Abstract
Purpose
Previous studies have shown that ischemia alters gene expression in normal and malignant tissues. There are no studies that evaluated effects of ischemia in renal tumors. This study examines the impact of ischemia and tissue procurement conditions on RNA integrity and gene expression in renal cell carcinoma.
Experimental Design
Ten renal tumors were resected without renal hilar clamping from 10 patients with renal clear cell carcinoma. Immediately after tumor resection, a piece of tumor was snap frozen. Remaining tumor samples were stored at 4C, 22C and 37C and frozen at 5, 30, 60, 120, and 240 minutes. Histopathologic evaluation was performed on all tissue samples, and only those with greater than 80% tumor were selected for further analysis. RNA integrity was confirmed by electropherograms and quantitated using RIN index. Altered gene expression was assessed by paired, two-sample t-test between the zero time point and aliquots from various conditions obtained from the same tumor.
Results
One hundred and forty microarrays were performed. Some RNA degradation was observed 240 mins after resection at 37C. The expression of over 4,000 genes was significantly altered by ischemia times or storage conditions. The greatest gene expression changes were observed with longer ischemia time and warmer tissue procurement conditions.
Conclusion
RNA from kidney cancer remains intact for up to 4 hours post surgical resection regardless of storage conditions. Despite excellent RNA preservation, time after resection and procurement conditions significantly influence gene expression profiles. Meticulous attention to pre-acquisition variables is of paramount importance for accurate tumor profiling.
Keywords: Ischemia, gene expression microarrays, tissue procurement, renal cell carcinoma
INTRODUCTION
High throughput technologies such as gene expression microarrays have been utilized to obtain prognostic and predictive signatures that often outperform any combination of clinical or pathologic variables currently available (1-2). In oncology, these technologies play an increasingly important clinical role in providing patients with individualized information about their disease, thereby guiding therapy. As gene expression studies become clinically applicable, the accuracy and relevance of findings need to be addressed.
Gene expression microarray data is well-known to be subject to inter-platform variation, laboratory processing variation, and variation from statistical analysis (3-4). An area that has not been thoroughly explored is the effect of tissue procurement conditions and pre-acquisition variables on tumor gene expression profiles. Most published gene expression studies indicate that surgical specimens were “snap frozen” after surgery, but few specify how long and under what conditions the specimen were kept prior to “snap-freezing”. Furthermore, with the advent of laparoscopic and robotic surgery, the acquisition variables prior to tissue processing have become even more complex, as these specimen are subjected to prolonged intervals of ischemia at body temperature.
This study seeks to investigate the effect of ischemia and tissue procurement conditions on gene expression profiling in renal cell carcinoma.
MATERIALS AND METHODS
Study Design and Tissue Procurement
Solid renal tumors were obtained from ten patients with von Hippel-Lindau undergoing open partial nephrectomy at the National Cancer Institute (NCI) between 2007 and 2009. The tumors were selected for study only if the following inclusion criteria were met: 1) Preoperative evaluation with CT and intraoperative evaluation with ultrasound confirmed presence of a solid homogeneous renal mass; 2) The tumor was resected without clamping of the renal hilum (to minimize ischemia time); 3) Immediate sectioning of tumor in the operating room confirmed gross tumor homogeneity.
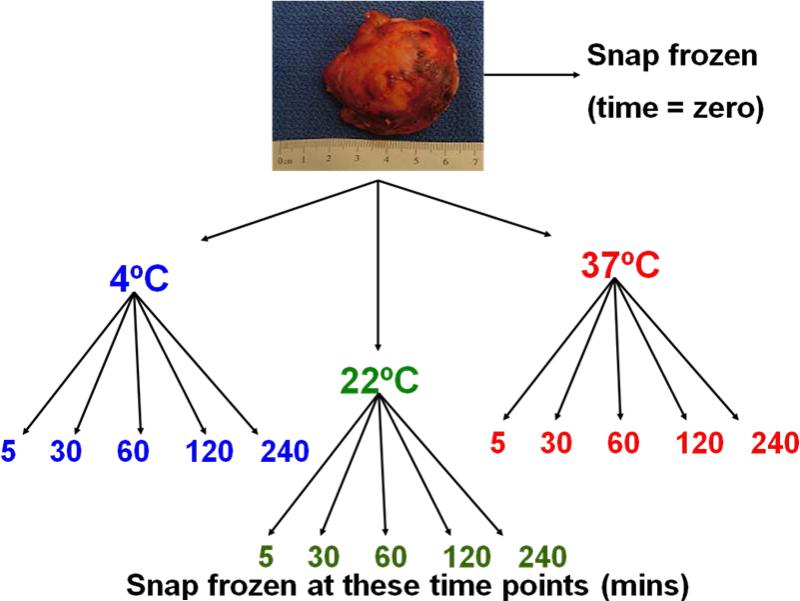
To ensure adequate amount of available tissue for analysis and to minimize tumor heterogeneity, tumors with a diameter less than 2 cm or greater than 6 cm, evidence of cystic components, areas of necrosis, or hemorrhage on gross inspection were excluded. No patient received previous chemotherapy, targeted therapy or radiotherapy. Resected tumors were evaluated and procured immediately in the operating room by a tissue procurement team consisting of a pathologist, a tissue banking technician, and an assistant. The tumor procurement process is shown in Figure 1.
