In 2 large human immunodeficiency virus (HIV)–discordant couple cohorts in Africa, time to treatment response after penicillin therapy for a positive rapid plasma reagin test result was similar irrespective of HIV status. Despite effective therapy, serofast state and syphilis reinfection were common.
Keywords: HIV/AIDS, syphilis, discordant couples, Zambia, Rwanda
Abstract
Background. Syphilis continues to be a common sexually transmitted infection, despite the availability of inexpensive and effective treatment. Infection in human immunodeficiency virus (HIV)–discordant couples is important because syphilis increases the risk of HIV acquisition. Current US treatment guidelines recommend 1 dose of benzathine penicillin for early syphilis, irrespective of HIV status, but data from coinfected patients are limited.
Methods. Retrospective analysis of 1321 individuals in 2 African HIV-discordant couple cohorts was performed. Cox proportional hazards analysis and multivariable modeling were used to assess predictors of serologic response to treatment at 180 days and 400 days. Modeling was performed for all episodes of positive rapid plasma reagin (RPR) test results and on a subset with higher RPR titers (≥1:4).
Results. A total of 1810 episodes of syphilis among 1321 individuals were treated with penicillin between 2002 and 2008. Although a positive RPR was more common in the HIV-infected partners, HIV infection did not impact the likelihood of serologic response to therapy (odds ratio [OR], 1.001; P = .995). By 400 days, 67% had responded to therapy, 27% were serofast, and 6.5% had documented reinfection. Prevalent infections were more likely to remain serofast than incident infections (33% vs 20% at 400 days).
Conclusions. In 2 HIV-serodiscordant couple cohorts in Africa, incident syphilis had a very good likelihood of response to penicillin therapy, irrespective of HIV infection. This supports current Centers for Disease Control and Prevention treatment guidelines. A high proportion of prevalent RPR-positive infections remain serofast despite treatment.
Human immunodeficiency virus (HIV) and syphilis are common infections in sub-Saharan Africa, and increasing rates of coinfection have been reported in many countries over the past decade [1–6]. In Zambia during 2009, HIV prevalence was approximately 14.3% and rapid plasma reagin (RPR) test positivity had been documented in 18%–32% of HIV-discordant couples [7–9]. In Rwanda, HIV prevalence was approximately 3% in 2005, and syphilis prevalence had been reported in up to 18% of patients with genital ulcers [10, 11].
Literature on HIV and syphilis coinfection shows that the clinical presentation and outcomes of syphilis infection are similar in monoinfected and coinfected individuals, although HIV patients with syphilis may have multiple chancres in primary disease and are more likely to have a delayed serologic response following therapy [12–14]. Ulcerative sexually transmitted infections (STIs) have been shown to increase the risk of HIV acquisition (adjusted risk ratio, 2.2–11.3 compared to 3–4 for nonulcerative STIs) [15].
The US Centers for Disease Control and Prevention's (CDC) 2010 Sexually Transmitted Disease Treatment Guidelines recommend the same course of therapy for early syphilis (a single intramuscular injection of 2.4 million units of benzathine penicillin), irrespective of HIV status, an unchanged recommendation from 2006 [16]. However, many providers continue to treat early infection in the coinfected patient with additional penicillin out of concern for potential treatment failure. In one 2009 study of 390 practicing infectious diseases specialists, 62% reported treating HIV patients with secondary syphilis with 3 doses of penicillin [17].
In the setting of some clinical uncertainty and increasing rates of syphilis and HIV in many communities worldwide, larger studies can help inform treatment recommendations for coinfected patients. The aim of this study is to examine treatment response among individuals in 2 large HIV-discordant couple cohorts in central Africa who received benzathine penicillin for the treatment of syphilis based on a positive RPR test result [18, 19].
METHODS
Study Design and Setting
This was a retrospective cohort analysis of individuals enrolled in HIV-serodiscordant couple cohorts in Lusaka, Zambia, and Kigali, Rwanda.
Participants
The Rwanda Zambia HIV Research Group is one of the largest cohorts of HIV-serodiscordant couples in Africa, with follow-up available for couples referred from a longstanding program of free couples voluntary counseling and testing (CVCT) for HIV, described elsewhere [8, 20–25]. The study period for this analysis was from January 2002 to October 2008 in Zambia and from August 2002 to April 2008 in Rwanda. During this study period, there were 3483 individuals enrolled in Lusaka and 2925 of them had at least 1 follow-up visit (84%); in Kigali, 3205 individuals were enrolled, with 3091 having at least 1 follow-up visit (96%). Couples were followed longitudinally with quarterly visits for ongoing HIV prevention education and for the collection of clinical and laboratory data [26, 27]. Participants received free treatment for STIs as well as counseling and condom provision at each visit.
