Abstract
Background:
The aim of this prospective audit was to investigate clinical practice related to muscle relaxant reversal and the impact made by the recent introduction of sugammadex on patient outcome at a tertiary teaching hospital.
Methods:
Data from all patients intubated at our institution during two epochs of seven consecutive days each was collected prospectively. Directly prior to extubation, the train-of-four (TOF) ratio was assessed quantitatively by an independent observer. Postoperative outcome parameters were complications in the recovery room and radiological diagnosed atelectasis or pneumonia within 30 days.
Results:
Data from 146 patients were analysed. Three reversal strategies were used: no reversal, neostigmine or sugammadex. The TOF ratio was less than 0.7 in 17 patients (nine no reversal, eight neostigmine) and less than 0.9 in 47 patients (24 no reversal, 19 neostigmine, four sugammadex). Those reversed with sugammadex showed fewer episodes of postoperative oxygen desaturation (15% vs. 33%; P<0.05). TOF ratios of less than 0.7 (P<0.05) and also <0.9 (P<0.01) were more likely associated with X-ray results consistent with postoperative atelectasis or pneumonia.
Conclusions:
Our results suggest a significant impact of residual paralysis on patient outcome. The use of sugammadex resulted in the lowest incidence of residual paralysis.
Keywords: Neostigmine, residual paralysis, sugammadex
INTRODUCTION
The incidence of postoperative residual neuromuscular blockade is still alarmingly high[1] with the prevalence of a train-of-four (TOF) ratio of less than 0.9 found in the postoperative recovery unit ranging from 3.5%[2] to up to 83%.[3] Volunteer studies as well as clinical investigations have been able to link even seemingly low levels of residual paralysis (TOF ratio <0.9) with significant impairment of pharyngeal muscle function, hypoxic ventilatory drive and decreased respiratory function in the immediate postoperative period.[1]
Despite the knowledge of such side effects, and despite the introduction of various new neuromuscular blocking agents (NMBA) such as rocuronium or mivacurium over the last 15 years, no significant reduction in the incidence of residual neuromuscular blockade has so far been observable.
The recent introduction of sugammadex, a γ-cyclodextrin with a high affinity to rocuronium and other amino-steroidal NMBA that allows the rapid and complete reversal of especially rocuronium-induced neuromuscular blockade,[4] has raised hopes to finally overcome the problem of residual neuromuscular blockade. However, the fear of yet unknown side effects, the limit of only reversing steroidal NBMA, delays in Food and Drug Administration approval in the United States, as well as the relatively high price of sugammadex have so far hindered its progress in becoming standard reversal agents in most operating theatres.
At our tertiary teaching hospital sugammadex was introduced as an unrestricted (use not restricted to a specific indication, e.g., airway emergency) alternative to neostigmine from February 2011. This meant that anaesthetists were given the free choice of using neostigmine, sugammadex or no reversal agent in all of their patients.
The aim of this prospective audit was to investigate the effects of sugammadex's introduction on the incidence of residual neuromuscular paralysis and postoperative patient outcome.
METHODS
After approval by the ethics committee (audit A 11-001, 4.3.2011; need for patient consent waived as non-interventional audit), data from two epochs of seven consecutive days each (in March and May 2011) were collected prospectively. The audit included data from all patients who were paralysed and tracheally intubated within main operating theatres at our hospital during the period of 8:00 am to 6:00 pm. Data from patients who remained intubated at the end of surgery were excluded. Data collection was done electronically via the theatre management system (details of operation, surgical and anaesthesia related times), via electronically documented radiological reports of pre-and postoperative chest X-rays, via direct observation/anaesthetist interview in theatres and by means of a logbook style questionnaire (acute airway/pulmonary related postoperative complications, e.g., episodes/duration of oxygen desaturation) completed by nursing staff in the recovery unit.
Patients received an anaesthetic as chosen by their attending anaesthetist. The choice of the NMBA for induction and, if required, intraoperative maintenance of neuromuscular blockade as well as the choice of whether or how to reverse a neuromuscular block at the end of surgery (available reversal agents: neostigmine and sugammadex) was entirely left to the attending anaesthetist. The same anaesthetist also decided whether and by which means of stimulation pattern neuromuscular paralysis was monitored. At our institution, only non-quantitative nerve stimulators for visual or tactile assessment of neuromuscular blockade were routinely available in each operating theatre (at the time of the audit).
At the end of the operation, and once extubation was considered safe by the attending anaesthetist, an independent observer performed three consecutive (at 12 second intervals) supramaximal train-of-four (TOF) stimulations of the ulnar nerve using quantitative neuromuscular monitoring (kinemyometric monitoring via NMT module (GE Healthcare, Helsinki, Finland)). The mean TOF ratio of the three stimulations was noted and also immediately reported to the attending anaesthetist prior to extubation.
