2008; Cao et al., 2010; Dirat et al., 2011). Preference for metastatic colonization is heavily influenced also by communication between circulating tumor cells and bone marrow-derived cells (BMDCs), which home to the tumor and promote progression, escape from the tumor, survival and ultimately metastatic growth (reviewed in Joyce and Pollard, 2009).
3. Tumor microenvironment facilitates the emergence of MDR
As discussed above, tumors exist in intimate relationship with the surrounding microenvironment, and it is the dynamics of this heterogeneous and ever changing ecosystem that provides additional but crucial information for mutated genes to exert their function. In addition to initiating and supporting the tumorigenic process, a permissive microenvironment can also affect the sensitivity of tumor cells to drug treatment. The composition and organization of the ECM and stromal components contribute to marked gradients in drug concentration, increased interstitial fluid pressure and metabolic changes, all of which can strongly enhance the resistance of tumor cells to drug agents (Dang and Semenza, 1999; Heldin et al., 2004; Di Paolo and Bocci, 2007). That the three-dimensional structure of the tissue itself could also account for tumor resistance to radiation and chemotherapy was first recognized by Sutherland and co-workers in the early 1970s. Using Chinese hamster lung fibroblasts and EMT-6 mammary tumor cells, they showed that multicellular spheroids were markedly more resistant to radiation and distinct doses of adriamycin than the same cells cultured in monolayer (Durand and Sutherland, 1972; Sutherland et al., 1979). This finding led Teicher and colleagues to hypothesize that resistance to anticancer drugs could develop through mechanisms operative only in vivo. By deriving a series of alkylating agent-resistant variants of the EMT-6 mouse mammary tumor, they surprisingly found that those cells plated on plastic culture dishes were no more resistant than the parental EMT-6 cell line, but they would reexpress their drug resistance properties upon reinjection in mice or when grown in three-dimensional conditions (Teicher et al., 1990; Kobayashi et al., 1993). Further work demonstrated that this rapid reappearance of resistance represents a physiological strategy of adaptation implicit to a multicellular tissue, involving cell-cell and cell-ECM interactions, and it may be one reason to explain the seemingly rapid development of drug resistance in some patients who are initially responsive to chemotherapy (Graham et al., 1994; Durand and Olive, 2001; Kerbel et al., 1996).
3.1. Cell adhesion-mediated drug resistance (CAM-DR)
While adhesion is essential for normal cells to grow and survive, anchorage-independence for growth and survival is considered an essential feature of malignant cells (Frisch and Francis, 1994). We demonstrated in 1995, that loss of β1-integrin-mediated adhesion in non-malignant mammary cells leads to apoptosis and that Laminin-111 specifically was needed for survival (Boudreau et al., 1995). Some tumor cells lose β1-integrin altogether (Howlett et al., 1995), others dramatically upregulate the level, but driving the level down using inhibitory antibodies allows these cells to reversibly ‘revert’ to a ‘normal’ phenotype, and reduce tumor take and size appreciably despite the malignant genome (Weaver et al., 1997; Wang et al., 1998, 2002; Weaver and Bissell, 1999; Bissell et al., 2005). Adhesion to ECM via β1-integrins can also enhance the tumorigenicity and resistance of multiple myeloma and small cell lung cancer (SCLC) cells to chemotherapeutic agents doxorubicin and melphalan (Fridman et al., 1990; Sethi et al., 1999). Conversely, preventing tumor cell adhesion by blocking integrin binding to ECM and stromal cells results in a dramatic reduction in tumor burden and increases considerably the overall survival in a mouse model of multiple myeloma (Mori et al., 2004). The combination of this anti-adhesion approach with conventional cytotoxic melphalan proves even greater efficiency, reducing tumor load substantially more than either treatment alone. Similar observations by Park et al. (2006, 2008) show that inhibition of β1-integrin allows also for a significant reduction in tumor volume and increases sensitivity to ionizing radiation (IR) in human breast cancer xenografts. Recent work has also showed that inhibition of β1-integrin significantly increases the sensitivity of HER2-amplified breast cancer cell lines to Trastuzumab and Pertuzumab. This study has also reported dramatic differences in response to therapeutic agents for cells grown in monolayer as opposed to three-dimensional matrices, highlighting again that cellular response to drugs is context dependent (Weigelt et al., 2010).
Simple culture models have been used to delineate specific molecular mechanisms of cell adhesion-mediated drug resistance (CAM-DR) – the term coined to describe a rapid form of drug resistance mediated by adhesion. For example, allowing adhesion of human SCLC to the ECM components fibronectin or laminin confers those cells a survival advantage under acute exposure to cytotoxic drugs, by inhibiting drug-induced apoptosis (Sethi et al., 1999). Not unique to SCLC, resistance-promoting effects by integrin-mediated adhesion to ECM were also observed in cancers of the pancreas (Cordes and Meineke, 2003), ovary (Maubant et al., 2002; Sherman-Baust et al., 2003), prostate (Miyamoto et al., 2004), breast (Aoudjit and Vuori, 2001; Menendez et al., 2005), liver (Zhang et al., 2002), brain (Uhm et al., 1999) and leukemia (Damiano et al., 1999; de la Fuente et al., 2003). Further studies in leukemia cell lines showed that β1-integrin-mediated adhesion could modulate cellular localization and availability of several apoptotic regulators (such as CASP8, c-FLIPL and BIM), preventing tumor cells from apoptosis and favoring MDR (Shain et al., 2002; Hazlehurst et al., 2007). Interestingly, this mechanism of CAM-DR identified in cell culture models is consistent with patterns of low expression of apoptotic promoters in patients with resistant acute lymphoblastic lymphoma or acute myeloid leukemia (Flotho et al., 2006, 2007; van Stijn et al., 2005).
Integrin binding to ECM and stromal cells can also control cell cycle progression in both hematological and epithelial malignancies. Work by Hazlehurst et al. (2003) reported that G1 arrest of myeloma cells induced by β1-integrin adhesion to fibronectin correlates with upregulated levels of cell cycle regulator p27, and enhanced resistance to etoposide (Hazlehurst et al., 2003). Later studies showed that integrin-mediated adhesion could also interfere with ubiquitin-proteasome proteolytic pathways. For example, preventing p27 proteosomal degradation induces cell cycle arrest in non-Hodgkin B cell lymphoma and hepatocellular carcinoma cell lines, leading to extreme drug resistance (Lwin et al., 2007; Fu et al., 2007). In addition to being significantly less chemosensitive, tumor cells grown on certain ECM components show prolonged radiation-induced cell cycle arrest in contrast to cells growing on non-specific substrates (Cordes and van Beuningen, 2004; Kremer et al., 2006; Dimitrijevic-Bussod et al., 1999). This delay appears to provide more time for DNA damage repair at distinct cell cycle checkpoints after genotoxic injury (Bartek and Lukas, 2001). Experiments in non-tumorigenic lung endothelial and hemopoietic cancer cell lines demonstrated that integrin-ECM interactions can strongly affect the machinery of DNA damage recognition and repair (Hoyt et al., 1997; Hazlehurst et al., 2003; Jones et al., 2001). Activation of these pathways by adhesion of tumor cells to ECM is likely to accelerate and optimize the efficacy of DNA damage repair after irradiation, providing for a more stable genome and thus cell survival.