Figure 1.
Study design and tissue procurement process
Briefly, immediately after surgical resection, a piece of tumor was embedded in an OCT-filled cassette (Tissue-Tek, OCT Compound, Sakura Finetek, Torrance, CA USA) and snap-frozen in isopentane solution that was chilled by dry ice. This tissue was used as the zero-time point reference sample. Remaining tumor samples were stored in phosphate buffered saline at three temperature conditions: 1) 4C to simulate tumor storage on ice, 2) 22C to simulate room temperature, and 3) 37C to simulate extracorporeal storage of tumors resected during laparoscopic or robotic surgery. At 5, 30, 60, 120 and 240 minutes after surgical resection, a piece of tissue from each temperature condition was “snap-frozen” in similar fashion as described above. Subsequently, all tissue samples were transferred to and stored in liquid nitrogen at -160C until histopathologic evaluation and RNA extraction. Tissue acquisition, processing and analysis were approved under an NCI institutional review board approved protocol.
Specimen Handling and Pathologic Confirmation
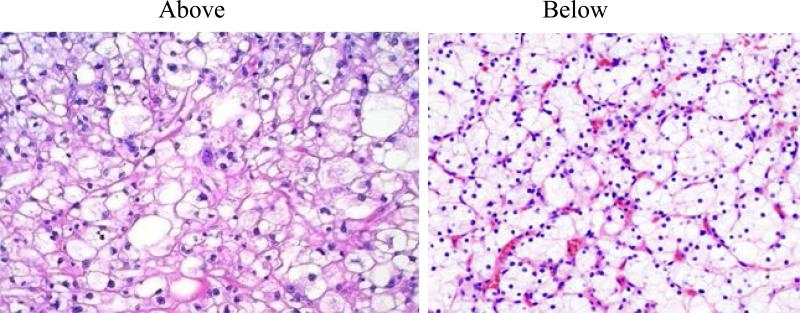
After specimen retrieval from liguid nitrogen, the representative slides of the tumor aliquot were prepared using a cryostat microtome in the laboratory. The tissue obtained from the tumor aliquot was immediately used for RNA extraction. Histopathologic evaluation, including tumor content, stromal contribution and absence of tumor necrosis, was performed by a single pathologist (CB) on hematoxylin and eosin (H&E)-stained frozen sections from the procured samples. To assure microscopic homogeneity of the tumor as well as presence of the tumor in the analyzed tissue fragment, two H&E slides, above and below the tissue used for RNA extraction, were prepared from each sample (Figure 2). Microscopic evaluation confirmed that all H&E slides contained greater than 80% clear cell renal cell carcinoma.
Figure 2.
Pictures of the H&E stained sections of the tissue samples above and below the tissue block used for RNA extraction and analysis.
RNA Isolation
Fifteen 20μm thick frozen sections from each tissue sample were homogenized in TRIZOL reagent (Invitrogen Life Techonolgies, Carlsbad, CA USA). Total RNA was isolated using a standard chloroform extraction protocol followed by a cleanup process with Qiagen RNeasy Mini Kit (QIAGEN Inc, Valencia, CA USA). RNA purity was assessed by the ratio of spectrophotometric absorbance at 260 and 280 nm (A260/280nm) using NanoDrop ND-1000 (NanoDrop Inc, Wilmington, DE USA). RNA integrity was evaluated by using RNA 6000 Nano LabChips on an Agilent 2100 Bioanalyzer (Agilent Technologies, Foster City, CA USA). All chips were prepared according to the manufacturer's instructions. Total RNA degradations were evaluated by reviewing the electropherograms and the RNA integrity number (RIN): only samples with preserved 18S and 28S peaks and RIN values greater than 7 were selected for gene expression analysis (5-6).
Microarray hybridization and Image Acquisition
Messenger RNA expression levels were analyzed using the GeneChip® HG-U133 plus 2.0 platform following the manufacturer's protocol (Affymetrix Inc, Santa Clara, CA USA). Briefly, double-stranded cDNA was synthesized from 100ng of total RNA from each tumor sample using GeneChip Two-Cycle cDNA Target Synthesis Kit (Affymetrix Inc, Santa Clara, CA USA). After two rounds of amplifications, in vitro transcription was performed using IVT Labeling Kit (Affymetrix Inc, Santa Clara, CA USA) to synthesize biotin-labeled cRNA. An aliquot of 15ug labeled cRNA was fragmented and subsequently hybridized for 16 hours at 45C in a hybridization oven to a HG-U133 plus 2.0 olgionucleotide array. Each array was then washed, stained and scanned by the Affymetrix GeneChip 3000 Scanner. Raw intensities for each probe were stored as .DAT and .CEL files by the GeneChip Operating Software v1.4 (Affymetrix Inc, Santa Clara, CA USA).