Patients were then extubated and transferred to the postoperative recovery unit (PACU). PACU nurses were given a logbook-style questionnaire and asked to note the number and approximate duration of episodes of oxygen desaturation (all patients initially on 6 l oxygen/min via Hudson mask), any other airway related incidents (e.g., airway related review by anaesthetist, need for ventilatory support) as well as episodes of cardiac arrhythmia and nausea and vomiting.
As stated above, data from postoperative chest X-rays performed within 30 days from the operation date were reviewed for findings consistent with atelectasis or pneumonia. No radiological investigation was prescribed by the audit. All investigations were based on clinical symptoms, and referrals to X-ray were made by clinicians who were unaware of the survey and its outcome parameters. Radiological reports of postoperative atelectasis or pneumonia were noted as potential sequelae of residual paralysis, thus used to define undesirable outcome. In all cases with pathological radiological findings attempts were made to retrieve pre-operative X-ray results in order to exclude cases with pre-existing pulmonary disease.
Statistical analysis
This audit was planned as a prospective, cross-sectional pilot investigation; therefore no formal sample size estimation was performed. Investigating two separate epochs of seven days each was preferred over the observation of just one episode of fourteen days as we also aimed to investigate the “over-time-uptake rate” of sugammadex amongst anaesthetists at our institution.
IBM SPSS version 19 was used for statistical analysis. Data are presented as mean (standard deviation), median (interquartile range) or number (proportion), as appropriate. The alpha error was set at 5%.
Data was tested for normal distribution by means of the Kolmogorov-Smirnov test.
For comparison of means/medians Students T test or Mann-Whitney U test were used, as appropriate. Proportional data was compared using Chi-Square or Fisher's Exact test, as appropriate.
In order to minimize bias by uncontrolled confounders, data collected collaterally (in this model: age, American Society of Anaesthesiology (ASA) physical score, surgical specialty, urgency of operation, preoperative pulmonary morbidity, smoking, duration of operation, experience of anaesthetist) were investigated for their relationship with the defined X-ray pathology using exact logistic regression analysis. Based on this, the parameters “age, ASA score, urgency of operation, smoking habits, duration of operation and experience of the anaesthetist” were used to model a propensity score (bootstrapped model to limit the risk of score over-fitting). This score was then used to post-hoc adjust the results related to the relationship of residual paralysis with post-operative X-ray pathology (see results).
RESULTS
Data of 146 patients (53 (20) years; 38 ASA I, 55 ASA II, 43 ASA III, and 5 ASA IV) were analysed. These cases were predominantly orthopaedic- (38), general- (37), plastic- (18) and ear nose and throat surgical cases, with an additional 37 cases from other surgical specialties. 85 cases were booked as an elective procedure, with the remaining cases being urgent (44) or emergency (17) procedures.
The majority of procedures were performed using a volatile anaesthetic agent (sevoflurane or desflurane) for maintenance of anaesthesia (133) and eleven cases as total intravenous anaesthesia (propofol/opioid).
Rocuronium was the most often chosen NMBA for muscle relaxation following induction of anaesthesia (108), followed by suxamethonium (11), cis-atracurium (8), vecuronium (5), atracurium (4) and mivacurium (3). Seven patients did not receive any NMBA at the time of induction (awake fiberoptic intubation or use of high dose remifentanil).
Following the initial bolus of the muscle relaxant, 38 patients received further doses of NMBA (26 rocuronium, 10 cis-atracurium, 2 vecuronium). NMBA were administered predominantly on initiative of the anaesthetist, with 16 cases triggered by specific surgical request.
As described in the methods, attending anaesthetists were free to choose whether and how to monitor neuromuscular blockade. The most frequently chosen pattern to define readiness for extubation was the TOF (79), followed by double burst (37) and tetanic stimulation (13) patterns.
Prior to extubation, the attending anaesthetist defined (either by “experience” or neuromuscular monitoring) the need for reversal of residual neuromuscular blockade in 90 cases, whereas no reversal agent was deemed necessary in 53 instances.
If a reversal agent was used, neostigmine (2.5 [2.5/2.5], 1.25-5 mg) was chosen in 33 patients and sugammadex (200 [200/200], 110-400 mg) in 57 patients.
No differences were found between the groups of “no reversal”, “neostigmine” and “sugammadex” regarding age, ASA score, smoking status and pre-existing pulmonary co-morbidities. Surgical details (e.g., type, urgency and duration of operation) as well as anaesthesia related data (e.g., duration of anaesthesia, anaesthetic used for maintenance, training status of attending anaesthetist) were also not significantly different between the groups.
