Abstract
Background:
Recent manuscripts have described the use of ultrasound imaging to evaluate airway structures. Ultrasound training tools are necessary for practitioners to become proficient at obtaining and interpreting images. Few training tools exist and those that do can often times be expensive and rendered useless with repeated needle passes.
Methods:
We utilised inexpensive and easy to obtain materials to create a gel phantom model for ultrasound-guided airway examination training.
Results:
Following creation of the gel phantom model, images were successfully obtained of the thyroid and cricoid cartilages, cricothyroid membrane and tracheal rings in both the sagittal transverse planes.
Conclusion:
The gel phantom model mimics human airway anatomy and may be used for ultrasound-guided airway assessment and intervention training. This may have important safety implications as ultrasound imaging is increasingly used for airway assessment.
Keywords: Airway imaging, airway management, ultrasonography
INTRODUCTION
The spectrum of applications for the use of ultrasound imaging has experienced tremendous growth. This is likely secondary to the low cost, portability and relatively short learning curve associated with its use. At many institutions, the use of ultrasound imaging to guide central line placement is rapidly becoming standard practice, as it allows one to easily visualise vascular structures and improve procedural safety and efficiency.[1] Ultrasound imaging has also recently been reported as a sensitive tool for the detection of pneumothorax and is used for that purpose in emergency departments, ICUs and operating rooms.[2–5] Within anaesthesia, ultrasound guidance has truly ushered in a renaissance for peripheral nerve blockade. Ultrasound guidance has allowed non-experts in the field of regional anaesthesia to perform advanced peripheral nerve blocks with a large degree of confidence, safety and efficacy with resultant decreases in necessary local anaesthetic volumes and decreased block setup times.[6,7]
Recently, there has been increased interest in utilising ultrasound imaging to evaluate airway anatomy. Ultrasound imaging has been demonstrated to be effective for procedures such as percutaneous tracheostomy or cricothyroidotomy, visualisation of the epiglottis and larynx, prediction of airway difficulties, confirmation of correct airway device placement, selection of appropriately sized standard or double-lumen endotracheal tubes, detection of subglottic stenosis and performing nerve blocks for awake fibre optic intubations.[8–11] Ultrasound guidance may also be helpful for everyday routine procedures, such as providing cricoid pressure, as recent evidence has demonstrated that anaesthesia providers can have difficulty locating the thyroid and cricoid cartilages and cricothyroid membrane.[12] Three recent publications have thoroughly discussed strategies and techniques to systematically approach ultrasound-guided airway assessment, but true mastery can only be obtained through practice.[11,13,14]
Unfortunately, despite a growing number of practitioners attempting to incorporate ultrasound imaging into their everyday practices, few ultrasound training tools exist and those that do exist can be very expensive and quickly rendered useless once needle tracks are created.[15] As such, there is a need for a low-cost, air-filled training tool that simulates laryngeal anatomy. With this in mind, we set out to construct an inexpensive gel phantom model that can be used for ultrasound-guided airway assessment and intervention. The purpose of this report is to describe our construct.
METHODS
An adult pig laryngotracheal complex was obtained from a local butcher shop. An oblong balloon (>15 cm in length) was then inserted into the complex and inflated with air to maintain an air-filled interphase within the gel phantom. The laryngotracheal/balloon unit was then sutured to the bottom of an adequately sized plastic container [Figure 1]. Using a ratio of 250 ml of boiling water to 10 g of sugar-free psyllium fibre to 20 g of unflavoured gelatine, a phantom material solution was created. The solution was stirred by hand until smooth and all large particles were dissolved.[16] Red food colouring was then added until the material took on a desired colour. The liquid material was poured over the laryngotracheal/balloon unit until a desired surface-to-unit thickness was achieved to simulate human conditions. The material was allowed to cool and harden at 12 degrees Celsius for 120 minutes, producing a final gel phantom model [Figure 2]. A Sonosite M-Turbo® ultrasound machine (Sonosite, Bothell, WA, USA) with a 13.6 MHz linear probe was then used to obtain images of the laryngotracheal structures.
Figure 1.