The absence of unit tissue architecture inherent in two-dimensional (2D) cell culture systems used in the majority of the studies referred to above explains why so many of these cells do not express tissue specific functions (for review see Bissell, 1981; Bissell et al., 2005). When normal mammary cells were cultured in a laminin-rich ECM gel (3D lrECM) (Barcellos-Hoff et al., 1989) the cells reorganized, and both form and function were restored. This concept was used to develop an assay that could distinguish between non-malignant and malignant cells on the basis of their structural integrity. Whereas non-malignant cells formed polarized growth-arrested acini in lrECM, primary breast tumor cells or breast cancer cell lines formed highly disorganized and proliferative colonies (Petersen et al., 1992; Weaver et al., 1995). Under these conditions the balance of signaling pathways are deranged in tumor cells. Antagonizing one or more of the many signaling pathways that are deregulated in tumor cells causes them to functionally revert to a ‘normal’ phenotype, despite their malignant genome (Howlett et al., 1995; Weaver et al., 1997; Wang et al., 1998, 2002; Kirshner et al., 2003; Weaver and Bissell, 1999; Muschler et al., 2002; Liu et al., 2004; Weir et al., 2006; Itoh et al., 2007; Kenny and Bissell, 2007; Beliveau et al., 2010). Interestingly, there is a reciprocal interaction between any oncogenic pathway and all the rest in 3D and the changes do not occur in 2D (Anders et al., 2003). Together, these data show that tissue architecture can override the proliferative and invasive malignant phenotype of breast tumor cells, but that reversion to a ‘normal’ phenotype is dependent upon sensing of the appropriate spatial and biochemical cues from the microenvironment.
The same concepts were later used to demonstrate that survival and sensitivity to drugs used in the clinic of human breast cells is dependent on cell and tissue polarity as well as integrin-mediated adhesion to BM and does not correlate with the rate of growth or quiescence (Weaver et al., 2002) (Fig. 1). Briefly, when non-malignant and malignant cells were treated with three immunomodulators (Trail peptide, anti-FAS antibody and tumor necrosis factor TNF-α) and three chemical drugs (the topoisomerase II inhibitor etoposide, the microtubule modulator paclitaxol and actin cytoskeleton disruptor cytochalasin B) on 2D, the rate of apoptosis was equivalent in both cell types with high statistical significance. However, when placed in 3D lrECM, the cells that become polarized (either non-malignant or reverted tumor cells) were resistant to all six agents, whereas disorganized cells both normal and malignant were equally sensitive. It was shown that the resistance to apoptosis depends upon the 3D organization of the acini and is functionally linked to β4-integrin-directed hemidesmosome formation and NFκB activation. Expression of a β4-integrin that lacked the hemidesmosome-targeting domain disrupted tissue polarity and triggered apoptosis by all drugs tested (Weaver et al., 2002).
Fig. 1.
Polarized mammary structures are resistant to apoptosis induced by chemotherapeutics. When cultured on 2D monolayer, both non-malignant (A) and malignant (B) human breast cells show a similar rate of apoptosis upon treatment with distinct immunomodulators and chemical agents. However, when placed in 3D lrECM, S-1 non-malignant cells form polarized growth-arrested acini resistant to drug cytotoxic effects (C), whereas T4-2 malignant cells appear highly disorganized, proliferative and sensitive to therapeutic drugs (D). Perturbing apical–basal polarity of S-1 acini, by treatment with E-cadherin function-blocking antibody, results in a dramatic increase of sensitivity to drug agents (E). Conversely, restoring cell and tissue polarity in T4-2 structures, by treatment with β1 integrin inhibitory antibody, induces malignant cells to ‘revert’ and provides them resistance to chemotherapeutic agents (F). Polarized mammary epithelial cells are resistant to apoptosis induced by cytotoxic agents, either growth-arrested (C) or proliferating (G). In contrast, growth-arrested but reversely polarized S-1 cells grown in collagen I ECM undergo apoptosis (H, upper panel); once exposed to lrECM, these S-1 non-polar structures polarize and become resistant to apoptosis (H, lower panel).
Aside from determining cell and tissue architecture, the way cell surface adhesion molecules perceive ECM also affects nuclear structure and chromatin organization. Experiments in mammary epithelial cells demonstrate that ECM can modulate the transcription of the β-casein gene by activating an ECM/response element inducing rapid histone modifications (Schmidhauser et al., 1992; Myers et al., 1998). Studies by Maniotis et al. (1997) in fibroblasts and endothelial cells have also confirmed that alterations in surface-adhesion receptors are channeled along cytoskeletal filaments and ultimately concentrate at the nucleus to reorganize chromatin structure and gene expression. The work that followed brought the first demonstration that cells experience a complete and global reorganization of chromatin in response to a certain ECM composition and thickness (Maniotis et al., 2005; Sandal et al., 2007). Laminin specifically, but not fibronectin or Type I collagen, greatly increased the resistance of chromatin digestion by AluI restriction enzyme in breast cancer cells. This suggests chromatin reorganization as another mechanism by which cells develop CAM-DR, particularly to drug agents that bind to or disrupt DNA.
3.2. ‘Forcing’ malignant progression and therapeutic resistance
Cells and ECM exert positive and negative tension on each other. Cells sense force through mechanoreceptors and respond by generating mechanical tension in their actin cytoskeleton and adhesions to ECM (Ingber, 1991, 1997). This phenomenon of mechanoreciprocity maintains tensional homeostasis in the tissue and is crucial for normal tissue-specific development (Krieg et al., 2008). Each tissue has a particular ‘stiffness phenotype’ and each cell type is finely tuned to the specific tissue in which it resides. An increase in ECM protein concentration, matrix crosslinking or reorientation of matrix fibrils can stiffen a tissue locally to alter cell growth or direct cell migration (Discher et al., 2005). This has important implications for development and frequently leads to disease progression, including cancer. For example, malignant transformation of the breast has been associated with a dramatic and chronic increase in mammary gland tension and ECM stiffening (Krouskop et al., 1998; Plewes et al., 2000) (Fig. 2). Here we describe the variety of physical stresses experienced by transformed mammary epithelial cells (MECs) within a breast tumor, which can dramatically enhance cell growth, survival, motility, invasion, and ultimately compromise therapeutic response.
Fig. 2.
Mammary gland tissue becomes increasingly stiffer during tumor progression. Each tissue has a particular ‘stiffness phenotype’ (stiffness measured in Pascals – Pa) and each cell type is finely tuned to the specific tissue in which it resides. For example, fat tissue is much softer than cartilage. Thus, a highly compliant matrix favors adipogenesis, whereas osteoblast differentiation is optimal on stiffer ECM. Similarly, normal mammary gland development is optimally supported by interaction of epithelial cells with a soft matrix. During tumor progression, breast tissue becomes increasingly stiffer and tumor cells become significantly more contractile and hyper-responsive to highly compliance signals. Although breast tumors are much stiffer than the normal mammary gland, the material properties of a breast tumor or any other physiological environment remain significantly softer than those of glass or plastic culture dishes.