At the Zambia site, RPR testing was performed for all participants every 3 months from 2002 to 2005 and annually from 2006 to 2008. In Rwanda, RPR testing was performed every 6 months from 2002 to 2005 and annually from 2006 to 2008. In both cohorts, patients with signs or symptoms of syphilis received diagnostic testing and treatment in addition to routine testing. Treponemal-specific confirmatory testing was not performed at the time of RPR titer, but was tested retrospectively on a subset of samples as described below. All clients with RPR-positive undiluted serum were offered a single dose of 2.4 million units of benzathine penicillin, although a few patients with prevalent infection were treated with 2 or 3 doses of penicillin weekly. RPR testing was repeated at subsequent visits and retreatment offered if there was no drop in titer. When incident syphilis was serologically diagnosed in a client during follow-up, his or her sexual partners were treated with penicillin. When prevalent cases were detected at enrollment, only RPR-positive individuals were treated unless they reported a recent genital ulcer, in which case their partner was treated as well. During the time of the study, the circumcision rates among adult men in both countries were quite low, with Zambia at 16% and Rwanda at 12% [28–30].
Inclusion and Exclusion Criteria
Eligibility criteria for the parent study included men (aged 18–65) and women (aged 16–45) in HIV-serodiscordant couples who were cohabitating and planned to stay in the area for at least 1 year. If the HIV-seronegative member of the couple became HIV positive (approximately 7.5% of Zambian and 3.5% of Rwandan couples per year), they were no longer included in this analysis [31]. CD4 and HIV monitoring was performed infrequently. When antiretroviral therapy (ART) became available, clients with stage III–IV HIV disease as classified by the World Health Organization were referred to their district health clinic for ART assessment. Those started on ART were excluded from further analysis. ART became available on a small scale starting in 2003 in Kigali and 2004 in Lusaka, with access expanding steadily thereafter [32–34]. All patients with a positive RPR test at any dilution were included in the initial analysis if they had a follow-up visit 14–400 days after treatment.
Laboratory Testing
Laboratories were GCLP (Good Clinical Laboratory Practices) certified, laboratory technicians were GCLP trained and certified, and standard operating procedures were used for all testing and clinical procedures. RPR testing was performed via card method (RPR test Macro-Vue, Becton-Dickinson Europe). Treponema pallidum hemagglutination assays (TPHAs) were performed in both sites on banked plasma samples from 2007 to 2008 (stored at −80°C) with the Biotec Laboratories test kit. In Zambia, historical TPHA data from 1994 to 1998 were included, as were available data from 2003 to 2006 in Rwanda (TPHA test, Fujirebio, Tokyo, Japan). HIV testing was performed with a rapid antibody test that was confirmed by a second rapid antibody test if it was positive or indeterminate and a third test as indicated for discrepant results (Determine and Capillus tests in Zambia, Determine and Unigold tests in Rwanda) [35].
Case Definitions
Syphilis was defined as the presence of positive RPR at any titer and cases were subset into incident disease (previously RPR negative) and prevalent disease (RPR positive at first testing). Response to therapy was defined according to CDC recommendations, as a 4-fold fall in RPR titer (ie, from 1:64 to 1:16) or reversion to nonreactive [16]. Treatment was documented receipt of 2.4 million units of benzathine penicillin delivered intramuscularly. Treatment data were directly verified from pharmacy records in Zambia and with medical records review in Rwanda.
Outcomes
The primary outcomes of interest were the time to treatment response following the receipt of penicillin therapy and the likelihood of various serologically defined outcomes (response, serofast, or reinfection) at 180 days and 400 days after therapy. For the multivariate and Cox proportional hazards models, patients with RPR-positive titers with undiluted serum only were excluded. Only RPR titers ≥1:2 were included for increased specificity because confirmatory treponemal specific testing was not available The proportion of responders stratified by city, sex, HIV status, and prevalent versus incident infection is also presented.