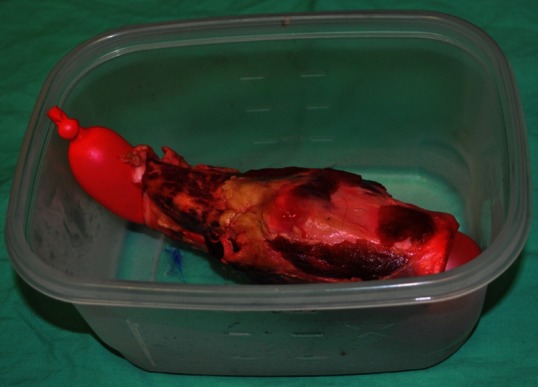
Adult pig laryngotracheal complex with air filled balloon sutured to bottom of plastic container
Figure 2.

Completed gel phantom model
RESULTS
Images demonstrating steps required for phantom creation and maintenance of air interface are provided in Figures 1 and 2. Following creation of the gel phantom model, images of the larynx demonstrating the thyroid and cricoid cartilages (with intervening cricothyroid membrane) and tracheal rings were obtained in the sagittal plane in the phantom model [Figure 3].
Figure 3.
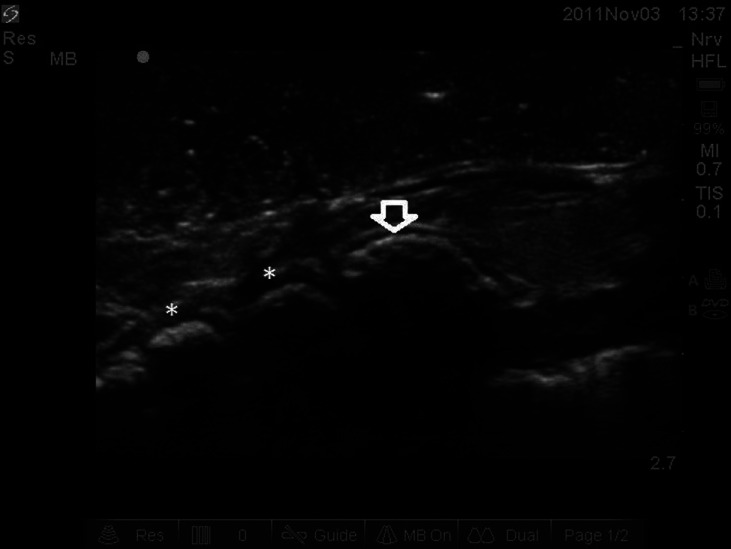
Ultrasound image in sagittal plane of phantom model. Note arrow representing cricoid cartilage and asterisk representing tracheal rings
DISCUSSION
We have produced a gel phantom model for use in ultrasound-guided airway assessment and intervention. The popularity and clinical indications for ultrasound imaging within the field of medicine has experienced a tremendous increase over the past decade. The advantages of ultrasound imaging include low-cost, portability and relatively short learning curve associated with the ability to obtain images in “real-time.” Anaesthesiologists have utilised ultrasound for a variety of procedures, but the area of greatest utilisation thus far has been for ultrasound–guided peripheral nerve blockade. In this application, ultrasound guidance has demonstrated multiple benefits, including higher success rates, shorter block placement times, shorter block onset times, decreased local anaesthetic requirements, decreased pain with block placement, decreased number of needle passes required and improved patient satisfaction.[6,7] Anaesthesiologists also commonly utilise ultrasound guidance to reduce the risk of complications associated with central venous cannulation.