At the tissue level, actively proliferating transformed MECs exert gradually increased compression stresses in the ductal tree. Once the tension becomes large enough, the tumor mass compresses intratumoral vessels, and prevents the blood flow, producing regions of tissue hypoxia and compromising the efficacy of tumor therapy (Roose et al., 2003; Shannon et al., 2003). Likewise, compression stress also increases the interstitial pressure, blocking tissue vasculature and lymphatic networks, and impairing drug delivery and immune cell infiltration and clearance (Jain, 2001; Padera et al., 2004). When a tumor forms within a breast, even non-malignant cells within that breast experience fields of increased resistance force (increased stiffness) in their ECM microenvironment that alternate with pockets of high compliancy (decreased stiffness). Such fluctuations in the ECM elastic properties likely arise from the activation of resident stromal fibroblasts and infiltrating immune cells, as well as the increased deposition, processing and cross-linking of ECM proteins (Ebihara et al., 2000; Chiquet et al., 2009). All these changes can strongly influence the behavior of transformed MECs, either by directly activating mechanotransduction pathways or by indirectly stimulating resident mammary gland stromal fibroblasts to release various cytokines, growth factors and ECM degrading enzymes (Decitre et al., 1998; Yeung et al., 2005; Wozniak et al., 2003). For example, increases in matrix stiffness that enhance cell contractility have been found as sufficient to induce transformation of mammary epithelial cells (Paszek et al., 2005; Samuel et al., 2011). Conversely, a decrease in tissue stiffness by inhibition of collagen crosslinking prevents malignant growth and tumor progression in a murine model of breast cancer (Levental et al., 2009). Increased matrix stiffness has been recently implicated also in the modulation of chemotherapeutic resistance in hepatocarcinoma cells (Schrader et al., 2011). The relation of the biomechanical properties of the microenvironment with the emergence of therapeutic resistance is still in its infancy and requires much more attention.
3.3. Plasticity of cell phenotype and the emergence of MDR
The tumor microenvironment is extraordinarily heterogeneous: different numbers and types of infiltrating normal cells, distinct densities of blood and lymphatic vasculature, and singular composition of extracellular matrix. For this reason, cells within a given tumor are expected to experience an array of microenvironmental cues, which will in turn translate into several phenotypic manifestations. In epithelial cancers, these adaptive changes may involve, at least in part, a stepwise cycle of epithelial plasticity, governed by epithelial to mesenchymal transitions (EMT) and the reverse mesenchymal to epithelial transitions (MET). It is now believed that this state is a reversible change of cell phenotype (Petersen et al., 2003), characterized by loose cell–cell adhesion, disruption of apical–basal polarity and cytoskeleton reorganization. Cells become isolated, motile and resistant to apoptosis (Thiery et al., 2009). Although EMT was initially defined to support normal tissue remodeling and diversification during development, an intermediate EMT-like process, meaning transient plasticity, is also evoked during tumor progression, metastasis and recently drug resistance (Lee et al., 2006). For instance, an EMT-like signature was identified as a determinant of insensitivity of non-small cell lung carcinoma (NSCLC) cell lines and xenografts to the small molecule-EGFR-inhibitor Erlotinib (Tarceva) (Yauch et al., 2005; Thomson et al., 2005). These results were also confirmed in other types of tumors, such as head and neck squamous cell carcinoma (HNSCC) and hepatocellular carcinoma, as well as for treatment with other EGFR inhibitors such as Gefitinib (Iressa) (Frederick et al., 2007) and Cetuximab (Erbitux) (Fuchs et al., 2008). The implication of EMT in therapeutic drug resistance has been increasingly reported, for example gemcitabine resistance in pancreatic tumor cell lines (Shah et al., 2007), Oxaliplatin resistance in colorectal cancer cells (Yang et al., 2006), Lapatinib resistance in breast cancer (Konecny et al., 2008) and Paclitaxel resistance in both breast (Cheng et al., 2007) and epithelial ovarian carcinoma (Kajiyama et al., 2007).
3.4. Microenvironment-induced protective quiescence
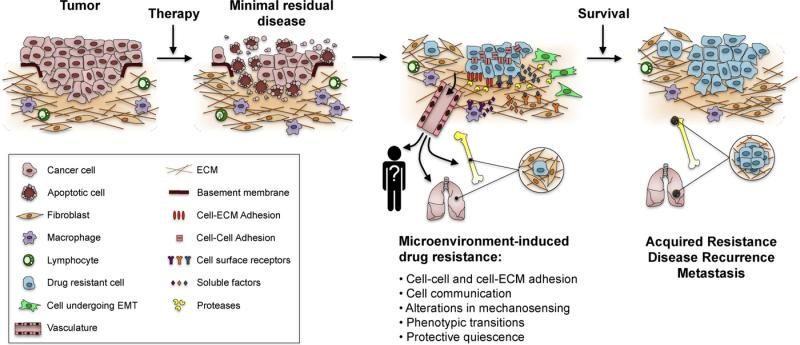
The selective pressure imposed by conventional chemotherapy regimes eliminates certain cells within the tumor population. This surviving population following chemotherapy is referred to as minimal residual disease; these cells either can stay within the tumor or most likely have already found refuge in protective microenvironments in specific organs, depending on the origin of the primary tumor. These dormant cells remain in a state of quiescence until they sense signals to start a burst of growth. Failure of the initial therapy to eradicate these cells to prevent tumor recurrence is clearly one of the main barriers to effective cancer treatment. For example, the presence of bone marrow micrometastases in about 30% of patients with breast cancer at the time of diagnosis is a strong predictor of disease recurrence (Karrison et al., 1999; Bidard et al., 2008). It is reported that 15–20% of patients still have disseminated tumor cells in the bone marrow regardless of the aggressiveness of the treatment (Wiedswang et al., 2004). But how do some cells manage to become quiescent to survive the selective pressure of therapeutics? We suggest that, despite the malignant genome, some tumor cells can find a microenvironment to allow them to remain polar, blend into an organized context and survive in a quiescent state similar to our studies on reversion described above. Polar cells are quiescent. However, cells with reverse polarity in 3D cultures are also quiescent, and yet they die when treated with chemotherapeutic agents, whereas the polar cells do not (Gudjonsson et al., 2002; Weaver et al., 2002). Studies with metastatic hematopoietic, colon adenocarcinoma and breast cancer cells show that tumor-ECM interactions indeed determine a state of quiescence associated with CAM-DR (Nefedova et al., 2003; Korah et al., 2004; Schmidt et al., 2001). Similar observations in mouse models of breast cancer micrometastasis confirmed that microenvironments that induce dormancy harbor cells that become quiescent and tolerant to doxorubicin (Naumov et al., 2003; Goodison et al., 2003). Further support for the concept of microenvironmental-induced quiescence comes from data from bone marrow specimens from breast, gastric and colorectal cancers, in which micrometastasis with marked signs of quiescence were found in 34% of the patient cohort (Pantel et al., 1993). Whereas some cells interpret the microenvironment as conducive to quiescence, others may remodel and/or reproduce a microenvironment that would be permissive for growth of dormant cells. Experiments in myeloma cells showed that a dynamic interaction with their surrounding stroma allows tumor cells to proliferate in response to IL-6 stimulation while still adhered to a fibronectin matrix (Shain et al., 2009). In vivo studies demonstrated that single mammary epithelial tumor cells can remain dormant in metastatic sites for long periods of time, but retain their ability to proliferate when transplanted to their tissue of origin (Naumov et al., 2002). A similar switch between proliferation and growth arrest controlled by the tumor cell–microenvironment crosstalk was observed in head and neck carcinoma (Aguirre Ghiso et al., 1999). In this model, the metastasis-associated urokinase receptor (uPAR) drives tumor growth by interacting and activating α5β1-integrins, whereas blocking this interaction results in tumor suppression due to induction of dormancy. A more detailed analysis of the mechanisms and markers of dormant cancers will be important for the choice of therapy when patients are known to have minimal residual disease.