Statistical Analysis
SAS Base 9.3 and Enterprise Guide 4.2 were used for analysis. Survival analysis technique was used to analyze time to treatment response. Time to response was right-censored if we did not observe any serologic response within 400 days. Cox proportional hazards modeling with random intercept was performed with time-independent variables: HIV status, sex, age, and initial RPR titer [36]. The random intercept was to take care of the correlations of the response times within a subject. RPR titer was recoded as a binary variable. Assumptions for the model were tested by goodness of fit and Schoenfeld residuals.
Log-rank test was used to determine whether the 2 curves were significantly different. Confounding by age, sex, initial RPR titer, city, and incident or prevalent syphilis was analyzed graphically one at a time to evaluate for a meaningful difference between the groups. Meaningful confounders were then adjusted by the model to reanalyze magnitude of effect. Missing ages (n = 44) were imputed with median age in all analyses. The generalized estimating equation method was used to handle within-subject correlations for logistic regressions.
Ethics
The study was approved by the Office for Human Research Protections registered institutional review boards at Emory University, and in Rwanda and Zambia.
RESULTS
The prevalence of positive RPR was higher in Zambia (18% vs 9% in Rwanda) and higher among HIV-positive individuals (16% vs 10% in HIV-negative individuals). In Zambia, RPR prevalence was higher among women (20% vs 15% among men), a difference that was not noted in Rwanda. Among prevalent cases, 35% of RPR-positive participants had RPR-positive spouses and this differed significantly by sex: 27% of RPR-positive women had RPR-positive husbands, whereas 45% of RPR-positive men had RPR-positive wives. Most incident cases (62.9%) occurred in individuals whose spouses were and remained RPR-negative; 9.9% had spouses with baseline prevalent positive RPR results; and in the remaining 27%, both husband and wife had incident syphilis detected during follow-up.
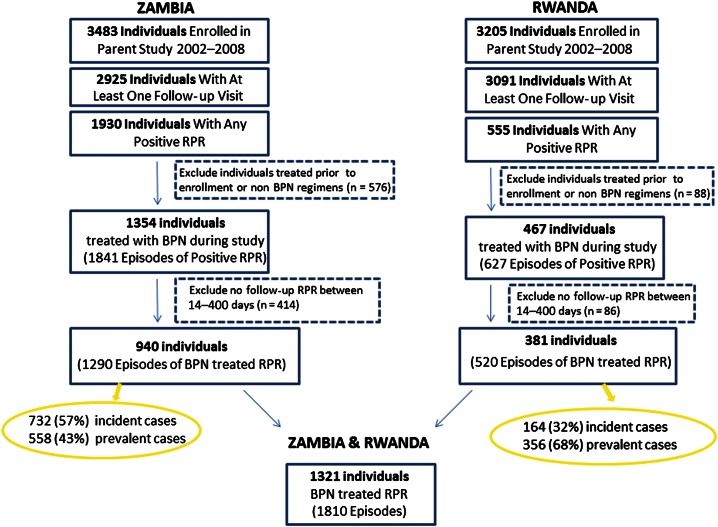
Overall, 1321 individuals in HIV-discordant couples had 1810 episodes of positive RPR treated with benzathine penicillin during the 6-year study period (Figure 1). Nearly all received a single dose of benzathine penicillin, but 44 individuals with prevalent disease were treated with 2 weekly doses and 24 individuals received 3 weekly doses of benzathine penicillin. Demographic and clinical data are shown in Table 1 for RPR-positive individuals. In both cities, the average woman was 7–8 years younger than her male partner [37]. Acute genital ulcers over the past year were reported by 12.8%, and 7.3% had a genital ulcer present on physical examination. Minimal CD4 and viral load data were available, but in a subset of 39 individuals, median CD4 count was 367 cells/mm³ in Zambia and 446 cells/mm³ in Rwanda. Of 1810 episodes, 896 (49.5%) were incident positive RPR titers and the remainder were first detected at the time of CVCT. A majority of positive tests were low titer RPR (56.8% positive only with undiluted serum) (Table 2).
Figure 1.
Flowchart of treated episodes of positive rapid plasma reagin test result. Abbreviations: BPN, benzathine penicillin; RPR, rapid plasma reagin.
Table 1.