The use of ultrasound imaging is now also becoming of interest in the area of airway evaluation and management. Ultrasound assessment of pretracheal soft tissue depth has been demonstrated to correlate with difficult laryngoscopy.[10] Ultrasound-guided tracheal puncture has been utilised for non-surgical percutaneous dilational tracheostomy and the use of ultrasound may allow practitioners to avoid unseen and dangerous vascular structures. One study demonstrated that vulnerable anterior internal jugular veins were present in 27% of patients evaluated by ultrasound for percutaneous dilational tracheostomy.[8] Ultrasound imaging has also been found to perform similarly to computed tomography imaging with regard to structure identification with the advantages of portability, decreased radiation exposure and ability to assist in airway intervention.[17] Finally, ultrasound imaging has been demonstrated to be more sensitive than chest X-ray at detecting the presence of a pneumothorax and it may prove invaluable in this regard in the operating room setting as well.[2]
Unfortunately, ultrasound imaging is not without dangers and inadequate training in ultrasonography can result in unintended adverse consequences. Most ultrasound machines emit a beam width of 0.2-1.2 mm. This narrow beam width can lead to difficult or lack of needle tip localisation, resulting in unintended needle tip position. A study of residents trained in ultrasound-guided central venous catheterisation demonstrated that 64% of study participants penetrated the posterior wall of the target vessel in a model.[18] To address this issue, ultrasound machine software enhancements and needles with enhanced echogenicity are being developed; however, a baseline ultrasonographic skill level requirement remains and must be acquired for these enhancements to be helpful.
Ultrasonographic training devices are therefore required for those practitioners intending to utilise ultrasound guidance for procedures in their practice. Many regional anaesthesia programs believe that practitioners must first spend time with phantom models prior to attempting regional anaesthesia techniques on patients. Interventional radiologists have demonstrated that phantom training results in improvement in confidence, needle visualisation, success and safety.[15] Unfortunately, available training devices are either expensive, have a limited use life and/or are not available for the entire array of possible applications. Thus, low-cost training models that allow intervention are of interest.
Low-cost methods utilised to simulate various anatomical structures include water-filled balloons for cysts, olives for soft tissue masses, rubber tubing or pasta for vessels and pasta or electrical wires for nerves.[15] Similarly, we have created a low-cost, gel phantom training model for airway assessment (i.e., identification of the thyroid, cricoid and tracheal cartilages, the cricothyroid membrane and interthyroid cartilage spaces) that could also be used to teach airway interventions (e.g., transtracheal blocks for subglottic airway topicalisation, percutaneous tracheotomy, cricothyroidotomy and trans-cricothyroid membrane puncture for wire-guided retrograde intubation) that require aspiration of air to confirm correct needle tip location. Qualitatively, this model is consistent with its human counterpart. It benefits from the utilisation of common and inexpensive raw materials and a short preparation time requirement. The cost of the gelatine and psyllium required is less than $5 US per phantom model. Outdated suture, balloons, plastic containers and the laryngotracheal complex itself can typically be obtained without any or very little cost. When the quality of the model degrades, the laryngotracheal complex is easily recovered by dissolution of the gel material and the model can be quickly regenerated. Finally, adjusting the length of the retaining sutures, depth of phantom poured over laryngotracheal complex or selecting a different container allows the creator to vary the surface-to-laryngotracheal complex depth, simulating thin to thick or obese neck anatomy.