4. Treating the tumor microenvironment to overcome MDR
We believe that the same mechanisms that help us not to develop more cancers (Bissell and Hines, 2011) can also help keeping dormant cells dormant (Fig. 3). The initial attempts to reconstruct the correct microenvironment were based on co-culture assays. Analyzing combinatorial products of human prostate epithelial and fibroblast cells, Olumi et al. (1999) showed that normal stromal cells inhibit the progression to epithelial malignancy. Similarly, Javaherian et al. (1998) were able to suppress early stages of neoplastic progression of malignant keratinocytes by introducing an excess of normal keratinocytes. However, it became evident that the 3D architecture and the complex network of interactions that characterize both organs and tumors were traits simply not possible to recapitulate in traditional 2D cultures. In the last two decades, engineered animal models and 3D culture systems have become commonplace, making it possible to start dissecting the plasticity of the tumor ecosystem and mechanisms by which microenvironmental signals could lead to tumor cell reprogramming and ‘reversion’. In addition to the work from Bissell laboratory described above, Hendrix and colleagues conduced a series of elegant studies elucidating the regulation of tumor cell plasticity by an embryonic milieu of human stem cells (hESCs), zebrafish or chick (Topczewska et al., 2006; Kulesa et al., 2006). Her laboratory has also developed a 3D model to demonstrate that the microenvironment of hESCs can reprogramme aggressive melanoma cells toward a less aggressive melanocytic-like phenotype (Postovit et al., 2006). Work from Gil Smith laboratory has demonstrated that human carcinoma cells could be redirected to produce progeny capable of typical mammary epithelial cell function by interaction with the microenvironment of a mouse mammary gland developing in vivo (Bussard et al., 2010).
Fig. 3.
Postulated steps in drug resistance and dormancy. Cancer cells exist in intimate relationship with other cells within the tumor and the surrounding microenvironment. This dynamic coalition ensures tumor survival and proliferation, but may determine also the overall sensitivity to anti-cancer drugs. The selective pressure imposed by conventional chemotherapy regimes eliminates certain cells within the tumor population. The surviving population following chemotherapy is referred to as minimal residual disease; despite the malignant genome, these cells can find a microenvironment to allow them to remain polar, blend into an organized context and survive therapeutic insults. These protective microenvironments facilitate the development of drug resistance by distinct molecular mechanisms, including: intercellular and cell-ECM adhesion; cell communication by various soluble factors and overproduction of proteolytic enzymes; alterations in mechanosensing that disrupt tensional homeostasis in the tissue; phenotypic transitions for cells to become isolated, motile and resistant to apoptosis; and a state of protective quiescence, either within the tumor or in specific organs depending on the origin of the primary tumor. Over time, drug resistant cells develop even more permanent mechanisms of resistance (acquired resistance), and eventually cause disease recurrence and metastatic growth.
Collectively, the above observations reaffirm the dominance of tissue microenvironment and architecture over the genotype, and suggest that differentiation therapy, a concept used in treating some forms of leukemia by administration of retinoic acid, vitamin D compounds and PPARγ agonists (reviewed in Nowak et al., 2009), may also be a powerful strategy for therapeutic intervention in solid cancers. This ‘microenvironmental therapy’ might potentially reverse subtle, but critical, imbalances in tumor–microenvironment interactions, and provide a higher specificity that can help minimizing collateral toxicity to normal adjacent tissues. Additionally, stromal cells are not as genetically unstable as cancer cells, and are therefore less likely to develop drug resistance. There already have been several exciting reports of success in the clinical targeting of tumor stroma. For example, inhibition of inflammatory cells and cytokines by treatment with non-steroidal anti-inflammatory drugs (NSAIDs) has been shown to lower the risk for colon and breast cancer, and might help preventing lung, oesophageal and stomach cancers (Ricchi et al., 2003). Likewise, the angiogenesis inhibitor bevacizumab (Avastin) has proven successful in the treatment of colorectal (Salgaller, 2003) and kidney tumors (Mass et al., 2004). However, there have been also some disappointments, such as the inefficacy and severe intolerable side effects of MMP inhibitors in patients with late-stage cancers (Stetler-Stevenson and Yu, 2001). This may be due to the many contradictory roles that MMPs play in modulating tumor microenvironment, and that were not taken into account when the broad-spectrum MMP inhibitors were designed (Coussens et al., 2002; Morrison et al., 2009). In addition, selecting patients in advanced stage of the disease is not likely to be successful.
In summary, normalizing tumor microenvironment represents an important new direction for cancer therapy. Despite the fact that the microenvironment comprises many different components, it is still possible to reduce the severity of malignant cells using a single effective agent. Of course it is best always to combine drugs that target distinct aspects of the reactive stroma with the conventional cytotoxic drugs designed the kill tumor cells, thereby treating the tumor as the organ we now recognize it to be.
5. Concluding remarks
We now appreciate tumors as true ecosystems, harboring a plethora of cells and stromal components that coexist and engage in dynamic and reciprocal interactions. It is the product of these interactions from a very early stage of the disease that clearly determines the fate of the tumor as well as the patient. The data we have summarized here suggests that tumor microenvironment also is a prominent shelter for the population of surviving tumor cells following initial chemotherapy. As such, the microenvironment can facilitate the development of therapeutic resistance. Given the increased knowledge of the signaling cues and components that comprise the tumor and its microenvironment, it would be important to incorporate this knowledge into organ-specific and physiological culture models of human cells together with appropriate animal models for drug testing. These systems represent the toolkit to more successfully translate fundamental research findings into therapies in the clinic, and may have great potential in providing answers before proceeding into costly clinical trials. So far, most 3D models available allow co-culture of epithelial cells only with one other cell type. Thus heterotypic culturing systems that more closely mimic the heterogeneity of the tumor microenvironment still need to be developed. Tailoring drugs to target the tumor microenvironment represents a new direction for anti-cancer drug development, and may hold significant therapeutic promise.
Acknowledgments
To the Portuguese Foundation for Science and Technology for the research grant awarded to ALC (SFRH/BD/33249/2007). The work from MJB's laboratory is supported by grants from the U.S. Department of Energy, Office of Biological and Environmental Research and Low Dose Radiation Program (contract no. DE-AC02-05CH1123); by National Cancer Institute (awards R37CA064786, U54CA126552, R01CA057621, U54CA112970, U01CA143233, and U54CA143836 – Bay Area Physical Sciences – Oncology Center, University of California, Berkeley, California); and by U.S. Department of Defense (W81XWH0810736).
Footnotes
Conflict of interest
The authors declare no competing financial interests.
