Abstract
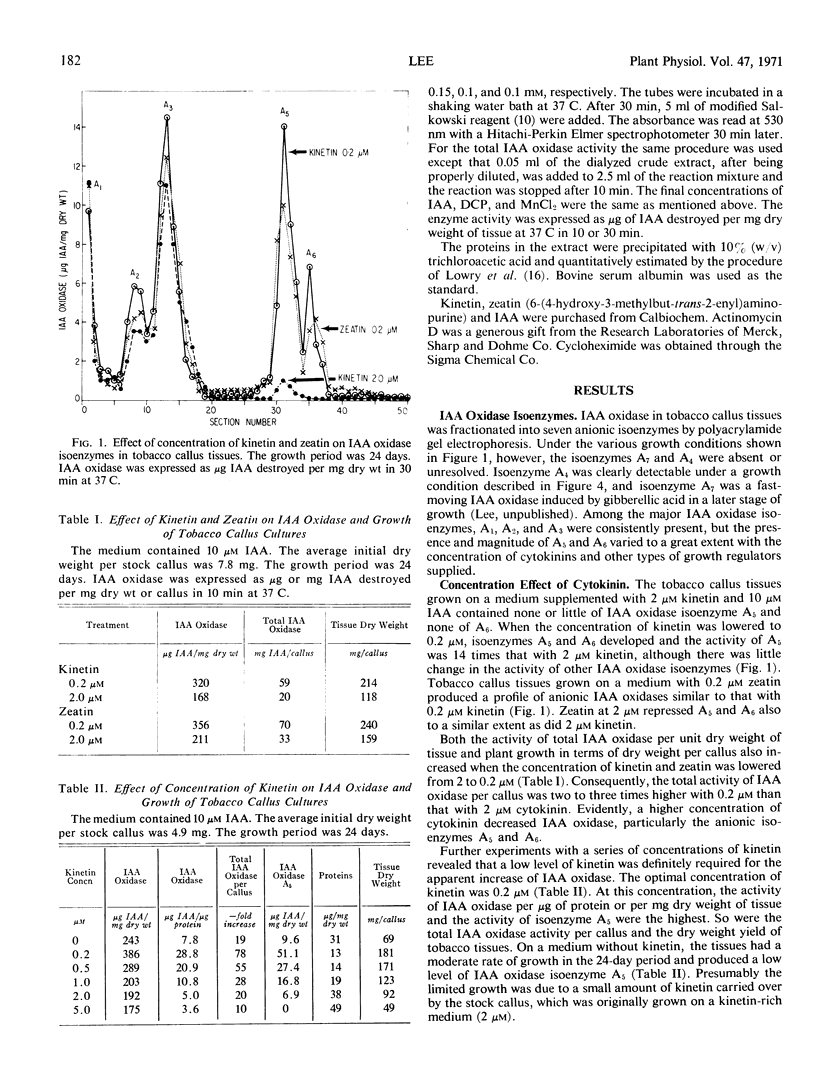
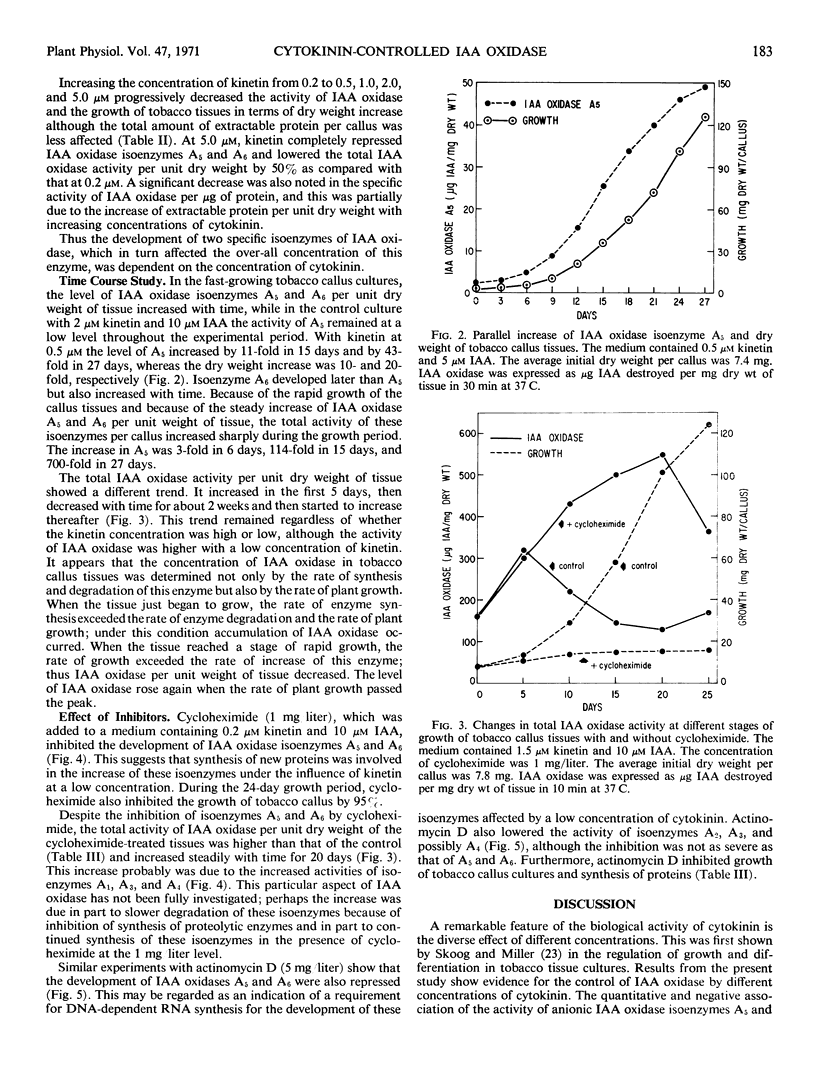
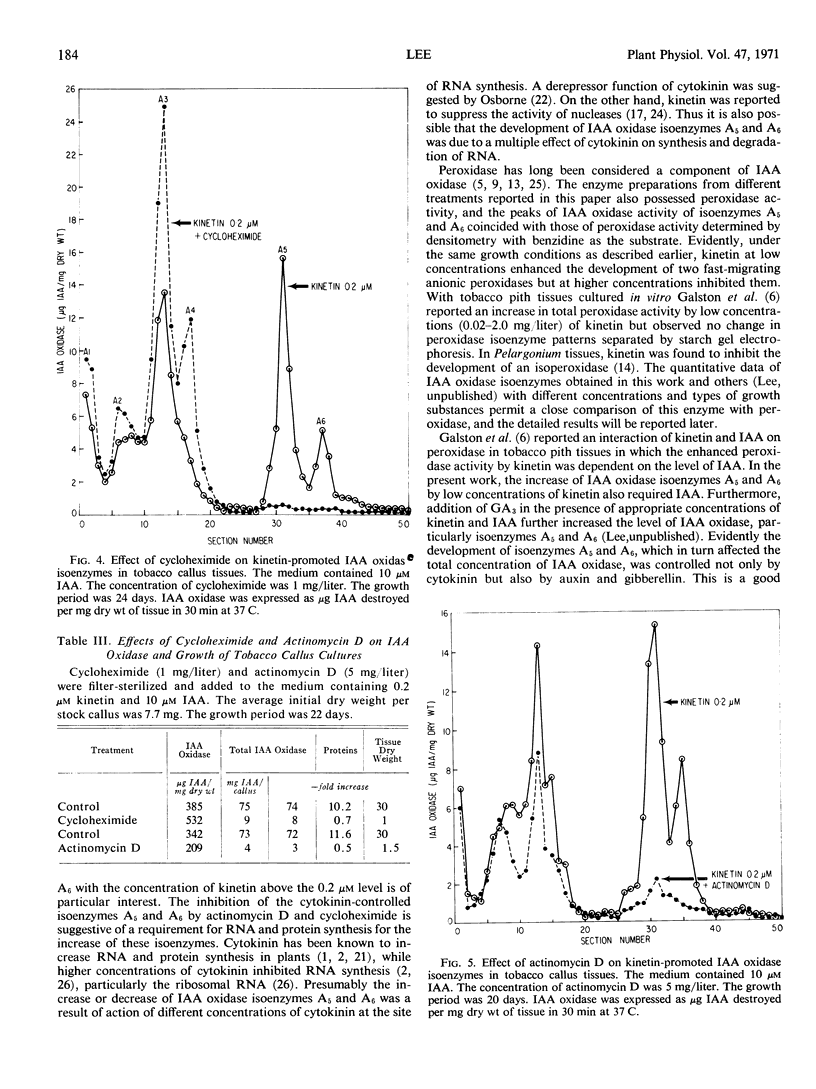
Indoleacetic acid oxidase in tobacco callus tissues (Nicotiana tabacum L., cultivar White Gold) was resolved into seven anionic isoenzymes by polyacrylamide gel disc electrophoresis. Different concentrations of kinetin and zeatin in the presence of indoleacetic acid affected the level of this enzyme, particularly two fast-moving isoenzymes, A5 and A6. The optimal concentration of kinetin was 0.2 μm; increasing concentrations above this level progressively lowered the total activity of indoleacetic acid oxidase and repressed the development of isoenzymes A5 and A6. Actinomycin D and cycloheximide inhibited the development of these two isoenzymes under the influence of 0.2 μm kinetin, suggesting a requirement for RNA and protein synthesis. The cytokinin-promoted indoleacetic acid oxidase isoenzymes A5 and A6 increased with time and paralleled the dry weight increase of tobacco callus tissues, but the total activity of indoleacetic acid oxidase per unit dry weight of tobacco callus varied with time depending on the stage of