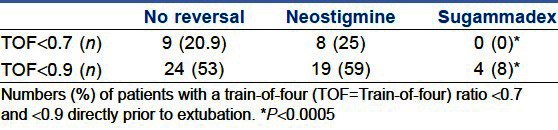
Directly prior to extubation, the TOF ratio was assessed quantitatively by an independent observer. The use of sugammadex resulted in significantly lower numbers of patients with TOF ratios <0.7 and also <0.9 when compared with neostigmine-based or no reversal [Table 1]. Remarkably, no such differences were found between not using a reversal agent and neostigmine.
Table 1.
Train-of-four ratio prior to extubation
Episodes of oxygen desaturation during the patients’ stay in the recovery room were defined as SpO2 of <96 % (from previously higher values, patients on 6 l O2/min via Hudson mask). The use of sugammadex resulted in significantly fewer episodes of desaturation when compared to the use of no reversal agent or neostigmine (15% vs. 33 %; P<0.05). No differences between the reversal groups were found regarding postoperative nausea or vomiting, significant (anaesthetist required for patient review) airway complications and episodes of new-onset cardiac arrhythmia.
Out of the 146 included patients, 30 patients had postoperative chest X-rays performed, with 11 reporting findings (reported by a radiologist) consistent with either pneumonia (defined as consolidation/opacification consistent with a pneumonic infiltrate) or atelectasis (4 patients ASA I or II, 6 ASA III, 1 ASA IV).
Compared to patients with no abnormal X-ray results (either not performed or reported as “normal”) patients with reported pneumonia or atelectasis had a significantly lower median TOF ratio prior to extubation (0.71 [0.44/0.86] vs. 0.94 [0.84/0.98]; P<0.001).
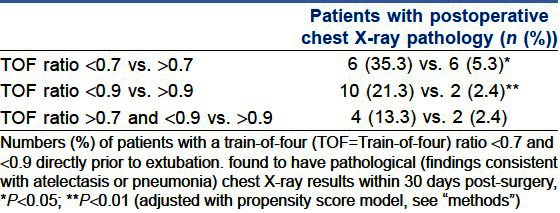
Significantly more patients with TOF ratios <0.7 and <0.9 (P<0.05 and <0.01, resp.), were found to have radiological signs of atelectasis or pneumonia within 30 days after surgery [Table 2]. The odds for postoperative X-ray pathology were 6.2 and 6.9 times higher for patients with TOF ratios <0.7 and <0.9, respectively.
Table 2.
Train-of-four ratios and X-ray pathology
Though fewer patients showed pathological X-ray results after the use of neostigmine or sugammadex (6.1 and 7.1%, respectively) when compared to cases in which no reversal had been used (14%), this difference did not reach the level of statistical significance.
DISCUSSION
Since Beecher and Todd[5] described a significantly increased mortality after the use of NMBA, the need to monitor the effects of such drugs has been well recognised. The TOF stimulation pattern, the pattern most often chosen by anaesthetists in our institution, has been used since around 1970 to monitor residual neuromuscular blockade.[6] However, over the past decade the limit of acceptable TOF ratios has shifted from >0.7 to >0.9 due to the recognition of significant pathology associated with even shallow blocks.[1]
In contrast to scientific evidence, a high number of anaesthetists still perceive TOF ratios of >0.7 as appropriately safe, as the risk of clinically evident complications in this group of patients is often considered acceptably small.[7]
This audit does challenge this assertion as we demonstrated pulmonary complications even at shallow residual blocks. As symptoms of mild (TOF ratio >0.7 & <0.9) residual neuromuscular blockade are very likely to be overshadowed by the residual influence of other anaesthetic agents or mimic the effects of such agents (e.g., opioids, volatiles), the prevalence of residual neuromuscular blockade during this period, and its impact on patient outcome is likely to be underestimated by clinicians. It is also of note that in our audit these postoperative pulmonary complications were not confined to patients with significant preoperative co-morbidities. Nearly half the patients with reports of atelectasis or pneumonia were rated as ASA 1 or 2.
The impact of residual neuromuscular blockade on pulmonary complications beyond the acute recovery period has been previously reported by Berg et al.[8] However, the authors found only cases of severe residual paralysis (TOF ratio <0.7) in combination with a long-acting NMBA (pancuronium) to cause a significantly increase in risk. In contrast to our study in which chest X-rays were solely triggered by clinical indicators, Berg et al., performed this investigation in all patients, regardless of the presence or absence of clinical symptoms. It is not unlikely that, following an approach similar to that of Berg et al., we may have seen higher rates of pathology, atelectasis in particular, as this may often remain clinically undiagnosed.
