Abstract
Background:
Patients undergoing corrective surgery for scoliosis of spine are commonly ventilated in our institute after the operation. Postoperative mechanical ventilation (PMV) and subsequent prolongation of intensive care unit stay are associated with increase in medical expenditure and complications such as ventilator-associated pneumonia. Identification of factors which may contribute to PMV and their modification may help in allocation of resources effectively. The present study was performed to identify preoperative and intraoperative factors associated with early PMV after scoliosis surgery.
Methods:
One hundred and two consecutive patients who underwent operation for scoliosis correction between January 2006 to July 2011 were reviewed retrospectively. Patients requiring PMV included patients who were not extubated in the operating room and were continued on mechanical ventilation. Preoperative and intraoperative factors which were analysed included age, gender, weight, cardiorespiratory function, presence of kyphosis, number and level of vertebrae involved, surgical approach, whether thoracoplasty was done, duration of surgery, blood loss, fluids and blood transfused, hypothermia and use of antifibrinolytics.
Results:
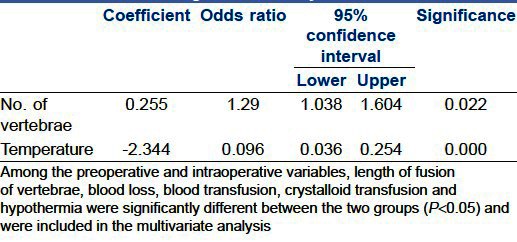
The average age of the patients was 14.31±3.78 years with female preponderance (57.8%). Univariate analysis found that longer fusions of vertebrae (more than 8), blood loss, amount of crystalloids infused, blood transfused and hypothermia were significantly associated with PMV (P<0.05). Independent risk factors for PMV were longer fusion (Odds Ratio (OR), 1.290; 95% confidence interval (CI), 1.038-1.604) and hypothermia (OR, 0.096; 95% CI, 0.036-0.254; P<0.05).
Conclusion:
The authors identified that longer fusions and hypothermia were independent risk factors for early PMV. Implementation of measures to prevent hypothermia may result in decrease in PMV.
Keywords: Postoperative mechanical ventilation, risk factors, scoliosis surgery
INTRODUCTION
Anaesthesia for correction of scoliosis is a challenge as it is a complex reconstructive procedure with inherent potential for massive blood loss and the need to facilitate intraoperative neurophysiological monitoring of the spinal cord. Longstanding scoliosis results in significant cardiorespiratory impairment and many of the patients in our institute were routinely ventilated postoperatively. Very few studies have focused on the risk factors which may result in postoperative mechanical ventilation (PMV) after scoliosis surgery.[1] Poor cardiorespiratory function (forced vital capacity <30% and fractional shortening of <25% on preoperative echocardiography), duration of surgery and type of surgery may influence the need for postoperative ventilation.[2–4] As the operations for correction of scoliosis become common, PMV becomes an important issue for allocation of resources and correlates with significant postoperative morbidity. The aim of this study was to identify preoperative and intraoperative factors that are associated with early PMV after scoliosis surgery.
METHODS
The current study is a retrospective review of the medical records of 102 patients who underwent spinal fusion of the thoracolumbar region at our institute from January 2006 to July 2011. The main outcome measure was the need for early PMV. Preoperative investigation consisted of spirometry and echocardiography, full blood examination and cross match. The aetiology of scoliosis included congenital, idiopathic, neuromuscular and others. In all patients, a similar anaesthesia technique was used. Following premedication with oral alprazolam, patients were anaesthetised with intravenous thiopentone, fentanyl, muscle relaxant, nitrous oxide and isoflurane. Apart from the American society of Anaesthesiologists recommended standard monitors, invasive arterial pressure (ABP) via radial artery, central venous pressure with 7 Fr triple lumen via right internal jugular vein, temperature, arterial blood gases (ABG) and urine output were monitored. The mean ABP was maintained between 60 and 80 mmHg. The depth of anaesthesia was monitored either by bispectral index (40-60) or by entropy (50-60). Stagnara's wake up test was performed on all patients to test for spinal cord integrity after the scoliosis correction. Fluid warmers in all patients and forced warm air blankets in some patients were used to prevent hypothermia. Blood loss was measured by weighing the sponges and blood in the suction bottles. In patients where tranexamic acid was used to reduce blood loss, it was given in three doses (10 mg/kg before skin incision, 5 mg/kg during instrumentation and 5 mg/kg after instrumentation). Blood was transfused when the estimated blood loss was more than 25% of total blood volume. Postoperative pain control was achieved with a combination of intravenous tramadol, postoperative wound infiltration with local anaesthesia and non-steroidal anti-inflammatory agents.