References
- Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell Biol. 1999;147:89–103. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders M, Hansen R, Ding RX, Rauen KA, Bissell MJ, Korn WM. Disruption of 3D tissue integrity facilitates adenovirus infection by deregulating the coxsackievirus and adenovirus receptor. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1943–1948. doi: 10.1073/pnas.0337599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartek J, Lukas J. Mammalian G1- and S-phase checkpoints in response to DNA damage. Curr. Opin. Cell Biol. 2001;13:738–747. doi: 10.1016/s0955-0674(00)00280-5. [DOI] [PubMed] [Google Scholar]
- Beliveau A, Mott JD, Lo A, Chen EI, Koller AA, Yaswen P, et al. Raf-induced MMP9 disrupts tissue architecture of human breast cells in three-dimensional culture and is necessary for tumor growth in vivo. Genes Dev. 2010;24:2800–2811. doi: 10.1101/gad.1990410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Bidard FC, Vincent-Salomon A, Gomme S, Nos C, de Rycke Y, Thiery JP, et al. Disseminated tumor cells of breast cancer patients: a strong prognostic factor for distant and local relapse. Clin. Cancer Res. 2008;14:3306–3311. doi: 10.1158/1078-0432.CCR-07-4749. [DOI] [PubMed] [Google Scholar]
- Bissell MJ. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int. Rev. Cytol. 1981;70:27–100. doi: 10.1016/s0074-7696(08)61130-4. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hall HG. Nevell MC, Neville CWD, editors. Form and function in the mammary gland: the role of extracellular matrix. The Mammary Gland: Development, Regulation and Function. 1987.
- Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Kenny PA, Radisky DC. Microenvironmental regulators of tissue structure and function also regulate tumor induction and progression: the role of extracellular matrix and its degrading enzymes. Cold Spring Harb. Symp. Quant. Biol. 2005;70:343–356. doi: 10.1101/sqb.2005.70.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky D. Putting tumours in context. Nat. Rev. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Radisky DC, Rizki A, Weaver VM, Petersen OW. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537–546. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P, McPherson J, Sage H. Synthesis and secretion of structural macromolecules by endothelial cells in culture. Pathobiology of the Endothelial Cell, P and S Biomedical Sciences Symposia. 1982.
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussard KM, Boulanger CA, Booth BW, Bruno RD, Smith GH. Reprogramming human cancer cells in the mouse mammary gland. Cancer Res. 2010;70:6336–6343. doi: 10.1158/0008-5472.CAN-10-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps JL, Chang SM, Hsu TC, Freeman MR, Hong SJ, Zhau HE, et al. Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc. Natl. Acad. Sci. U.S.A. 1990;87:75–79. doi: 10.1073/pnas.87.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Liu X, Lin ED, Wang C, Choi EY, Riban V, et al. Environmental and genetic activation of a brain-adipocyte BDNF/Leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim. Biophys. Acta. 2009;1793:911–920. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Cordes N, Meineke V. Cell adhesion-mediated radioresistance (CAM-RR). Extracellular matrix-dependent improvement of cell survival in human tumor and normal cells in vitro. Strahlenther Onkol. 2003;179:337–344. doi: 10.1007/s00066-003-1074-4. [DOI] [PubMed] [Google Scholar]
- Cordes N, van Beuningen D. Arrest of human lung fibroblasts in G2 phase after irradiation is regulated by converging phosphatidylinositol-3 kinase and beta1-integrin signaling in vitro. Int. J. Radiat. Oncol. Biol. Phys. 2004;58:453–462. doi: 10.1016/j.ijrobp.2003.09.069. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int. J. Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93:1658–1667. [PMC free article] [PubMed] [Google Scholar]
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem. Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- de la Fuente MT, Casanova B, Cantero E, Hernandez del Cerro M, Garcia-Marco J, Silva A, et al. Involvement of p53 in alpha4beta1 integrin-mediated resistance of B-CLL cells to fludarabine. Biochem. Biophys. Res. Commun. 2003;311:708–712. doi: 10.1016/j.bbrc.2003.10.054. [DOI] [PubMed] [Google Scholar]
- Decitre M, Gleyzal C, Raccurt M, Peyrol S, Aubert-Foucher E, Csiszar K, et al. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Lab. Invest. 1998;78:143–151. [PubMed] [Google Scholar]
- Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS. Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science. 1996;274:2057–2059. doi: 10.1126/science.274.5295.2057. [DOI] [PubMed] [Google Scholar]
- Di Paolo A, Bocci G. Drug distribution in tumors: mechanisms, role in drug resistance, and methods for modification. Curr. Oncol. Rep. 2007;9:109–114. doi: 10.1007/s11912-007-0006-3. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic-Bussod M, Balzaretti-Maggi VS, Gadbois DM. Extracellular matrix and radiation G1 cell cycle arrest in human fibroblasts. Cancer Res. 1999;59:4843–4847. [PubMed] [Google Scholar]
- Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Dolberg DS, Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309:552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- Dolberg DS, Hollingsworth R, Hertle M, Bissell MJ. Wounding and its role in RSV-mediated tumor formation. Science. 1985;230:676–678. doi: 10.1126/science.2996144. [DOI] [PubMed] [Google Scholar]
- Durand RE, Olive PL. Resistance of tumor cells to chemo- and radiotherapy modulated by the three-dimensional architecture of solid tumors and spheroids. Methods Cell Biol. 2001;64:211–233. doi: 10.1016/s0091-679x(01)64015-9. [DOI] [PubMed] [Google Scholar]
- Durand RE, Sutherland RM. Effects of intercellular contact on repair of radiation damage. Exp. Cell Res. 1972;71:75–80. doi: 10.1016/0014-4827(72)90265-0. [DOI] [PubMed] [Google Scholar]
- Ebihara T, Venkatesan N, Tanaka R, Ludwig MS. Changes in extracellular matrix and tissue viscoelasticity in bleomycin-induced lung fibrosis. Temporal aspects. Am. J. Respir. Crit. Care Med. 2000;162:1569–1576. doi: 10.1164/ajrccm.162.4.9912011. [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Flotho C, Coustan-Smith E, Pei D, Cheng C, Song G, Pui CH, et al. A set of genes that regulate cell proliferation predicts treatment outcome in childhood acute lymphoblastic leukemia. Blood. 2007;110:1271–1277. doi: 10.1182/blood-2007-01-068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotho C, Coustan-Smith E, Pei D, Iwamoto S, Song G, Cheng C, et al. Genes contributing to minimal residual disease in childhood acute lymphoblastic leukemia: prognostic significance of CASP8AP2. Blood. 2006;108:1050–1057. doi: 10.1182/blood-2006-01-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J, Kalluri R. Tumor angiogenesis. In: Holland JF, Frei E III, Bast RC Jr., Kufe DW, Pollock RE, Weichselbaum RR, editors. Cancer Medicine. 6th ed. PC Decker Inc.; Hamilton, Ontario, Canada: 2002. [Google Scholar]
- Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- Frederick BA, Helfrich BA, Coldren CD, Zheng D, Chan D, Bunn PA, Jr., et al. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Mol. Cancer Ther. 2007;6:1683–1691. doi: 10.1158/1535-7163.MCT-07-0138. [DOI] [PubMed] [Google Scholar]