Data Analysis and Statistical Methods
Complete analyses of array data were carried out in BRB-Array Tools v3.7(7). The expression intensities for all probe sets from Affymetrix CEL-files were normalized using the robust multiarray average (RMA) algorithm (8). To determine which genes were differentially expressed at each temperature and time point, class comparison analysis was performed using a permutation paired, two-sample t-test (9). Next, a multivariate analysis was carried out using the temperature condition as a blocking variable. The null hypothesis tested in the multivariate analysis assumes there were no gene expression changes over time within a specific condition. Differential expression was considered significant at P <0.001. The MIAME-compliant microarray data are available at http://www.ncbi.nlm.nih.gov/geo/ under accession number GSE41137.
Microarray Data Validation with Quantitative Real-Time PCR
Quantitative real-time PCR (qRT-PCR) was used to validate microarray results for selected genes with known prognostic significance. An aliquot of 500ng of total RNA from snap frozen as well as samples frozen at 1 hour post surgical resection from 4C, 22C and 37C was reverse transcribed to synthesize cDNA using High Capacity cDNA Transcription Kit (Applied Biosystems Inc, Foster City, CA USA) according to manufacturer's protocol. Primer sets specific for 3 selected genes and a reference gene, Cyclophilin A, were obtained from TaqMan Gene Expression Assay using UMapIt, an online microarray-to-TaqMan assays mapping tool (Applied Biosystems, Foster City, CA USA) (10). Relative quantification of gene expression by qRT-PCR was performed in triplicate using ABI PRISM 7000 Sequence Detection System according to manufacturer's protocol (Applied Biosystems, Foster City, CA USA). The 2–ΔΔ CT method (11) was used to calculate the relative expression between snap frozen and samples with an hour of ischemic time from all three temperature conditions.
RESULTS
Patient Demographic and Clinical Characteristics
The demographic characteristics of the 10 patients in this study are listed in Table 1. Median patient age was 42 years. Median tumor size (in largest diameter) was 3.25 cm and 70% of the specimens were taken from the left kidneys. The pathologic grade for all tumor samples was Furhman grade 2.
Table 1.
Patient Demographics
| Patient ID | Age | Histology | Specimen Site | Fuhrman Grade | Tumor Size (largest diameter in cm) |
|---|---|---|---|---|---|
| 1 | 53 | ccRCC | Left Kidney | 2 | 3.0 |
| 2 | 27 | ccRCC | Right Kidney | 2 | 2.5 |
| 3 | 44 | ccRCC | Left Kidney | 2 | 3.3 |
| 4 | 68 | ccRCC | Left Kidney | 2 | 3.2 |
| 5 | 29 | ccRCC | Right Kidney | 2 | 5.0 |
| 6 | 43 | ccRCC | Left Kidney | 2 | 2.2 |
| 7 | 41 | ccRCC | Left Kidney | 2 | 4.5 |
| 8 | 28 | ccRCC | Left Kidney | 2 | 3.1 |
| 9 | 79 | ccRCC | Left Kidney | 2 | 3.5 |
| 10 | 37 | ccRCC | Left Kidney | 2 | 5.0 |
RNA Integrity of Tumor Samples from Various Storage Conditions
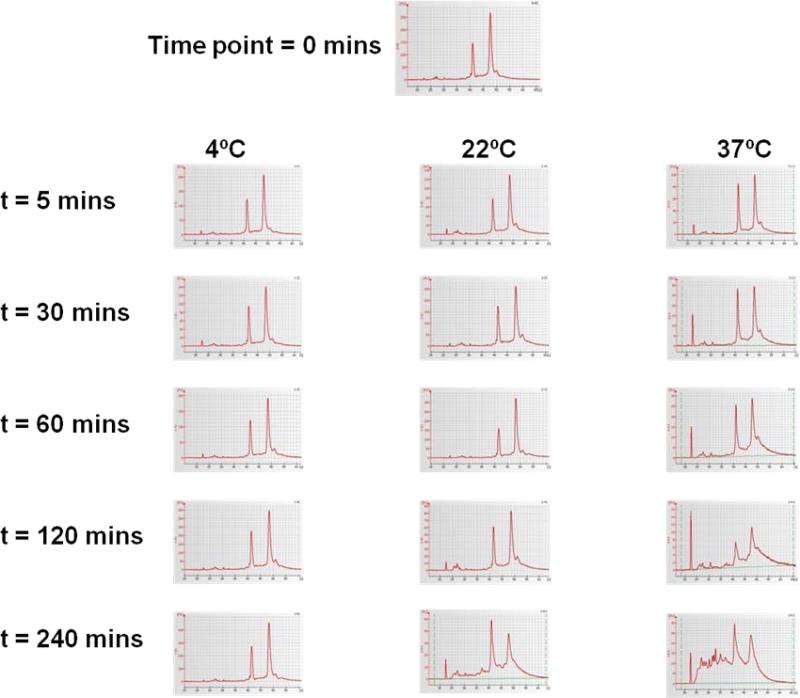
Electropherograms of various tissue procurement conditions are shown in Figure 3. No signs of RNA degradation were evident in snap frozen tissue samples and tissue samples stored in 4C. The RIN values for all snap frozen and 4C samples greater or equal to 8. Intact RNA with RIN values greater than 7 was observed in all tumors samples stored for up to 120 mins. Some RNA degradation was seen on electropherograms in those samples obtained 240 mins after surgical resection at 22C and 37C (median RIN for 37C equal to 6.1). For these reasons, samples obtained 240 mins after surgical extirpation from 22C and 37C were not selected for microarray analysis.