The following preoperative variables were evaluated: Age, gender, aetiology, presence of kyphosis, preoperative cardiopulmonary function. Intraoperative data included number of vertebrae, involvement of upper thoracic levels, surgical approach (anterior, posterior or both), whether thoracoplasty was done and duration of surgery. The non-surgical intraoperative factors included were blood loss, amount of crystalloid infusion, colloid infusion, blood transfused, use of antifibrinolytic agents and hypothermia. The postoperative parameters studied were reintubations, duration of intensive care unit (ICU) stay, duration of hospital stay and any other complications. Perioperative data were collected from the case files retrospectively. Patients requiring early PMV included patients who were not extubated in the operating room and were continued on mechanical ventilation. The decision to extubate was made by the attending anaesthesiologist. To be considered for extubation, the patients had to be fully awake, warm, with normal ABGs (pH >7.3, pO2 >80 mmHg, pCO2<50 mmHg), sufficient pain relief, complete reversal of neuromuscular blockade and haemodynamically stable.
All statistical analyses were performed by SPSS version 17.0 and a P<0.05 was considered significant. The continuous variables were presented as mean±standard deviation or median and interquartile range, as appropriate. The categorical data were presented as numbers and percentages. Preoperative and intraoperative data were evaluated univariately as predictors for early PMV and then a logistic regression model was developed based on identified univariate predictors. Forward selection within the regression model was stepwise where variables were retained if their associated P values were <0.05.
RESULTS
A total of 102 patients were included in the study. The average age of patients was 14.31±3.78 years with female preponderance (57.8%). Sixty one patients (59.8%) were ventilated after the operation. None of the 102 patients required reintubation. The mean duration of ventilation in the early PMV group was 6.7±4.8 hours [Figure 1]. One patient in the early PMV group developed pneumonia after extubation, but did not require ventilation. There were no perioperative deaths. Except for one patient who required inotropic support after an anaphylactic reaction, none of the patients developed haemodynamic disturbances. Idiopathic scoliosis was the commonest cause for scoliosis (44.1%), followed by congenital (35.3%). Majority of the patients (93.1%) underwent posterior fusion, while 2.9% had anterior and 3.9% had both anterior and posterior fusion. In all the patients with an anterior fusion (n=7), the vertebral column was accessed anteriorly by a thoracotomy and postoperatively an intercostal drainage was left in place. The level of involvement was classified as high (upper extent of vertebral involvement as T1-4), mid (T5-8) and low thoracic (including T9-12 and lumbar segments) as more respiratory complications were expected with operations on thoracic than lumbar segments. 40.1% of the patients had upper thoracic, 41.1% had midthoracic and 18.8% had lower thoracic involvement. The complete patient demographics and preoperative and intraoperative variables for extubation on table (ET) and early PMV are shown in Table 1. Among the preoperative and intraoperative variables, length of fusion of vertebrae, blood loss, blood transfusion, crystalloid transfusion and hypothermia were significantly different between the two groups (P<0.05). The duration of ICU stay was also significantly prolonged in the ventilated group (P<0.05). Independent risk factors for early PMV were number of vertebrae involved (P<0.05) (Odds Ratio (OR), 1.290; 95% confidence interval (CI), 1.038-1.604) and hypothermia (OR, 0.096; 95% CI, 0.036-0.254; P<0.05) [Table 2].
Figure 1.
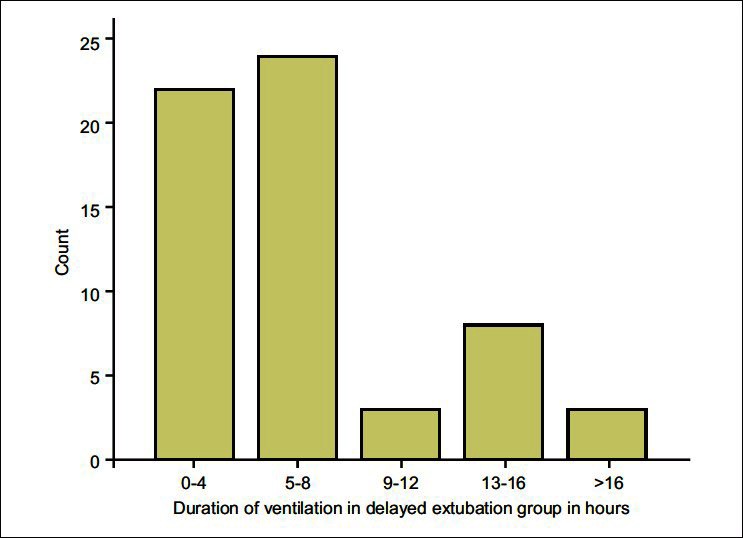
The frequency distribution of duration of ventilation in the early postoperative mechanical ventilation group
Table 1.