The proportion of residual paralysis found in our investigation is high, but in line with previously published data.[1] Apart from the aforementioned denial of the clinical significance of the problem, other factors resulting in the described outcome can been identified from our data:
The majority of anaesthetists in our audit used the TOF pattern to determine the time point for safe extubation. As it has been shown that clinicians are unlikely to detect fade if the TOF ratio is >0.4,[9] this method is clearly unsuitable to detect TOF ratios between 0.7 and 0.9. If, like in our hospital, only (at the time of the audit) qualitative monitoring is routinely available in every theatre, the use of double-burst stimulation may be beneficial as it has been described to detect fade even if the TOF ratio is between 0.6 and 0.7.[10] However, any qualitative monitoring as well as all clinical signs of neuromuscular recovery (e.g., head lift, hand grip) have been demonstrated to be insufficient to detect TOF ratios between 0.7 and 0.9 with reasonable sensitivity and specificity.[11]
Only quantitative methods such as mechanomygraphic, acceleromyographic or kinemyographic approaches to monitoring TOF ratios can be regarded as sufficiently accurate for detection of mild residual paralysis.[11]
The fact that we did not find significant differences between pre-extubation TOF ratios achieved when no reversal agent had been used and the use of neostigmine points out another common problem of NMBA reversal: if neostigmine is used to reverse neuromuscular blockade at the end of surgery, its timing is crucial, but difficult.[11] Depending on the NMBA used, and depending on the degree of spontaneous recovery, TOF ratios achieved with standard doses of neostigmine as well as the time needed for satisfactory reversal of neuromuscular blockade vary greatly.[11] Only 55% of patients with a TOF count of 4 twitches achieve TOF ratios >0.9 within 10 minutes of reversal with neostigmine.[12] In order to avoid significantly longer theatre turnover times, administration of neostigmine may have to commence at a time when a distinct neuromuscular block is still desirable (e.g., during closure of fascia after laparotomy, or during eye surgery).
Our hospital has permitted unrestricted use of sugammadex since February 2011, and it was the aim of this audit to study the “take up rate” by anaesthetists as well as clinical patient outcome. The majority of patients who were identified as being in need of reversal of neuromuscular blockade received sugammadex. This shows a high acceptance rate as an alternative to neostigmine, as previously published by[13] and.[14]
The use of sugammadex in our study resulted in the lowest rate of TOF ratios <0.7 and <0.9, and correspondingly, a lower rate of acute episodes of oxygen desaturation in the recovery room. Despite this, we did not find significant benefits for sugammadex regarding the rate of (X-ray defined) postoperative pulmonary complications. In case of neostigmine, this could be due to a time-delayed (hence not monitored by us) onset of action which may have prevented longer-term (beyond recovery) outcome differences. In case of “no reversal” it is likely to be largely due to the overall low numbers of included patients and thus postoperative pathological X-ray results in this pilot investigation.
Our audit had several limitations: Although prospective in design, we only studied a non-randomized convenience sample (two weeks) which limits the conclusions that can be firmly drawn from our results. Furthermore, we used kinemyometric (KMG) quantitative neuromuscular monitoring for assessment of TOF ratios. Although KMG monitoring has been mentioned to be suitable for clinical monitoring, it may be less reproducible than other forms of quantitative monitoring (e.g., mechanomyographic) and hence not ideal for research purposes.[11] Another potential limitation of this audit is the definition of “outcome”: with reference to the study by Berg et al.[8] we defined X-ray findings consistent with atelectasis or pneumonia as undesirable “mid-term” outcome. The clinical consequences of mild atelectasis reported in a chest X-ray may ultimately be small, especially in ASA 1 patients. However, very strong evidence exists for the detrimental acute effects of residual paralysis on respiratory physiology[1] and our data suggesting medium term consequences at least very much supports such reports.
When compared with neostigmine, the significantly higher price of sugammadex is often quoted as a major setback. It was not our aim to investigate economic data; however, the confirmed significantly lower rate of TOF ratios <0.7 and <0.9 after the use of sugammadex and the overwhelming evidence[1] for the detrimental impact of residual paralysis on patient outcome should prompt the question of the acceptable price for patient safety.
CONCLUSION
We report evidence for a reduction of residual neuromuscular blockade following the use of sugammadex, and that residual blockade was associated with findings suggestive of pulmonary pathology, even in patients with only mildly impaired TOF ratios.