- Fridman R, Giaccone G, Kanemoto T, Martin GR, Gazdar AF, Mulshine JL. Reconstituted basement membrane (matrigel) and laminin can enhance the tumorigenicity and the drug resistance of small cell lung cancer cell lines. Proc. Natl. Acad. Sci. U.S.A. 1990;87:6698–6702. doi: 10.1073/pnas.87.17.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Fang Z, Liang Y, Zhu X, Prins P, Li Z, et al. Overexpression of inte-grin beta1 inhibits proliferation of hepatocellular carcinoma cell SMMC-7721 through preventing Skp2-dependent degradation of p27 via PI3K pathway. J. Cell Biochem. 2007;102:704–718. doi: 10.1002/jcb.21323. [DOI] [PubMed] [Google Scholar]
- Fuchs BC, Fujii T, Dorfman JD, Goodwin JM, Zhu AX, Lanuti M, et al. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008;68:2391–2399. doi: 10.1158/0008-5472.CAN-07-2460. [DOI] [PubMed] [Google Scholar]
- Goodison S, Kawai K, Hihara J, Jiang P, Yang M, Urquidi V, et al. Prolonged dormancy and site-specific growth potential of cancer cells spontaneously disseminated from nonmetastatic breast tumors as revealed by labeling with green fluorescent protein. Clin. Cancer Res. 2003;9:3808–3814. [PubMed] [Google Scholar]
- Graham CH, Kobayashi H, Stankiewicz KS, Man S, Kapitain SJ, Kerbel RS. Rapid acquisition of multicellular drug resistance after a single exposure of mammary tumor cells to antitumor alkylating agents. J. Natl. Cancer Inst. 1994;86:975–982. doi: 10.1093/jnci/86.13.975. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J. Cell Sci. 2002;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harach HR, Franssila KO, Wasenius V. Occult papillary carcinoma of the thyroid: a “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Cunha GR, Wong YC. Influence of male genital tract mesenchymes on differentiation of Dunning prostatic adenocarcinoma. Cancer Res. 1990;50:4747–4754. [PubMed] [Google Scholar]
- Hazlehurst LA, Argilagos RF, Dalton WS. Beta1 integrin mediated adhesion increases Bim protein degradation and contributes to drug resistance in leukaemia cells. Br. J. Haematol. 2007;136:269–275. doi: 10.1111/j.1365-2141.2006.06435.x. [DOI] [PubMed] [Google Scholar]
- Hazlehurst LA, Enkemann SA, Beam CA, Argilagos RF, Painter J, Shain KH, et al. Genotypic and phenotypic comparisons of de novo and acquired melphalan resistance in an isogenic multiple myeloma cell line model. Cancer Res. 2003;63:7900–7906. [PubMed] [Google Scholar]
- Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat. Rev. Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Blelloch R, Brennan C, Yamada Y, Kim M, Chin L, et al. Reprogramming of a melanoma genome by nuclear transplantation. Genes Dev. 2004;18:1875–1885. doi: 10.1101/gad.1213504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by beta 1 integrins in normal human breast epithelium but not in breast carcinoma. J. Cell Sci. 1995;108(Pt 5):1945–1957. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- Howlett AR, Carter VC, Martin GS, Bissell MJ. pp60v-src tyrosine kinase is expressed and active in sarcoma-free avian embryos microinjected with Rous sarcoma virus. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7587–7591. doi: 10.1073/pnas.85.20.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt DG, Rizzo M, Gerritsen ME, Pitt BR, Lazo JS. Integrin activation protects pulmonary endothelial cells from the genotoxic effects of bleomycin. Am. J. Physiol. 1997;273:L612–L617. doi: 10.1152/ajplung.1997.273.3.L612. [DOI] [PubMed] [Google Scholar]
- Ingber D. Integrins as mechanochemical transducers. Curr. Opin. Cell Biol. 1991;3:841–848. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- Itoh M, Nelson CM, Myers CA, Bissell MJ. Rap1 integrates tissue polarity, lumen formation, and tumorigenic potential in human breast epithelial cells. Cancer Res. 2007;67:4759–4766. doi: 10.1158/0008-5472.CAN-06-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar P, Espina V, Williams TW, Lin Y, Berry D, Jelicks LA, et al. Adipocyte-derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J. Clin. Invest. 2005;115:1163–1176. doi: 10.1172/JCI23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J. Natl. Cancer Inst. 1989;81:570–576. doi: 10.1093/jnci/81.8.570. [DOI] [PubMed] [Google Scholar]
- Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv. Drug Deliv. Rev. 2001;46:149–168. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- Javaherian A, Vaccariello M, Fusenig NE, Garlick JA. Normal keratinocytes suppress early stages of neoplastic progression in stratified epithelium. Cancer Res. 1998;58:2200–2208. [PubMed] [Google Scholar]
- Jones CB, McIntosh J, Huang H, Graytock A, Hoyt DG. Regulation of bleomycin-induced DNA breakage and chromatin structure in lung endothelial cells by integrins and poly(ADP-ribose) polymerase. Mol. Pharmacol. 2001;59:69–75. doi: 10.1124/mol.59.1.69. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, et al. Chemoresistance to paclitaxel induces epithelial–mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int. J. Oncol. 2007;31:277–283. [PubMed] [Google Scholar]
- Karrison TG, Ferguson DJ, Meier P. Dormancy of mammary carcinoma after mastectomy. J. Natl. Cancer Inst. 1999;91:80–85. doi: 10.1093/jnci/91.1.80. [DOI] [PubMed] [Google Scholar]
- Kenny PA, Bissell MJ. Targeting TACE-dependent EGFR ligand shedding in breast cancer. J. Clin. Invest. 2007;117:337–345. doi: 10.1172/JCI29518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS, St Croix B, Florenes VA, Rak J. Induction and reversal of cell adhesion-dependent multicellular drug resistance in solid breast tumors. Hum. Cell. 1996;9:257–264. [PubMed] [Google Scholar]
- Kirshner J, Chen CJ, Liu P, Huang J, Shively JE. CEACAM1-4S, a cell-cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc. Natl. Acad. Sci. U.S.A. 2003;100:521–526. doi: 10.1073/pnas.232711199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Man S, Graham CH, Kapitain SJ, Teicher BA, Kerbel RS. Acquired multicellular-mediated resistance to alkylating agents in cancer. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3294–3298. doi: 10.1073/pnas.90.8.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konecny GE, Venkatesan N, Yang G, Dering J, Ginther C, Finn R, et al. Activity of lapatinib a novel HER2 and EGFR dual kinase inhibitor in human endometrial cancer cells. Br. J. Cancer. 2008;98:1076–1084. doi: 10.1038/sj.bjc.6604278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korah R, Boots M, Wieder R. Integrin alpha5beta1 promotes survival of growth-arrested breast cancer cells: an in vitro paradigm for breast cancer dormancy in bone marrow. Cancer Res. 2004;64:4514–4522. doi: 10.1158/0008-5472.CAN-03-3853. [DOI] [PubMed] [Google Scholar]