Demographic characteristics and preoperative and intraoperative data for extubation on table patients and early postoperative mechanical ventilation patients
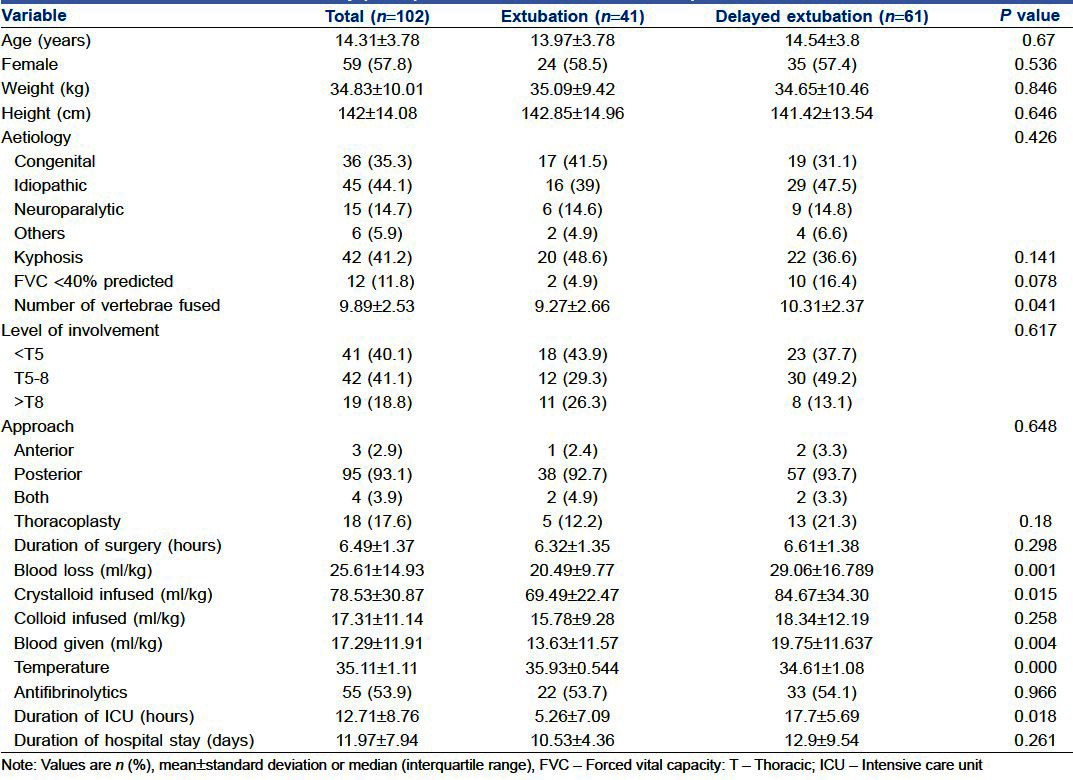
Table 2.
Multivariate predictors of early postoperative mechanical ventilation obtained by logistic regression analysis
DISCUSSION
Patients are often ventilated after correction of scoliosis due to extensive resection and reconstruction, massive blood loss and compromised cardiorespiratory function. PMV and prolongation of ICU stay are associated with significant increase in medical expenditure.[5] Many adverse effects are associated with mechanical ventilation such as decreased cardiac output, respiratory alkalosis and hepatic and renal dysfunction.[6] Perhaps, most feared among complications occurring during mechanical ventilation are pneumothorax and nosocomial pneumonia. Early PMV was defined as any extubation which was not done in operating room. The authors thought that this cut-off would help them identify preoperative and intraoperative factors associated with ventilation postoperatively and therefore, allotment of ventilators and intensive care beds before surgery can be planned with more rationale. Early extubation not only reduces the complications associated with mechanical ventilation, but also helps in spinal cord monitoring and detection of neurological deficits as soon as possible in the postoperative period. The present study was performed to identify all the preoperative and intraoperative factors associated with early PMV after scoliosis surgery.
