Abstract
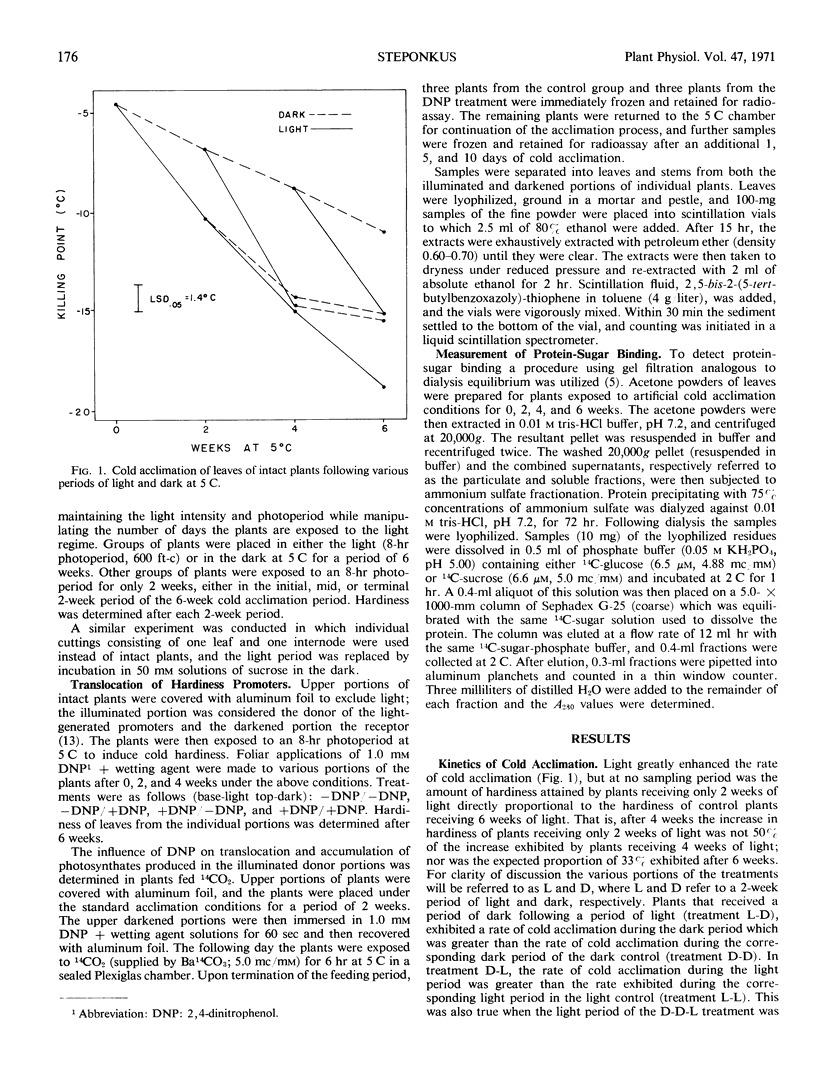
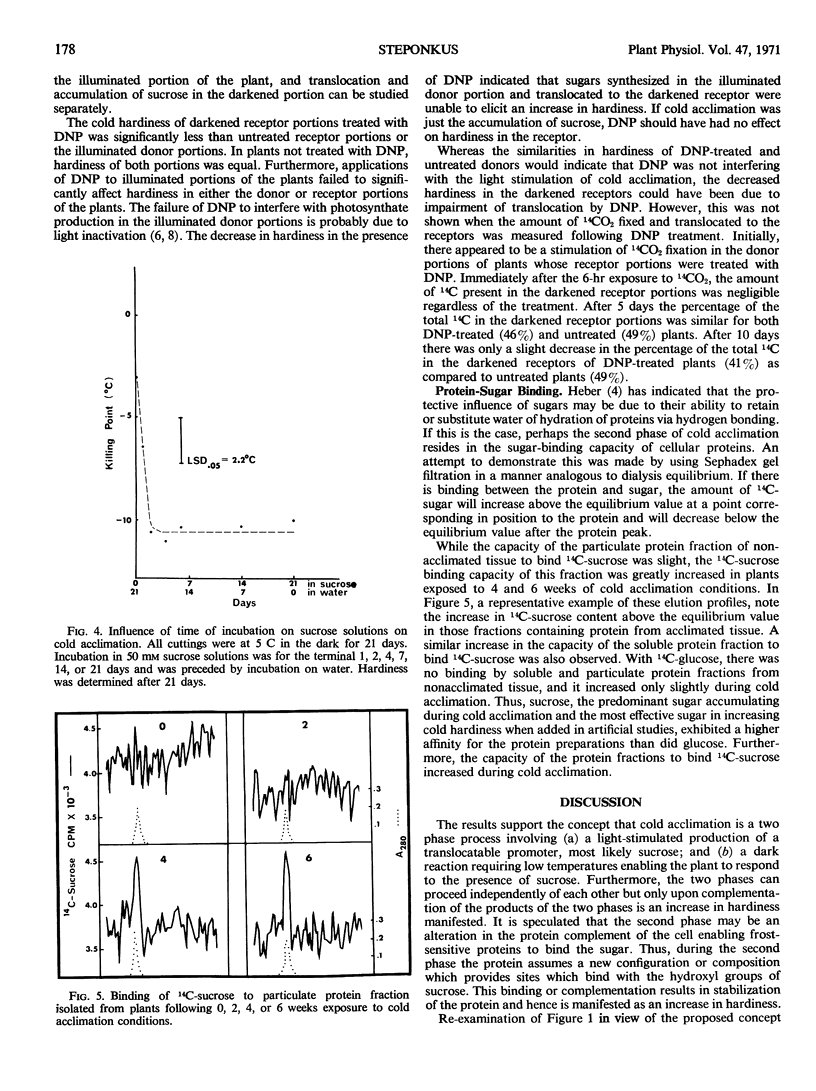
The light-enhanced production and accumulation of sugars is only one step in the process of cold acclimation in Hedera helix L. var. Thorndale (English ivy). Applications of 2,4-dinitrophenol to plants with different portions exposed to light and dark indicated that the mere presence or accumulation of the light-generated promoters did not invoke an increase in hardiness. Kinetics of cold acclimation during alternating periods of light and dark also indicate that the light stimulation of cold acclimation is only a partial component of the total process. Incubation on 50 mm solutions of sucrose can replace the light requirement. A second phase which can proceed in the dark is thought to result in the production of proteins which, due to an altered composition or configuration, have a greater capacity to bind sugars. This is evidenced by the fact that protein from cold acclimated tissue exhibited a higher sugar-binding capacity than protein from nonacclimated tissue. Furthermore, the two phases can proceed independently of each other, but only upon complementation of the products of the two phases is an increase in cold hardiness manifested.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dexter S. T. DECREASING HARDINESS OF WINTER WHEAT IN RELATION TO PHOTOSYNTHESIS, DEFOLIATION, AND WINTER INJURY. Plant Physiol. 1933 Apr;8(2):297–304. doi: 10.1104/pp.8.