Figure 3.
Assessment of RNA Integrity using electrophernogram
Gene Expression Changes Associated with Tissue Procurement Conditions
Table 2 demonstrates the results from the class comparison analysis between snap frozen tissue and tissue from each of the procurement conditions. The number of differentially expressed genes increased with longer ischemia time and warmer procurement conditions. Although all genes in table 2 are statistically significant with p-values <0.001, only those that are differentially expressed at longer ischemic time or warmer temperatures have False Discovery Rate (FDR) less than 0.20. Similar trends were observed on multivariate analysis, with the number of differentially expressed genes in the samples stored at 37C significantly higher than in samples stored in 4C and 22C. Once again, the FDR decreased with warmer storage conditions.
Table 2.
Differentially Expressed Genes over Four Hours in Three Temperature Conditions
| Univariate Analysis | ||||
|---|---|---|---|---|
| Temperature | Time | Number of Differentially Expressed Genes at each time point | P-value | FDR |
| 4C | 5 mins 30 mins 60 mins 120 mins 240 mins |
30 6 29 32 633 |
<0.001 <0.001 <0.001 <0.001 <0.001 |
0.99 0.96 0.94 0.94 <0.08 |
| 22C | 5 mins 30 mins 60 mins 120 mins |
15 164 469 352 |
<0.001 <0.001 <0.001 <0.001 |
0.91 <0.29 <0.11 <0.15 |
| 37C | 5 mins 30 mins 60 mins 120 mins |
387 375 1048 3945 |
<0.001 <0.001 <0.001 <0.001 |
<0.14 <0.14 <0.05 <0.01 |
| Multivariate Analysis using a Blocking Variable | ||||
|---|---|---|---|---|
| Temperature | Time | Number of Differentially Expressed Genes across all time points | P-value | FDR |
| 4C | All time points | 130 | <0.001 | <0.40 |
| 22C | All time points | 302 | <0.001 | <0.18 |
| 37C | All time points | 4797 | <0.001 | <0.01 |
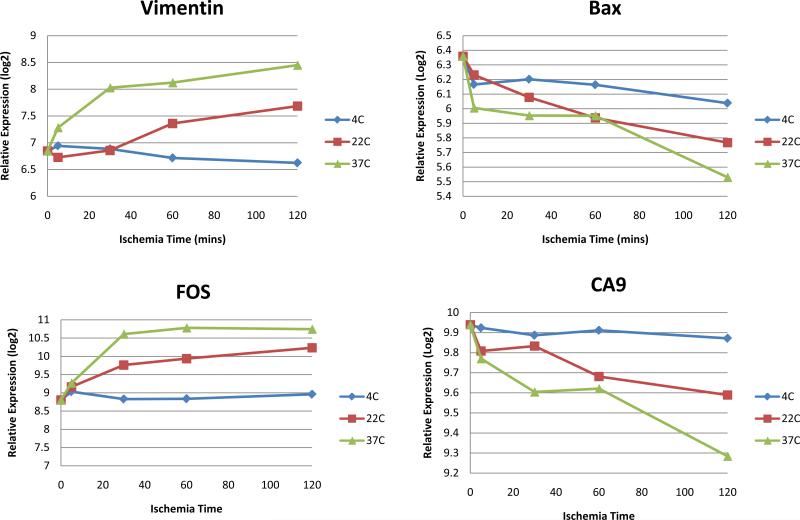
Figure 4 demonstrates the microarray gene expression summaries for four selected prognostic genes at various temperature conditions and ischemia time. The expression levels for all 4 genes remained relatively constant when the specimen was stored at 4C. Greater expression changes were observed at warmer temperature and longer ischemia time.
Figure 4.
Gene expression summaries for four selected prognostic genes at various temperature conditions and ischemia time obtained from the gene expression microarrays from 10 tumors (calculated averages from singlet experiments). Blue represents 4C, red represents 22C or room temperature, and green represents warm ischemia at 37C.
Confirmation with Quantitative Real Time-PCR
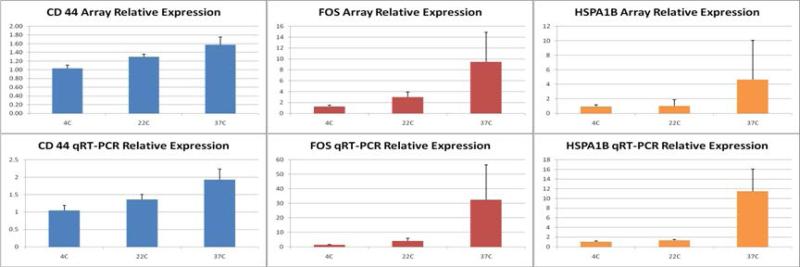
Figure 5 shows the mean relative expression levels of both microarray and qRT-PCR for three known prognostic RCC genes (CD44, FOS, and HSPA1B) from three different procurement temperature conditions at one hour post-surgical resection. For all three genes, the relative gene expression level increased with warmer tissue procurement conditions as demonstrated by both qRT-PCR and microarray analysis. Similar to the above findings, the greatest over-expression was observed at 37C whereas minimal gene expression changes were seen for specimens stored at 4C.