plant growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter W. J., Cherry J. H. Effects of benzyladenine on accumulation of 32P into nucleic acids of peanut cotyledons. Biochim Biophys Acta. 1966 Mar 21;114(3):640–642. doi: 10.1016/0005-2787(66)90115-8. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Evans J. J. Spectral similarities and kinetic differences of two tomato plant peroxidase isoenzymes. Plant Physiol. 1970 Jan;45(1):66–69. doi: 10.1104/pp.45.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALSTON A. W., BONNER J., BAKER R. S. Flavoprotein and peroxidase as components of the indoleacetic acid oxidase system of peas. Arch Biochem Biophys. 1953 Feb;42(2):456–470. doi: 10.1016/0003-9861(53)90373-7. [DOI] [PubMed] [Google Scholar]
- GOLDACRE P. L. Hydrogen peroxide in the enzymic oxidation of heteroauxin. Aust J Sci Res B. 1951 Aug;4(3):293–302. doi: 10.1071/bi9510293. [DOI] [PubMed] [Google Scholar]
- Gordon S. A., Weber R. P. COLORIMETRIC ESTIMATION OF INDOLEACETIC ACID. Plant Physiol. 1951 Jan;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENTEN R. H. The oxidation of indolyl-3-acetic acid by waxpod bean root sap and peroxidase systems. Biochem J. 1955 Jan;59(1):110–121. doi: 10.1042/bj0590110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay E., Shannon L. M., Lew J. Y. Peroxidase isozymes from horseradish roots. II. Catalytic properties. J Biol Chem. 1967 May 25;242(10):2470–2473. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macnicol P. K. Peroxidases of the Alaska pea (Pisum sativum L.). Enzymic properties and distribution within the plant. Arch Biochem Biophys. 1966 Nov;117(2):347–356. doi: 10.1016/0003-9861(66)90422-x. [DOI] [PubMed] [Google Scholar]
- Osborne D. J. Effect of Kinetin on Protein & Nucleic Acid Metabolism in Xanthium Leaves During Senescence. Plant Physiol. 1962 Sep;37(5):595–602. doi: 10.1104/pp.37.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Srivastava B. I., Ware G. The effect of kinetin on nucleic acids and nucleases of excised barley leaves. Plant Physiol. 1965 Jan;40(1):62–64. doi: 10.1104/pp.40.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz R. E. The Indole-3-Acetic Acid Oxidase of Lupinus albus L. Plant Physiol. 1957 Jan;32(1):31–39. doi: 10.1104/pp.32.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]