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILES C. H., McKAY R. B. Studies in hydrogen bond formation. XI. Reactions between a variety of carbohydrates and proteins in aqueous solutions. J Biol Chem. 1962 Nov;237:3388–3392. [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Heber U. Freezing injury in relation to loss of enzyme activities and protection against freezing. Cryobiology. 1968 Nov-Dec;5(3):188–201. doi: 10.1016/s0011-2240(68)80163-4. [DOI] [PubMed] [Google Scholar]
- Krul W. R. Increased root initiation in pinto bean hypocotyls with 2,4-dinitrophenol. Plant Physiol. 1968 Mar;43(3):439–441. doi: 10.1104/pp.43.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massini P., Voorn G. The effect of ferredoxin and ferrous ion on the chlorophyll sensitized photoreduction of dinitrophenol. Photochem Photobiol. 1967 Nov;6(11):851–856. [PubMed] [Google Scholar]
- Steponkus P. L., Lanphear F. O. Light stimulation of cold acclimation: production of a translocatable promoter. Plant Physiol. 1967 Dec;42(12):1673–1679. doi: 10.1104/pp.42.12.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus P. L., Lanphear F. O. Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol. 1967 Oct;42(10):1423–1426. doi: 10.1104/pp.42.10.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus P. L., Lanphear F. O. The Role of Light in Cold Acclimation of Hedera helix L. var. Thorndale. Plant Physiol. 1968 Feb;43(2):151–156. doi: 10.1104/pp.43.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser C. J. Cold Resistance and Injury in Woody Plants: Knowledge of hardy plant adaptations to freezing stress may help us to reduce winter damage. Science. 1970 Sep 25;169(3952):1269–1278. doi: 10.1126/science.169.3952.1269. [DOI] [PubMed] [Google Scholar]