Demographic and Clinical Characteristics of Rapid Plasma Reagin–Positive Men and Women From Lusaka, Zambia, and Kigali, Rwanda
| Lusaka, Zambia (n = 940) |
Kigali, Rwanda (n = 381) |
Total (n = 1321) |
|
|---|---|---|---|
| Sex | |||
| Women | 615 (65.4) | 177 (46.5) | 792 (60) |
| Men | 325 (34.6) | 204 (53.5) | 529 (40) |
| Mean agea ± SD (range) | |||
| Overall | 32 ± 7.4 (16–64) | 33 ± 8.6 (18–63) | 32 ± 7.8 (16–64) |
| Women | 29 ± 6.1 (16–53) | 29 ± 6.2 (18–45) | 29 ± 6.1 (16–53) |
| Men | 36 ± 7.7 (23–64) | 37 ± 8.8 (21–63) | 36 ± 8.1 (21–64) |
| History of acute genital ulcer in the past year | 109 (11.6) | 60 (15.7) | 169 (12.8) |
| Genital ulcer on exam | 58 (6.2) | 39 (10.2) | 97 (7.3) |
| HIV status (at RPR) | |||
| Positive | 714 (76) | 219 (57.5) | 933 (70.6) |
| Negative | 226 (24) | 162 (42.5) | 388 (29.4) |
| Mean CD4, cells/mm3, ± SD (range) | n = 27 471 ± 270 (86–1056) |
n = 12 516 ± 327 (140–1218) |
|
| Median | 367 | 446 | |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: HIV, human immunodeficiency virus; RPR, rapid plasma reagin; SD, standard deviation.
a Ages were missing from the dataset for 44 subjects (11 in Zambia and 33 in Rwanda).
Table 2.
Distribution of Titers and Incident Versus Prevalent Episodes of Positive Rapid Plasma Reagin
| Lusaka, Zambia: 1290 Episodes, No. (%) |
Kigali, Rwanda: 520 Episodes, No. (%) |
Total: 1810 Episodes, No. (%) |
|
|---|---|---|---|
| Rapid plasma reagin titer | |||
| Undiluted serum | 472 (36.6) | 132 (25.4) | 604 (33.4) |
| 1:2 | 297 (23) | 126 (24.2) | 423 (23.4) |
| 1:4 | 224 (17.4) | 78 (15) | 302 (16.7) |
| 1:8 | 136 (10.5) | 65 (12.5) | 201 (11.1) |
| 1:16 | 79 (6.1) | 41 (7.9) | 120 (6.6) |
| 1:32 | 34 (2.6) | 33 (6.4) | 67 (3.7) |
| 1:64 | 30 (2.3) | 24 (4.6) | 54 (3) |
| 1:128 | 14 (1.1) | 15 (2.9) | 29 (1.6) |
| ≥1:256 | 4 (0.3) | 6 (1.2) | 10 (0.6) |
| Stage | |||
| Incident | 732 (56.7) | 164 (31.5) | 896 (49.5) |
| Prevalent | 558 (43.3) | 356 (68.5) | 914 (50.5) |
Table 3 shows the results of TPHA confirmatory testing performed on banked samples and from historical data. In Zambia, there was a significant background TPHA positivity rate of 43% among clients with a negative RPR, and the higher titer RPRs were more likely to confirm with specific treponemal testing. In Rwanda, fewer TPHA results were available, but the background rate of positive RPR was significantly lower, at 14%. At both sites, TPHA positivity was high even at the lowest titer (undiluted serum).
Table 3.
Treponema pallidum Hemagglutination Assay Confirmatory Testing of Rapid Plasma Reagin Negative and Positive Samples
| Lusaka, Zambia |
Kigali, Rwanda |
||||
|---|---|---|---|---|---|
| RPR Titer | TPHA Positive/Total | % | TPHA Positive/Total | % | |
| Negative | 136/317 | 43 | 11/78 | 14 | |
| 1:1 | 93/140 | 66 | 9/12 | 75 | |
| 1:2 | 160/207 | 77 | 53/63 | 84 | |
| 1:4 | 166/185 | 90 | 42/44 | 95 | |
| 1:8 | 166/168 | 99 | 43/47 | 91 | |
| ≥1:16 | 212/215 | 99 | 48/48 | 100 | |
| Total No. of tests | 1396 | 292 | |||
Abbreviations: RPR, rapid plasma reagin; TPHA, Treponema pallidum hemagglutination assay.
Overall, 51% of individuals had a successful response to therapy within 180 days and 67% had responded by 400 days. The proportion of serofast responses was 26.6% at 400 days, and approximately 6.5% of cohort participants became reinfected during the 400-day follow-up period. Bivariate analyses of predictors of serologic response at 400 days showed no differences between HIV-positive and HIV-negative cases. Zambians were more likely to respond than Rwandans (68% vs 63%, P = .048), and women were more likely to respond than men (69% vs 64%, P = .035).