- Kremer CL, Schmelz M, Cress AE. Integrin-dependent amplification of the G2 arrest induced by ionizing radiation. Prostate. 2006;66:88–96. doi: 10.1002/pros.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, et al. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- Krouskop TA, Wheeler TM, Kallel F, Garra BS, Hall T. Elastic moduli of breast and prostate tissues under compression. Ultrason. Imaging. 1998;20:260–274. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Kasemeier-Kulesa JC, Teddy JM, Margaryan NV, Seftor EA, Seftor RE, et al. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3752–3757. doi: 10.1073/pnas.0506977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J. Cell Biol. 2006;172:973–981. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Radisky DC, Wang F, Bissell MJ. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J. Cell Biol. 2004;164:603–612. doi: 10.1083/jcb.200306090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwin T, Hazlehurst LA, Dessureault S, Lai R, Bai W, Sotomayor E, et al. Cell adhesion induces p27Kip1-associated cell-cycle arrest through down-regulation of the SCFSkp2 ubiquitin ligase pathway in mantle-cell and other non-Hodgkin B-cell lymphomas. Blood. 2007;110:1631–1638. doi: 10.1182/blood-2006-11-060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. U.S.A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniotis AJ, Valyi-Nagy K, Karavitis J, Moses J, Boddipali V, Wang Y, et al. Chromatin organization measured by AluI restriction enzyme changes with malignancy and is regulated by the extracellular matrix and the cytoskeleton. Am. J. Pathol. 2005;166:1187–1203. doi: 10.1016/S0002-9440(10)62338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mass RD, Fyfe G, Hambleton J, Kabbinavar F, Hurwitz H, Novotny W, et al. Bevacizumab in combination with 5-FU/leucovorin improves survival in patients with metastatic colorectal cancer: a combined analysis. Proc. Am. Soc. Clin. Oncol. 2004:A3616. [Google Scholar]
- Maubant S, Cruet-Hennequart S, Poulain L, Carreiras F, Sichel F, Luis J, et al. Altered adhesion properties and alphav integrin expression in a cisplatin-resistant human ovarian carcinoma cell line. Int. J. Cancer. 2002;97:186–194. doi: 10.1002/ijc.1600. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Vellon L, Mehmi I, Teng PK, Griggs DW, Lupu R. A novel CYR61-triggered ‘CYR61-alphavbeta3 integrin loop’ regulates breast cancer cell survival and chemosensitivity through activation of ERK1/ERK2 MAPK signaling pathway. Oncogene. 2005;24:761–779. doi: 10.1038/sj.onc.1208238. [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor–stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28:38–44. doi: 10.1097/00006676-200401000-00006. [DOI] [PubMed] [Google Scholar]
- Mori Y, Shimizu N, Dallas M, Niewolna M, Story B, Williams PJ, et al. Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood. 2004;104:2149–2154. doi: 10.1182/blood-2004-01-0236. [DOI] [PubMed] [Google Scholar]
- Morrison CJ, Butler GS, Rodriguez D, Overall CM. Matrix metalloproteinase proteomics: substrates, targets, and therapy. Curr. Opin. Cell Biol. 2009;21:645–653. doi: 10.1016/j.ceb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Motrescu ER, Rio MC. Cancer cells, adipocytes and matrix metalloproteinase 11: a vicious tumor progression cycle. Biol. Chem. 2008;389:1037–1041. doi: 10.1515/BC.2008.110. [DOI] [PubMed] [Google Scholar]
- Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 2002;62:7102–7109. [PubMed] [Google Scholar]
- Myers CA, Schmidhauser C, Mellentin-Michelotti J, Fragoso G, Roskelley CD, Casperson G, et al. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol. Cell. Biol. 1998;18:2184–2195. doi: 10.1128/mcb.18.4.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumov GN, MacDonald IC, Weinmeister PM, Kerkvliet N, Nadkarni KV, Wilson SM, et al. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62:2162–2168. [PubMed] [Google Scholar]
- Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VH, Groom AC, et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res. Treat. 2003;82:199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- Nefedova Y, Landowski TH, Dalton WS. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia. 2003;17:1175–1182. doi: 10.1038/sj.leu.2402924. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell. Dev. Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M, Thomsen JL, Primdahl S, Dyreborg U, Andersen JA. Breast cancer and atypia among young and middle-aged women: a study of 110 medicolegal autopsies. Br. J. Cancer. 1987;56:814–819. doi: 10.1038/bjc.1987.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak D, Stewart D, Koeffler HP. Differentiation therapy of leukemia: 3 decades of development. Blood. 2009;113:3655–3665. doi: 10.1182/blood-2009-01-198911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr JW. The changes antecedent to tumour formation during the treatment of mouse skin with carcinogenic hydrocarbons. J. Pathol. Bacteriol. 1938;46:495–515. [Google Scholar]
- Padera TP, Stoll BR, Tooredman JB, Capen D, di Tomaso E, Jain RK. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- Pander C. Beitrage zur Entwickelungsgeschichte des Huhnchens im Eye. H.L. Bronner; Wurzburg: 1817. [Google Scholar]
- Pantel K, Schlimok G, Braun S, Kutter D, Lindemann F, Schaller G, et al. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J. Natl. Cancer Inst. 1993;85:1419–1424. doi: 10.1093/jnci/85.17.1419. [DOI] [PubMed] [Google Scholar]
- Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–1535. doi: 10.1158/0008-5472.CAN-05-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68:4398–4405. doi: 10.1158/0008-5472.CAN-07-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, et al. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am. J. Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. U.S.A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard O, Rolland Y, Poupon MF. Fibroblast-dependent tumorigenicity of cells in nude mice: implication for implantation of metastases. Cancer Res. 1986;46:3290–3294. [PubMed] [Google Scholar]
- Plewes DB, Bishop J, Samani A, Sciarretta J. Visualization and quantification of breast cancer biomechanical properties with magnetic resonance elastography. Phys. Med. Biol. 2000;45:1591–1610. doi: 10.1088/0031-9155/45/6/314. [DOI] [PubMed] [Google Scholar]
- Postovit LM, Seftor EA, Seftor RE, Hendrix MJ. Influence of the microenvironment on melanoma cell fate determination and phenotype. Cancer Res. 2006;66:7833–7836. doi: 10.1158/0008-5472.CAN-06-0731. [DOI] [PubMed] [Google Scholar]
- Radisky D, Hagios C, Bissell MJ. Tumors are unique organs defined by abnormal signaling and context. Semin. Cancer Biol. 2001;11:87–95. doi: 10.1006/scbi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Ricchi P, Zarrilli R, Di Palma A, Acquaviva AM. Nonsteroidal anti-inflammatory drugs in colorectal cancer: from prevention to therapy. Br. J. Cancer. 2003;88:803–807. doi: 10.1038/sj.bjc.6600829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich AR. Classics in oncology: on the frequency of occurrence of occult carcinoma of the prostate. CA. Cancer J. Clin. 1979;29:115–119. doi: 10.3322/canjclin.29.2.115. [DOI] [PubMed] [Google Scholar]
- Roose T, Netti PA, Munn LL, Boucher Y, Jain RK. Solid stress generated by spheroid growth estimated using a linear poroelasticity model small star, filled. Microvasc. Res. 2003;66:204–212. doi: 10.1016/s0026-2862(03)00057-8. [DOI] [PubMed] [Google Scholar]
- Salgaller ML. Technology evaluation: bevacizumab, Genentech/Roche. Curr. Opin. Mol. Ther. 2003;5:657–667. [PubMed] [Google Scholar]
- Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandal T, Valyi-Nagy K, Spencer VA, Folberg R, Bissell MJ, Maniotis AJ. Epigenetic reversion of breast carcinoma phenotype is accompanied by changes in DNA sequestration as measured by AluI restriction enzyme. Am. J. Pathol. 2007;170:1739–1749. doi: 10.2353/ajpath.2007.060922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. A novel transcriptional enhancer is involved in the prolactin- and extra-cellular matrix-dependent regulation of beta-casein gene expression. Mol. Biol. Cell. 1992;3:699–709. doi: 10.1091/mbc.3.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Lu Y, Parant JM, Lozano G, Bacher G, Beckers T, et al. Differential roles of p21(Waf1) and p27(Kip1) in modulating chemosensitivity and their possible application in drug discovery studies. Mol. Pharmacol. 2001;60:900–906. doi: 10.1124/mol.60.5.900. [DOI] [PubMed] [Google Scholar]
- Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, Walsh S, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–1205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat. Med. 1999;5:662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann. Surg. Oncol. 2007;14:3629–3637. doi: 10.1245/s10434-007-9583-5. [DOI] [PubMed] [Google Scholar]
- Shain KH, Landowski TH, Dalton WS. Adhesion-mediated intracellular redistribution of c-Fas-associated death domain-like IL-1-converting enzyme-like inhibitory protein-long confers resistance to CD95-induced apoptosis in hematopoietic cancer cell lines. J. Immunol. 2002;168:2544–2553. doi: 10.4049/jimmunol.168.5.2544. [DOI] [PubMed] [Google Scholar]
- Shain KH, Yarde DN, Meads MB, Huang M, Jove R, Hazlehurst LA, et al. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009;69:1009–1015. doi: 10.1158/0008-5472.CAN-08-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat. Rev. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- Sherman-Baust CA, Weeraratna AT, Rangel LB, Pizer ES, Cho KR, Schwartz DR, et al. Remodeling of the extracellular matrix through overexpression of collagen VI contributes to cisplatin resistance in ovarian cancer cells. Cancer Cell. 2003;3:377–386. doi: 10.1016/s1535-6108(03)00058-8. [DOI] [PubMed] [Google Scholar]
- Skobe M, Fusenig NE. Tumorigenic conversion of immortal human keratinocytes through stromal cell activation. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1050–1055. doi: 10.1073/pnas.95.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Yu AE. Proteases in invasion: matrix metalloproteinases. Semin. Cancer Biol. 2001;11:143–152. doi: 10.1006/scbi.2000.0365. [DOI] [PubMed] [Google Scholar]
- Stoker AW, Hatier C, Bissell MJ. The embryonic environment strongly attenuates v-src oncogenesis in mesenchymal and epithelial tissues, but not in endothelia. J. Cell Biol. 1990;111:217–228. doi: 10.1083/jcb.111.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RM, Eddy HA, Bareham B, Reich K, Vanantwerp D. Resistance to adriamycin in multicellular spheroids. Int. J. Radiat. Oncol. Biol. Phys. 1979;5:1225–1230. doi: 10.1016/0360-3016(79)90643-6. [DOI] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, et al. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J. Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin D. Fine structure of murine mammary tumours: the relationship between epithelium and connective tissue in neoplasms induced by various agents. Br. J. Cancer. 1969;23:417–425. doi: 10.1038/bjc.1969.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher BA, Herman TS, Holden SA, Wang YY, Pfeffer MR, Crawford JW, et al. Tumor resistance to alkylating agents conferred by mechanisms operative only in vivo. Science. 1990;247:1457–1461. doi: 10.1126/science.247.4949.1457. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, Behrendtsen O, et al. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am. J. Pathol. 1998;153:457–467. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson S, Buck E, Petti F, Griffin G, Brown E, Ramnarine N, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–9462. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat. Med. 2006;12:925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- Uhm JH, Dooley NP, Kyritsis AP, Rao JS, Gladson CL. Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin. Cancer Res. 1999;5:1587–1594. [PubMed] [Google Scholar]
- van Stijn A, Feller N, Kok A, van der Pol MA, Ossenkoppele GJ, Schuurhuis GJ. Minimal residual disease in acute myeloid leukemia is predicted by an apoptosis-resistant protein profile at diagnosis. Clin. Cancer Res. 2005;11:2540–2546. doi: 10.1158/1078-0432.CCR-04-1973. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat. Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Wang F, Hansen RK, Radisky D, Yoneda T, Barcellos-Hoff MH, Petersen OW, et al. Phenotypic reversion or death of cancer cells by altering signaling pathways in three-dimensional contexts. J. Natl. Cancer Inst. 2002;94:1494–1503. doi: 10.1093/jnci/94.19.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington C, Dalbegue F, Abreo F, Taubenberger JK, Lichy JH. Loss of heterozygosity in fibrocystic change of the breast: genetic relationship between benign proliferative lesions and associated carcinomas. Am. J. Pathol. 2000;157:323–329. doi: 10.1016/S0002-9440(10)64543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Bissell MJ. Functional culture models to study mechanisms governing apoptosis in normal and malignant mammary epithelial cells. J. Mammary Gland Biol. Neoplasia. 1999;4:193–201. doi: 10.1023/a:1018781325716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Howlett AR, Langton-Webster B, Petersen OW, Bissell MJ. The development of a functionally relevant cell culture model of progressive human breast cancer. Semin. Cancer Biol. 1995;6:175–184. doi: 10.1006/scbi.1995.0021. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res. Treat. 2010;122:35–43. doi: 10.1007/s10549-009-0502-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir ML, Oppizzi ML, Henry MD, Onishi A, Campbell KP, Bissell MJ, et al. Dystroglycan loss disrupts polarity and beta-casein induction in mammary epithelial cells by perturbing laminin anchoring. J. Cell Sci. 2006;119:4047–4058. doi: 10.1242/jcs.03103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedswang G, Borgen E, Karesen R, Qvist H, Janbu J, Kvalheim G, et al. Isolated tumor cells in bone marrow three years after diagnosis in disease-free breast cancer patients predict unfavorable clinical outcome. Clin. Cancer Res. 2004;10:5342–5348. doi: 10.1158/1078-0432.CCR-04-0245. [DOI] [PubMed] [Google Scholar]
- Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J. Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, et al. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin. Cancer Res. 2006;12:4147–4153. doi: 10.1158/1078-0432.CCR-06-0038. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Januario T, Eberhard DA, Cavet G, Zhu W, Fu L, et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin. Cancer Res. 2005;11:8686–8698. doi: 10.1158/1078-0432.CCR-05-1492. [DOI] [PubMed] [Google Scholar]
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ozaki I, Mizuta T, Matsuhashi S, Yoshimura T, Hisatomi A, et al. Beta 1-integrin protects hepatoma cells from chemotherapy induced apoptosis via a mitogen-activated protein kinase dependent pathway. Cancer. 2002;95:896–906. doi: 10.1002/cncr.10751. [DOI] [PubMed] [Google Scholar]