Figure 5.
Confirmatory real time-PCR results from 3 temperature conditions at one hour post-surgical resection
DISCUSSION
The utilization of genome-wide microarray technology has proven to be a powerful tool in identifying cancer gene expression signatures associated with prognosis as well as defining therapeutic regimen for individual patients. Such an approach to molecular stratifications of cancer requires accurate expression profiling of surgical specimens (1-2).
Several studies have demonstrated methods of tissue acquisitions alone could significantly alter tumor gene expression. Spruessel et al. demonstrated differential expression changes in approximately 2,000 genes within 30 mins after surgical resection of colon cancer (12). Lin et al. identified 61 genes that were differentially expressed as a result of surgical resection of the prostate gland (13). They also found that surgical excision artificially induced the expression of genes belonging to the JNK pathway, which may be misinterpreted as aggressive disease and resistance to chemotherapy. In a time-course experiment involving surgically resected breast cancer tissue, De Cecco et al. demonstrated that ischemia coupled with room temperature storage affected the expression profile of 461 genes with important prognostic information (14). In our study, for the first time, we aimed at addressing both: the impact of ischemia time as well as procurement conditions on RNA quality and gene expression of renal cell carcinoma.
Our study is notable for two major findings. First, RNA integrity of renal tumors remained stable up to four hours after surgical extirpation. This finding is consistent with several studies on the effect of ischemia time on RNA stability (15-18). Micke et al., for example, similarly demonstrated that RNA extracted from normal tonsil and colon tissue remained stable up to 16 hours after surgical resection (15). Our second finding, however, revealed that despite excellent RNA quality on electropherogram with high RIN numbers, there were significant gene expression changes not depicted on RNA quality assays. In addition to ischemia time, the storage temperature conditions also had a profound effect on the levels of changes in gene expression (Table 2). This finding was further supported by low False Discovery Rate (FDR) observed at higher temperature storage conditions. FDR is an important additional statistical method used to ensure that some genes discovered as significant are indeed significantly different in their expression (3). The FDR in Table 2 decreased with longer ischemia time and higher temperature conditions, confirming that the number of genes differentially expressed at 37C were truly statistically significant (as reflected by smaller FDR values). Additionally, the number of differentially expressed genes identified at 37C vastly outnumbered that of 4C and 22C at each one of the respective time points. For instance, at two hours after surgical extirpation 1,048 genes were differentially expressed at 37C compared to only 29 and 469 genes at 4C and 22C, respectively. Notably, storage conditions mimicking both laparoscopy (37C) and room temperature (22C) resulted in significant alteration of tumor profiling.
The present study may have a profound clinical relevance because it demonstrates that the gene expression pattern obtained from tumors procured under laparoscopic or robotic procedures, or subjected to prolonged storage at room temperature, may be a reflection of “periprocedural insult” rather than true tumor biology. As more procedures in surgical oncology are performed using minimally-invasive techniques, the methods of tissue acquisitions must be carefully monitored and reviewed. Currently there are no published data that specifically examine the effect of storage conditions or laparoscopic surgery on tumor gene expression. Dash et al. addressed the effect of warm ischemia time on radical prostatectomy specimens, and found that 41 genes were overexpressed at one hour post-surgical resection (19). Their study, however, was done at room temperature rather than under true laparoscopic conditions. With more oncologic surgeries being performed using the minimally-invasive approach, the validity of gene expression data obtained from specimen resected via laparoscopic or robotic approach must be addressed.
The emergence of high throughput microarray technology has led to the discovery of a plethora of molecular prognosticating biomarkers for different cancer types. A recent literature review by Nogueira et al. described at least sixty RCC biomarkers with prognostic implications discovered by gene and protein expression experiments (20). In this study, we found that at least 25 of these known biomarkers were differentially expressed at 37C, underscoring the ischemia sensitive nature of these prognostic signatures. For example, our data demonstrated that laparoscopic conditions altered the expression of several genes with known important prognostic implications in patients with RCC (21-23). Vimentin (VIM), CD44, FOS, hypoxia-inducible factor 1-alpha (HIF-1a) and other genes have been used in prognostic nomograms or found to correlate with aggressiveness of RCC (21-23). In this situation, upregulation of these genes may be interpreted as aggressive disease and poor clinical outcomes, while our data showed that the overexpression of these genes is secondary to the procurement conditions and not due to underlying tumor biology.
Therefore, the use of expression data from tumors obtained under sub-optimal conditions to guide clinical judgment should be interpreted with caution. Additionally, prior studies on prognostic biomarkers using microarrays may need to be carefully re-evaluated as specimen procurement conditions and time from tumor resection to processing were rarely reported. Furthermore, tissue specimen in tumor banks collected years ago must be carefully assessed for use in gene expression profiling.