Footnotes
Source of Support: Nil
Conflict of Interest: Prof. T. Ledowski has consulted for and received (unrelated) research grants from MSD Australia. However, this study was neither funded nor solicited or influenced by any means by MSD or their affiliates.
REFERENCES
- 1.Murphy GS, Brull SJ. Residual neuromuscular block: Lessons unlearned. Part I: Definitions, incidence and adverse physiologic effects of residual neuromuscular block. Anaesth Analg. 2010;111:120–8. doi: 10.1213/ANE.0b013e3181da832d. [DOI] [PubMed] [Google Scholar]
- 2.Baillard C, Clec’h C, Catineau J, Salhi F, Gehan G, Cupa M, et al. Postoperative residual neuromuscular block: A survey of management. Br J Anaesth. 2005;95:622–6. doi: 10.1093/bja/aei240. [DOI] [PubMed] [Google Scholar]
- 3.Murphy GS, Szokol JW, Franklin M, Marymont JH, Avram MJ, Vender JS. Post Anaesthesia care unit recovery times and neuromuscular blocking drugs: A prospective study of orthopedical surgical patients randomized to receive pancuronium or rocuronium. Anaesth Analg. 2004;98:193–200. doi: 10.1213/01.ANE.0000095040.36648.F7. [DOI] [PubMed] [Google Scholar]
- 4.Pühringer FK, Gordon M, Demeyer I, Sparr HJ, Ingimarsson J, Klarin B, et al. Sugammadex rapidly reverses moderate rocuronium- or vecuronium induced neuromuscular block during sevoflurane anaesthesia: A dose-response relationship. Br J Anaesth. 2010;105:610–9. doi: 10.1093/bja/aeq226. [DOI] [PubMed] [Google Scholar]
- 5.Beecher HK, Todd DP. A study of the deaths associated with Anaesthesia and surgery: Based on a study of 599, 548 Anaesthesias in ten institutions 1948-1952, inclusive. Ann Surg. 1954;140:2–35. doi: 10.1097/00000658-195407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali HH, Utting JE, Gray C. Stimulus frequency in the detection of neuromuscular block in humans. Br J Anaesth. 1970;42:967–78. doi: 10.1093/bja/42.11.967. [DOI] [PubMed] [Google Scholar]
- 7.Kopman AF. Residual neuromuscular block and adverse respiratory events. Anaesth Analg. 2008;107:1756. doi: 10.1213/ane.0b013e318187ac1f. [DOI] [PubMed] [Google Scholar]
- 8.Berg H, Roed J, Viby-Mogensen J, Mortensen CR, Engbaek J, Skovgaard LT, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications: A prospective, randomized and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095–103. doi: 10.1111/j.1399-6576.1997.tb04851.x. [DOI] [PubMed] [Google Scholar]
- 9.Brull SJ, Silverman DG. Visual and tactile assessment of neuromuscular fade. Anaesth Analg. 1993;77:352–5. doi: 10.1213/00000539-199308000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Drenck NE, Ueda N, Olsen NV, Engbaek J, Jensen E, Skovgaard LT, et al. Manual evaluation of residual curarization using double-burst stimulation: A comparison with train of four. Anaesthesiology. 1998;70:578–81. doi: 10.1097/00000542-198904000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Brull SJ, Murphy GS. Residual neuromuscular block: Lessons unlearned. Part 2: Methods to reduce the risk of residual weakness. Anaesth Analg. 2010;111:129–40. doi: 10.1213/ANE.0b013e3181da8312. [DOI] [PubMed] [Google Scholar]
- 12.Kim KS, Cheong MA, Lee HJ, Lee JM. Tactile assessment for the reversibility of rocuronium-induced neuromuscular blockade during propofol or sevoflurane anaesthesia. Anaesth Analg. 2004;99:1080–5. doi: 10.1213/01.ANE.0000130616.57678.80. [DOI] [PubMed] [Google Scholar]
- 13.Watts RW, London JA, Van Wijk RM, Lui YL. The influence of unrestricted use of sugammadex on clinical anaesthetic practice in a tertiary teaching hospital. Anaesth Intensive Care. 2012;40:333–9. doi: 10.1177/0310057X1204000218. [DOI] [PubMed] [Google Scholar]
- 14.Ledowski T, Hillyard S, Kozman A, Johnston F, Gillies E, Greeaway M, et al. Unrestricted access to sugammadex: Impact on neuromuscular blocking agent choice, reversal practice and associated healthcare costs. Anaesth Intensive Care. 2012;40:340–3. doi: 10.1177/0310057X1204000219. [DOI] [PubMed] [Google Scholar]