In our study, hypothermia and extent of the involvement of vertebral column were key determinants for extubation. There was no association between the aetiology of scoliosis and extubation unlike Murphy et al. who in their study reported that 25% of patients with neuromuscular scoliosis required mechanical ventilation postoperatively.[7] Poliomyelitis and cerebral palsy were the predominant causes for neuromuscular scoliosis in our series. As the scoliosis curve worsens, more lung tissue is compressed leading to decrease in vital capacity. A decrease in forced vital capacity (FVC usually less than 30-40%) has been associated with prolonged ventilation.[2] In our study, patients with FVC <40% were not significantly associated with postoperative ventilation. This could probably be due to the small number of patients with FVC <40% in our study. But there was neither bias nor any fixed criteria in the selection of these cases, which could have led to the deliberate exclusion of patients with FVC <30%. Our patients probably presented early in the course of disease before the cardiorespiratory impairment was severe and all of them had undergone meticulous preoperative optimisation of pulmonary function. Literature suggests that the patients with poor preoperative PFTs may undergo operation as long as the heightened risk of postoperative pulmonary complications is accepted.[8,9] The level of vertebral involvement, the surgical approach (anterior, posterior or both) or thoracoplasty also did not have any impact on extubation. The assumption was that thoracic scoliosis, anterior approach[10] and thoracoplasty may all contribute to impairment of postoperative respiratory mechanics.
Hod-Feins et al.[10] reported that in both idiopathic and neuromuscular scoliosis, longer fusions correlated with lower pulmonary complications. But, the length of fusion of more than 8 vertebrae (P-0.048) was a definite risk for early PMV in this study. We hypothesise that as the number of vertebrae increase, the duration of operation is prolonged with the attendant increase in blood loss. Massive blood loss may lead to both haemodynamic instability and the need for transfusion of fluids, blood and blood products. In addition to the risk of disease transmission, transfusion is also associated with pulmonary complications (transfusion-related acute lung injury, ventilator-associated pneumonia)[11] and significant hypothermia all of which may lead to PMV. In previous studies, both tranexamic acid and epsilon amino caproic acid have been found to be effective in decreasing perioperative blood loss and transfusion requirements in idiopathic scoliosis.[12–15] It is expected that decrease in blood loss would hasten the surgery and facilitate early extubation. However, the use of tranexamic acid was not significantly associated with early extubation in our study.
Hypothermia is common in anaesthetised patients, irrespective of the anaesthetic technique. Core temperature decreases due to anaesthetic induced impairment of central thermoregulation and redistribution of heat from core to periphery. Heat loss not only occurs across the skin, but also as a result of cold intravenous fluids and loss from the surgical wound. Though mild hypothermia provides organ (spinal cord) protection[16] by decreasing both electrophysiological activity and basal metabolic rate, it has several other disadvantages. It causes increased bleeding due to impairment of coagulation.[17] The distribution and metabolism of most of the drugs is altered due to which recovery may be prolonged.[18] Hence, measures to prevent hypothermia may help reduce the risk of bleeding and facilitate early extubation. Hypothermia can be prevented by administration of warm intravenous fluids, covering exposed skin and active cutaneous warming. Forced air systems are more effective than passive insulation of skin and are superior to circulating water mattresses and fluid warmers in maintaining normothermia.[16] In our study, fluid warmers were used in all patients and forced warm air blankets were used in some patients to prevent hypothermia. Both bispectral index and entropy may be used for assessment of depth of anaesthesia, thus preventing over dosage of drugs and delayed recovery. Short acting drugs should be used to facilitate early extubation.
Postoperative pain control was achieved with a combination of intravenous tramadol, postoperative wound infiltration with local anaesthesia and non-steroidal anti-inflammatory agents. This regimen was found to be adequate for postoperative analgesia with good patient satisfaction.