Our model, however, is not without limitations. Clinicians have not independently verified this model and therefore further validation and attempts at model improvement are warranted. While this model does closely approximate human anatomy, there are differences in sonoanatomy (i.e., enhanced echogenicity of cartilaginous structures and air-to-mucosa interface in phantom model) that should be understood prior to attempting to perform interventional techniques on patients. Infective-free biological specimens cannot always be ensured. Accordingly, but consistent with clinical practice, appropriate personal protective equipment should be available and worn during creation and handling of the model. Equally, the materials of the model are subject to decomposition. Therefore, refrigeration is required to prolong the useful life of the model to approximately two weeks. In addition, the pig cartilages utilised in our model appeared brighter on ultrasound imaging and this likely represents increased calcification. Nonetheless, we have found this model to be inexpensive, easily created and qualitatively consistent with human anatomy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.McGee D, Gould M. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123–33. doi: 10.1056/NEJMra011883. [DOI] [PubMed] [Google Scholar]
- 2.Wilkerson G, Stone M. Sensitivity of bedside ultrasound and supine anteroposterior chest radiographs for the identification of pneumothorax after blunt trauma. Acad Emerg Med. 2010;17:11–7. doi: 10.1111/j.1553-2712.2009.00628.x. [DOI] [PubMed] [Google Scholar]
- 3.Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: A systematic review and meta-analysis. Chest. 2012;141:703–8. doi: 10.1378/chest.11-0131. [DOI] [PubMed] [Google Scholar]
- 4.Volpicelli G. Sonographic diagnosis of pneumothorax. Intensive Care Med. 2011;37:224–32. doi: 10.1007/s00134-010-2079-y. [DOI] [PubMed] [Google Scholar]
- 5.Nagarsheth K, Kurek S. Ultrasound detection of pneumothorax compared with chest x-ray and computed tomography scan. Am Surg. 2011;77:480–3. [PubMed] [Google Scholar]
- 6.Abrahams M, Aziz M, Fu R, Horn J. Ultrasound guidance compared with electrical neurostimulation for peripheral nerve block: A systematic review and meta-analysis of randomized control trials. Br J Anaesth. 2009;102:408–17. doi: 10.1093/bja/aen384. [DOI] [PubMed] [Google Scholar]
- 7.Koscielniak-Nielsen ZJ. Ultrasound-guided peripheral nerve blocks: What are the benefits? Acta Anaesthesiol Scand. 2008;52:727–37. doi: 10.1111/j.1399-6576.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- 8.Hatfield A, Bodenham A. Portable ultrasonic scanning of the anterior neck before percutaneous dilational tracheostomy. Anaesthesia. 1999;54:660–3. doi: 10.1046/j.1365-2044.1999.00859.x. [DOI] [PubMed] [Google Scholar]
- 9.Green J, Tsui B. Applications of ultrasonography in ENT: Airway assessment and nerve blockade. Anesthesiol Clin. 2010;28:541–53. doi: 10.1016/j.anclin.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Ezri T, Gewürtz G, Sessler D, Medalion B, Szmuk P, Hagberg C, et al. Prediction of difficult laryngoscopy in obese patients by ultrasound quantification of anterior neck soft tissue. Anaesthesia. 2003;58:1111–4. doi: 10.1046/j.1365-2044.2003.03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kundra P, Kumar Mishra S, Ramesh A. Ultrasound of the airway. Indian J Anaesth. 2011;55:456–62. doi: 10.4103/0019-5049.89868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott D, Baker P, Scott M, Birch C, Thompson M. Accuracy of surface landmark identification for cannula cricothyroidotomy. Anaesthesia. 2010;65:889–94. doi: 10.1111/j.1365-2044.2010.06425.x. [DOI] [PubMed] [Google Scholar]
- 13.Singh M, Jinn Chin K, Chan VW, Wong DT, Prasad GA, Yu E. Use of sonography for airway assessment. J Ultrasound Med. 2010;29:79–85. doi: 10.7863/jum.2010.29.1.79. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen MS. Ultrasonography in the management of the airway. Acta Anaesthesiol Scand. 2011;55:1155–73. doi: 10.1111/j.1399-6576.2011.02518.x. [DOI] [PubMed] [Google Scholar]
- 15.Hocking G, Hebard S, Mithell C. A review of the benefits and pitfalls in ultrasound-guided regional anesthesia. Reg Anesth Pain Med. 2011;36:162–70. doi: 10.1097/aap.0b013e31820d4207. [DOI] [PubMed] [Google Scholar]
- 16.Bude R, Adler R. An easily made, low-cost, tissue-like ultrasound phantom material. J Clin Ultrasound. 1995;23:271–3. doi: 10.1002/jcu.1870230413. [DOI] [PubMed] [Google Scholar]
- 17.Prasad A, Yu E, Wong DT, Karkhanis R, Gullane P, Chan VW. Comparison of sonography and computed tomography as imaging tools for assessment of airway structures. J Ultrasound Med. 2011;30:965–72. doi: 10.7863/jum.2011.30.7.965. [DOI] [PubMed] [Google Scholar]
- 18.Blaivas M, Adhikari S. An unseen danger: Frequency of posterior vessel wall penetration by needles during attempts to place internal jugular vein central catheters using ultrasound guidance. Crit Care Med. 2009;37:2345–9. doi: 10.1097/CCM.0b013e3181a067d4. [DOI] [PubMed] [Google Scholar]