We chose to validate our microarray data using quantitative RT-PCR for CD44, fos, and HSPA1B based on their expression pattern at 60 mins after surgical extirpation as well as prognostic implications. CD44 is a transmembrane glycoprotein involved in tumor progression, and expression of this gene in RCC is associated with adverse prognosis (24). The proto-oncogene c-fos plays an important role in the growth and differentiation of renal tissue and its overexpression has been implicated in the formation of RCC (25-26). HSPA1B is the gene that encodes Heat Shock Protein 70 (HSP70), which is an important prognostic gene for many cancer types including, ovarian cancer, gastric cancer, esophageal cancer and more recently, Wilm's tumor (27-30). All three genes were significantly over expressed at 37C underscoring the profound influence of tissue procurement conditions on gene expression changes of surgical specimens. Interestingly, the gene expression level at 4C for all 3 genes remained relatively stable compared to snap frozen suggesting that tumor expression profile may be preserved up to one hour after resection when stored on ice.
Thus far, our results demonstrated that the methods of tissue acquisition clearly have a profound impact on renal tumor gene expression and may allow for guidance regarding tissue collection and handling practices for renal cell carcinoma. Immediately after surgical resection, renal tumor should be removed and snap frozen to preserve gene expression profile. If immediate snap freezing is not possible in the operating room, renal tumor should be placed on ice immediately after surgical extirpation and frozen within one hour after resection. In the setting of laparoscopic or robotic surgery, where immediate removal of specimen is not always feasible resulting in warm ischemic conditions for greater than 30 minutes, interpretation of molecular profiling should be done with great caution.
We should acknowledge a few potential drawbacks of our study. First, we did not use laser capture microdissection (LCM) to specifically extract tumor cells from surgical specimen. We chose not to use LCM because the laser dissection process requires warming the tissue block to room temperature for approximately 30 minutes, enough time to confound the effect of tissue procurement conditions on tumor expression profile. Additionally, with increased understanding of tumor microenvironment we chose to preserve and analyze the “whole” tumor rather than tumoral cells alone. Instead, we have meticulously processed and confirmed that the analyzed aliquot consisted of tumor by preparing over 300 H&E slides above and below each tissue block containing the aliquot. Second, we chose not to utilize core biopsies to be able to adequately assess the composition of the studied aliquot with H&Es and ensure adequate amounts of tissue obtained for RNA studies. Third, we realize that despite our maximal efforts to procure from homogeneous mass there is inherent heterogeneity in each tumor. This was addressed by obtaining as many as 16 aliquots from each tumor and performing gene expression analysis on almost 90% of all aliquots (140 from 160). Fourth, since tumor samples were taken from patients with VHL, a hereditary disorder that is responsible for less than 4% of all RCC (31), one may wonder about applicability of our data to the general population with sporadic RCC. We should point out that the vast majority of sporadic clear cell RCC harbor mutations in the VHL gene (32) suggesting that our findings on the effect of ischemia on clear cell tumors are valid. Furthermore, the pseudohypoxic mechanism for tumor formation in VHL tumors is similar to their sporadic clear RCC counterparts (33). In fact, we chose to perform this experiment on VHL tumors because of their homogeneous nature, surgical accessibility, well characterized molecular pathways, and similarity with the most common type sporadic clear cell RCC (34). Finally, while our results may not be a true reflection of gene expression profiles in other types of RCC or other tumor types, we have deliberately kept our tumor selection as pure as we possibly could.
Several strengths of this study should also be noted. First, this study utilized the most robust gene expression technology available to date with 140 microarrays studied. Second, all tumors were resected without the effect of renal hilar clamping or vascular ischemia and were processed immediately by a dedicated tissue procurement team in the operated room; therefore, we have maximally controlled for every confounding variable potentially affecting our results. Third, this study allows for identification of ischemia-sensitive and procurement-sensitive genes as well as those genes that are resilient to procurement conditions. Such genes from various tumor types will hopefully become available to the scientific community, eventually influencing patient care. Creation of such libraries may become paramount importance in the future. Finally, our study demonstrates that simple tissue handling, such as placing tumor specimen on ice immediately after surgical resection may maximally preserve tumor biological profile for an extended period of time.
In summary, the present study is the first attempt to address the influence of both ischemia time and different storage conditions on gene expression profiles in renal cell carcinoma. We demonstrate that despite excellent RNA preservation, pre-acquisition variables significantly influence tumor profiles. This work underscores the importance of meticulous documentation of tumor handling and processing and minimizing warm ischemic conditions.
CONCLUSION
Our data demonstrate that RNA from renal cell cancer remains intact for up to 4 hours post surgical resection when stored on ice. Despite RNA preservation sufficient for gene expression analysis, prolonged warm ischemia is associated with significant changes in gene expression profiles. Placing tumor on ice immediately after surgical extirpation may allow for preservation of molecular profile. In the setting of laparoscopic or robotic surgery, where immediate removal of specimen is not always feasible resulting in warm ischemic conditions for greater than 30 minutes, interpretation of molecular profiling should be done with great caution.
Translational Relevance.