In a multivariate analysis of RPR titers ≥1:2, predictors of response to therapy included incident disease and country (Rwanda). RPR titer ≥1:4 was associated with response in the multivariate model (odds ratio, 1.33; P = .031), but not the Cox proportional hazards model (hazard ratio [HR], 1.15; P = .099). HIV status did not predict response to therapy, and the difference between sexes found in the multivariate analysis did not persist when adjusted for other covariates. Cox proportional hazards modeling for all episodes showed similar results, with the exception of female sex, which was significant. When the analysis was stratified for those treated for a single episode and those with multiple episodes during the study period, the findings were unchanged (Table 4).
Table 4.
Multivariate Models for Treatment Response (Including Rapid Plasma Reagin Titers ≥1:2)
| Multivariable Model |
Cox Proportional Hazards Model |
|||
|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value |
| Predictors of response to BPN treatmenta | ||||
| HIV status | ||||
| Negative | 1.0 (Ref) | |||
| Positive | 0.94 (.72–1.23) | .669 | 0.96 (.82–1.14) | .669 |
| Sex | ||||
| Male | 1.0 (Ref) | |||
| Female | 1.22 (.92–1.63) | .166 | 1.23 (1.04–1.46) | .016 |
| Country | ||||
| Zambia | 1.0 (Ref) | |||
| Rwanda | 1.46 (1.11–1.92) | .006 | 1.27 (1.08–1.50) | .005 |
| RPR titer | ||||
| 1:2 | 1.0 (Ref) | |||
| ≥1:4 | 1.33 (1.03–1.72) | .031 | 1.15 (.97–1.35) | .099 |
| Disease stage | ||||
| Incident infection | 1.0 (Ref) | |||
| Prevalent infection | 0.40 (.31–.52) | <.0001 | 0.52 (.44–.61) | <.0001 |
| Age, y | 0.99 (.97–1.01) | .300 | 0.99 (.98–1.01) | .334 |
| For subjects with only 1 episode (n = 575)a | ||||
| HIV status | ||||
| Negative | 1.0 (Ref) | |||
| Positive | 0.92 (.61–1.40) | .706 | 0.93 (.72–1.20) | .581 |
| Sex | ||||
| Male | 1.0 (Ref) | |||
| Female | 1.30 (.87–1.95) | .196 | 1.22 (.96–1.54) | .103 |
| Country | ||||
| Zambia | 1.0 (Ref) | |||
| Rwanda | 1.38 (.93–2.04) | .114 | 1.07 (.85–1.34) | .589 |
| RPR titer | ||||
| 1:2 | 1.0 (Ref) | |||
| ≥1:4 | 1.38 (.95–2.00) | .094 | 1.17 (.93–1.47) | .192 |
| Disease stage | ||||
| Incident infection | 1.0 (Ref) | 0.36 (.28, .45) | <.0001 | |
| Prevalent infection | 0.26 (.17–.40) | <.0001 | ||
| Age, y | 1.01 (.98–1.04) | .489 | 1 (.98–1.02) | .956 |
| For subjects with multiple episodes (n = 258)a | ||||
| HIV status | ||||
| Negative | 1.0 (Ref) | |||
| Positive | 0.88 (.62–1.25) | .482 | 0.96 (.77–1.20) | .728 |
| Sex | ||||
| Male | 1.0 (Ref) | |||
| Female | 1.22 (.82–1.83) | .327 | 1.24 (.96–1.59) | .097 |
| Country | ||||
| Zambia | 1.0 (Ref) | |||
| Rwanda | 1.59 (1.07–2.37) | .023 | 1.41 (1.09–1.82) | .009 |
| RPR titer | ||||
| 1:2 | 1.0 (Ref) | |||
| ≥1:4 | 1.40 (.98–2.01) | .063 | 1.23 (.98–1.55) | .075 |
| Disease stage | ||||
| Incident infection | 1.0 (Ref) | |||
| Prevalent infection | 0.39 (.26–.57) | <.0001 | 0.58 (.45–.74) | <.0001 |
| Age, y | 0.98 (.96–1.00) | .071 | 0.99 (.98–1.00) | .18 |
Abbreviations: BPN, benzathine penicillin; CI, confidence interval; HIV, human immunodeficiency virus; RPR, rapid plasma reagin.
a Adjusted for all covariates in the table.