CONCLUSION
Postoperative mechanical ventilation is commonly used after scoliosis correction. The present study identified longer fusions and hypothermia as factors associated with early PMV. This may help in optimal utilisation of resources by better planning and by measures to prevent perioperative hypothermia.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Almenrader N, Patel D. Spinal fusion surgery in children with non-idiopathic scoliosis: Is there a need for routine postoperative ventilation? Br J Anaesth. 2006;97:851–7. doi: 10.1093/bja/ael273. [DOI] [PubMed] [Google Scholar]
- 2.Gibson PR. Anaesthesia for correction of scoliosis in children. Anaesth Intensive Care. 2004;32:548–59. doi: 10.1177/0310057X0403200413. [DOI] [PubMed] [Google Scholar]
- 3.Jules-Elysee K, Urban MK, Urquhart BL, Susman MH, Brown AC, Kelsey WT. Pulmonary complications in anterior-posterior thoracic lumbar fusions. Spine J. 2004;4:312–6. doi: 10.1016/j.spinee.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Yuan N, Skaggs DL, Dorey F, Keens TG. Preoperative predictors of prolonged postoperative mechanical ventilation in children following scoliosis repair. Pediatr Pulmonol. 2005;40:414–9. doi: 10.1002/ppul.20291. [DOI] [PubMed] [Google Scholar]
- 5.Rajakaruna C, Rogers CA, Angelini GD, Ascione R. Risk factorsfor and economic implications of prolonged ventilation after cardiac surgery. J Thorac Cardiovasc Surg. 2005;130:1270–7. doi: 10.1016/j.jtcvs.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 6.Mutlu GM, Factor P. Complications of mechanical ventilation. Respir Care Clin N Am. 2000;6:213–52. doi: 10.1016/s1078-5337(05)70069-1. [DOI] [PubMed] [Google Scholar]
- 7.Murphy NA, Firth S, Jorgensen T, Young PC. Spinal surgery in children with idiopathic and neuromuscular scoliosis. What's the difference? J Pediatr Orthop. 2006;26:216–20. doi: 10.1097/01.bpo.0000206516.61706.6e. [DOI] [PubMed] [Google Scholar]
- 8.Udink ten Cate FE, van Royen BJ, van Heerde M, Roerdink D, Plotz FB. Incidence and risk factors of prolonged mechanical ventilation in neuromuscular scoliosissurgery. J Pediatr Orthop B. 2008;17:203–6. doi: 10.1097/BPB.0b013e328301e962. [DOI] [PubMed] [Google Scholar]
- 9.Marsh A, Edge G, Lehovsky J. Spinal fusion in patients with Duchenne'smuscular dystrophy and a low forced vital capacity. Eur Spine J. 2003;12:507–12. doi: 10.1007/s00586-003-0545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hod-Feins R, Abu-Kishk I, Eshel G, Barr Y, Anekstein Y, Mirovsky Y. Risk factors affecting the immediate postoperative course in pediatric scoliosis surgery. Spine (Phila Pa 1976) 2007;32:2355–60. doi: 10.1097/BRS.0b013e3181558393. [DOI] [PubMed] [Google Scholar]
- 11.Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, et al. Transfusion-related acute lung injury: Incidence and riskfactors. Blood. 2012;119:1757–67. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson GH, Florentino-Pineda I, Poe-kochert C. The role of amicar in decreasing perioperative blood loss in idiopathic scoliosis. Spine (Phila Pa 1976) 2005;30:S94–9. doi: 10.1097/01.brs.0000175188.05542.a9. [DOI] [PubMed] [Google Scholar]
- 13.Florentino-Pineda I, Thompson GH, Poe-Kochert C, Huang RP, Haber LL, Blakemore LC. The effect of amicar on perioperative blood loss in idiopathic scoliosis: The results of aprospective, randomized double-blind study. Spine (Phila Pa 1976) 2004;29:233–8. doi: 10.1097/01.brs.0000109883.18015.b9. [DOI] [PubMed] [Google Scholar]
- 14.Thompson GH, Florentino-Pineda I, Poe-Kochert C, Armstrong DG, Son-Hing JP. Role ofAmicar in surgery for neuromuscular scoliosis. Spine (Phila Pa 1976) 2008;33:2237–42. doi: 10.1097/BRS.0b013e318187c046. [DOI] [PubMed] [Google Scholar]
- 15.Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg. 2001;93:82–7. doi: 10.1097/00000539-200107000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Sessler DI. Deliberate mild hypothermia. J Neurosurg Anesthesiol. 1995;7:38–46. doi: 10.1097/00008506-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–92. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- 18.Leslie K, Sessler DI, Leslie K, Bjorksten AR, Moayeri A. Mild hypothermia alters propofol pharmacokinetics and increases the duration of action of atracurium. Anesth Analg. 1995;80:1007–14. doi: 10.1097/00000539-199505000-00027. [DOI] [PubMed] [Google Scholar]