The advent of high throughput technologies, such as gene expression profiling, have enabled the discovery of tumor markers that are associated with prognostic and predictive outcomes. As gene expression studies become clinically applicable, the accuracy and reliability of findings need to be addressed. An area that has not been thoroughly explored is the effect of tissue procurement conditions on tumor expression profiles. To discover the effect of tissue acquisition variables on tumor gene expressions, we conducted expression array analyses on renal cell carcinoma at three different temperature conditions and five different time points. Our study demonstrates that the method of tissue acquisition alone greatly influenced tumor gene expression profile. Our findings suggest that data derived from gene expression studies may be a reflection of tissue procurement conditions rather than true tumor biology and, therefore, highlight the importance of establishing standardized protocols for human specimen collection in clinical and translational research.
Acknowledgments
Role of the Funding Source: This study was supported entirely by the NIH Intramural Research Grant.
Footnotes
Disclosure of Potential Conflicts of Interest: None
Authors’ Contributions:
Conception and design: N.W. Liu, W.M. Linehan and G. Bratslavsky
Development of methodology: N.W. Liu, R. Srinivasan and G. Bratslavsky
Acquisition of data: N.W. Liu, T. Sanford, K. Khurana, J.L. Liu, and G. Bratslavsky
Analysis and interpretation of data: N.W. Liu, T. Sanford, C. Bechert, M. Merino, W.M. Linehan, and G. Bratslavsky
Writing, review, and/or revision of the manuscript: N.W. Liu, T. Sanford, R. Srinivasan, W.M. Linehan, and G. Bratslavsky.
Administrative, technical, or material support: O. Aprelikova, V. Valero, R. Worrell, P.A. Pinto, and Y. Yang
Study supervision: G. Bratslavsky
Reference List
- 1.van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 2.Beer DG, Kardia SL, Huang CC, Giordano TJ, Levin AM, Misek DE, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–24. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 3.Shi L, Tong W, Fang H, Scherf U, Han J, Puri RK, et al. Cross-platform comparability of microarray technology: intra-platform consistency and appropriate data analysis procedures are essential. BMC Bioinformatics. 2005;6(Suppl 2):S12. doi: 10.1186/1471-2105-6-S2-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi L, Reid LH, Jones WD, Shippy R, Warrington JA, Baker SC, et al. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat Biotechnol. 2006;24:1151–61. doi: 10.1038/nbt1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiewe P, Gueller S, Komor M, Stroux A, Thiel E, Hofmann WK. Prediction of qualitative outcome of oligonucleotide microarray hybridization by measurement of RNA integrity using the 2100 Bioanalyzer capillary electrophoresis system. Ann Hematol. 2009;88:1177–83. doi: 10.1007/s00277-009-0751-5. [DOI] [PubMed] [Google Scholar]
- 6.Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27:126–39. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Simon R. BRB-ArrayTools Data Archive for human cancer gene expression: a unique and efficient data sharing resource. Cancer Inform. 2008;6:9–15. doi: 10.4137/cin.s448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 9.Simon R, Lam A, Li MC, Ngan M, Menenzes S, Zhao Y. Analysis of gene expression data using BRB-ArrayTools. Cancer Inform. 2007;3:11–7. [PMC free article] [PubMed] [Google Scholar]
- 10. http://www4.appliedbiosystems.com/tools/umapit/
- 11.Pfaffl M. Relative quantification. In: Dorak T, editor. Real-time PCR. International University Line; La Jolla, CA: 2006. pp. 63–82. [Google Scholar]
- 12.Spruessel A, Steimann G, Jung M, Lee SA, Carr T, Fentz AK, et al. Tissue ischemia time affects gene and protein expression patterns within minutes following surgical tumor excision. Biotechniques. 2004;36:1030–7. doi: 10.2144/04366RR04. [DOI] [PubMed] [Google Scholar]
- 13.Lin DW, Coleman IM, Hawley S, Huang CY, Dumpit R, Gifford D, et al. Influence of surgical manipulation on prostate gene expression: implications for molecular correlates of treatment effects and disease prognosis. J Clin Oncol. 2006;24:3763–70. doi: 10.1200/JCO.2005.05.1458. [DOI] [PubMed] [Google Scholar]
- 14.De Cecco L, Musella V, Veneroni S, Cappelletti V, Bongarzone I, Callari M, et al. Impact of biospecimens handling on biomarker research in breast cancer. BMC Cancer. 2009;9:409. doi: 10.1186/1471-2407-9-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Micke P, Ohshima M, Tahmasebpoor S, Ren ZP, Ostman A, Ponten F, et al. Biobanking of fresh frozen tissue: RNA is stable in nonfixed surgical specimens. Lab Invest. 2006;86:202–11. doi: 10.1038/labinvest.3700372. [DOI] [PubMed] [Google Scholar]