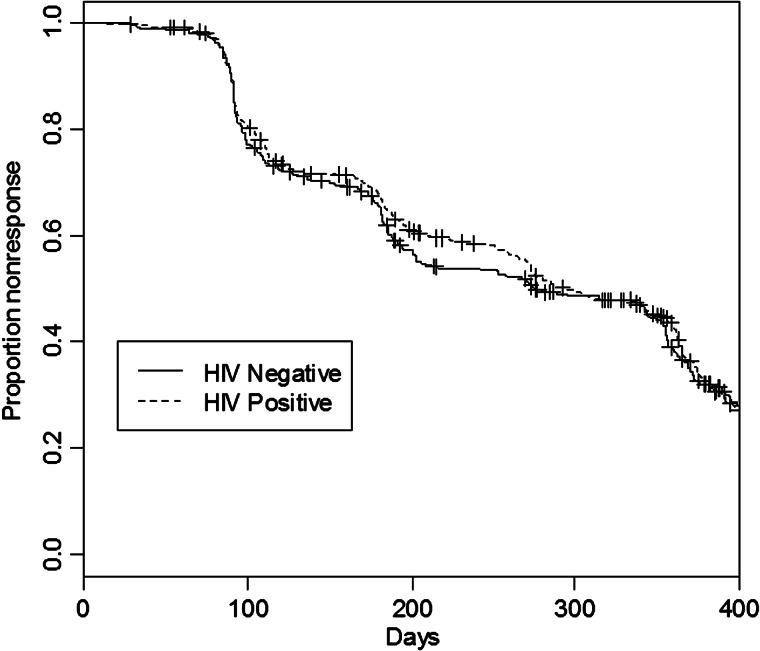
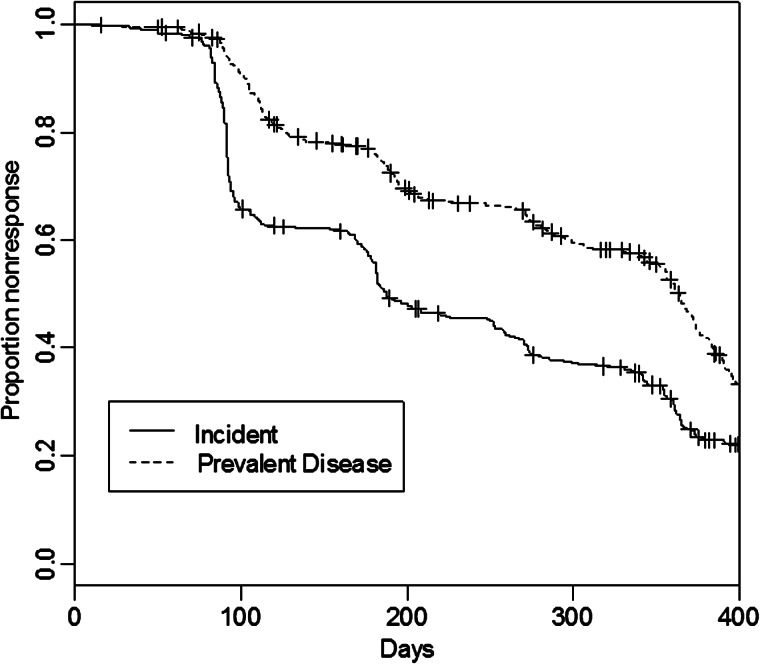
Figure 2 shows the Kaplan-Meier curve of likelihood of response by HIV status, showing no statistical difference between the 2 groups during the 400 days following therapy. In Figure 3, the KM curve showing the wide difference between likelihood of treatment response among incident and prevalent cases is presented.
Figure 2.
Adjusted survival curve for human immunodeficiency virus (HIV)–negative (solid curve) and HIV-positive patients (dashed curve). Abbreviation: HIV, human immunodeficiency virus.
Figure 3.
Adjusted survival curve by incident (solid curve) or prevalent (dashed curve) disease.
DISCUSSION
HIV and syphilis are common STIs among HIV-discordant couples in Zambia and Rwanda. This large retrospective cohort study documents equivalent efficacy of penicillin therapy for syphilis, irrespective of HIV coinfection. This supports current CDC treatment guidelines.