- 16.Fajardy I, Moitrot E, Vambergue A, Vandersippe-Millot M, Deruelle P, Rousseaux J. Time course analysis of RNA stability in human placenta. BMC Mol Biol. 2009;10:21. doi: 10.1186/1471-2199-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jewell SD, Srinivasan M, McCart LM, Williams N, Grizzle WH, LiVolsi V, et al. Analysis of the molecular quality of human tissues: an experience from the Cooperative Human Tissue Network. Am J Clin Pathol. 2002;118:733–41. doi: 10.1309/VPQL-RT21-X7YH-XDXK. [DOI] [PubMed] [Google Scholar]
- 18.Hatzis C, Sun H, Yao H, Hubbard RE, Meric-Bernstam F, Babiera GV, et al. Effects of tissue handling on RNA integrity and microarray measurements from resected breast cancers. J Natl Cancer Inst. 2011;103:1871–83. doi: 10.1093/jnci/djr438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dash A, Maine IP, Varambally S, Shen R, Chinnaiyan AM, Rubin MA. Changes in differential gene expression because of warm ischemia time of radical prostatectomy specimens. Am J Pathol. 2002;161:1743–8. doi: 10.1016/S0002-9440(10)64451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nogueira M, Kim HL. Molecular markers for predicting prognosis of renal cell carcinoma. Urol Oncol. 2008;26:113–24. doi: 10.1016/j.urolonc.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 21.Kim HL, Seligson D, Liu X, Janzen N, Bui MH, Yu H, et al. Using protein expressions to predict survival in clear cell renal carcinoma. Clin Cancer Res. 2004;10:5464–71. doi: 10.1158/1078-0432.CCR-04-0488. [DOI] [PubMed] [Google Scholar]
- 22.Lidgren A, Hedberg Y, Grankvist K, Rasmuson T, Vasko J, Ljungberg B. The expression of hypoxia-inducible factor 1alpha is a favorable independent prognostic factor in renal cell carcinoma. Clin Cancer Res. 2005;11:1129–35. [PubMed] [Google Scholar]
- 23.Lidgren A, Hedberg Y, Grankvist K, Rasmuson T, Bergh A, Ljungberg B. Hypoxia-inducible factor 1alpha expression in renal cell carcinoma analyzed by tissue microarray. Eur Urol. 2006;50:1272–7. doi: 10.1016/j.eururo.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 24.Rioux-Leclercq N, Epstein JI, Bansard JY, Turlin B, Patard JJ, Manunta A, et al. Clinical significance of cell proliferation, microvessel density, and CD44 adhesion molecule expression in renal cell carcinoma. Hum Pathol. 2001;32:1209–15. doi: 10.1053/hupa.2001.28957. [DOI] [PubMed] [Google Scholar]
- 25.Clifford SC, Czapla K, Richards FM, O'Donoghue DJ, Maher ER. Hepatocyte growth factor-stimulated renal tubular mitogenesis: effects on expression of c-myc, c-fos, c-met, VEGF and the VHL tumour-suppressor and related genes. Br J Cancer. 1998;77:1420–8. doi: 10.1038/bjc.1998.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vamvakas S, Bittner D, Koster U. Enhanced expression of the protooncogenes c-myc and c-fos in normal and malignant renal growth. Toxicol Lett. 1993;67:161–72. doi: 10.1016/0378-4274(93)90053-z. [DOI] [PubMed] [Google Scholar]
- 27.Canoz O, Belenli O, Patiroglu TE. General features of gastric carcinomas and comparison of HSP70 and NK cell immunoreactivity with prognostic factors. Pathol Oncol Res. 2002;8:262–9. doi: 10.1007/BF03036742. [DOI] [PubMed] [Google Scholar]
- 28.Elpek GO, Karaveli S, Simsek T, Keles N, Aksoy NH. Expression of heat-shock proteins hsp27, hsp70 and hsp90 in malignant epithelial tumour of the ovaries. APMIS. 2003;111:523–30. doi: 10.1034/j.1600-0463.2003.1110411.x. [DOI] [PubMed] [Google Scholar]
- 29.Noguchi T, Takeno S, Shibata T, Uchida Y, Yokoyama S, Muller W. Expression of heat shock protein 70 in grossly resected esophageal squamous cell carcinoma. Ann Thorac Surg. 2002;74:222–6. doi: 10.1016/s0003-4975(02)03641-x. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Niu ZB, Hou Y, Wang CL. The expression of HSP70 and HSP90alpha in children with Wilms tumor. J Pediatr Surg. 2006;41:1062–6. doi: 10.1016/j.jpedsurg.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 31.Linehan WM, Zbar B, Klausner RD. Renal cell carcinoma. In: Scriver CR, Beaudet A, Sly W, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 907–29. [Google Scholar]
- 32.van Houwelingen KP, van Dijk BA, Hulsbergen-van de Kaa CA, Schouten LJ, Gorissen HJ, Schalken JA, et al. Prevalence of von Hippel-Lindau gene mutations in sporadic renal cell carcinoma: results from The Netherlands cohort study. BMC Cancer. 2005;5:57. doi: 10.1186/1471-2407-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bratslavsky G, Sudarshan S, Neckers L, Linehan WM. Pseudohypoxic pathways in renal cell carcinoma. Clin Cancer Res. 2007;13:4667–71. doi: 10.1158/1078-0432.CCR-06-2510. [DOI] [PubMed] [Google Scholar]
- 34.Linehan WM, Bratslavsky G, Pinto PA, Schmidt LS, Neckers L, Bottaro DP, et al. Molecular diagnosis and therapy of kidney cancer. Annu Rev Med. 2010;61:329–43. doi: 10.1146/annurev.med.042808.171650. [DOI] [PMC free article] [PubMed] [Google Scholar]