Of the 2 areas studied, Zambian HIV-discordant couples had higher prevalence and incidence of both HIV and syphilis compared with their Rwandan counterparts. The increased incidence of syphilis in this urban population in Lusaka is further evidenced by the fact that 43% of people tested with a negative RPR had a positive treponemal test, suggestive of a high prevalence of prior infection (either treated or untreated), although false-positive TPHA or false-negative RPR are possible explanations as well. The increased rates of syphilis infection in Zambia likely contribute to reinfection rates, lower treatment response rates as documented (compared to Rwanda) and ongoing HIV transmission in their community.
The serologic response to therapy at 400 days was 67% overall and 76% among those with incident infection, comparable to other studies [19, 38]. There are several studies that evaluate response to syphilis therapy in monoinfected and coinfected patients, as outlined in 2 recent reviews. Blank et al were unable to make summary statistics owing to study heterogeneity, but they noted a consistent treatment failure rate in HIV patients of 7%–22% in early disease and 19%–31% in late disease [19, 38]. In a recent study in Switzerland by Knaute et al, HIV status was not associated with likelihood of response to therapy in the multivariate model [39].
Treatment for late-latent syphilis is less often associated with serologic response and trials including HIV patients with late stages have documented higher failure rates [40, 41]. González-López and colleagues [41] reviewed 347 cases of syphilis in Spain and showed that HIV coinfection was associated with an HR of 0.61 (95% confidence interval, .39–.96), male sex had an HR of 0.38, and late-stage syphilis had an HR of 0.46. This same study showed that patients receiving ART were more likely to have treatment response, as did a US-based study with 231 cases of syphilis reported by Ghanem et al [42]. In an Israeli HIV clinic with mostly African patients with late-latent syphilis or syphilis of unknown duration, treatment response was only 26%, with 41% experiencing serofast serologic results [43].
In our study, rates of serofast were 20% for those with incident infection and 33% for those with prevalent disease. Serologic response was also associated with higher initial RPR titer in one of the models. These findings are similar to those in a recent study by Sena et al of 231 HIV-negative persons treated with benzathine penicillin for early syphilis, which reported a 21% serofast rate at 6 months. Likelihood of response was associated with age (<30 years), RPR titer >1:32, and fewer sexual partners [44]. Women were more likely than men to respond to therapy in our Cox proportional hazards model, but this was not a significant finding in the multivariate model. Women did not have higher rates of incident disease or higher titer RPR on average.
The serofast state is a difficult one for clinicians, because it is unknown if it represents persistent organism burden or persistent antibody production despite cure. Many serofast clients in these cohorts were retreated multiple times over a period of months or years despite lack of evidence of reinfection. Much of this treatment may have been unnecessary but given high background rates of disease and reinfection, clinical decision making is complex. Another possible reason for serofast results may have been undiagnosed central nervous system disease (neurosyphilis). Neurological symptoms were infrequently reported on routine interval medical histories, but systematic assessment for neurosyphilis was not done.
Syphilis diagnosis in the field continues to rely on serologic testing. In a country such as Zambia, with a very high rate of syphilis, the use of the treponemal tests in detecting active infection is limited as most people have a lifelong positive Treponema pallidum particle agglutination assay or TPHA following a previously treated infection. Detecting active disease in the setting of recurrent infection using nontreponemal testing (most commonly the RPR) can be limited by false-positive tests at low titers, as seen in many common conditions (including pregnancy, autoimmune disease, and hepatitis) [45].
This study has several limitations, including the retrospective cohort design and the lack of confirmatory treponemal testing for all RPR-positive samples. A majority of the reactive RPR tests were of low titer and many could have been false-positive tests or serofast from previously treated cases. Among patients categorized as prevalent infection, duration of reactive RPR prior to study enrollment and information about prior antibiotics are not known. A good number of those with serofast serologies received multiple doses of penicillin, which did not increase serologic resolution. The limited CD4 data available from 39 individuals indicated that the cohort was not profoundly immunosuppressed. Because study participation was truncated at ART start date, the sickest patients were excluded from our cohort. Study findings may not be generalizable to areas with lower disease prevalence, to HIV-infected persons with lower CD4 counts, or to couples who are not in an HIV-discordant couple relationship.
Syphilis screening is often performed in the antenatal clinic setting, but more can be done to expand testing to male partners and in other clinic settings in order to reduce rates of reinfection Newer syphilis diagnostics with better specificity are needed, as well as further dissemination of currently available point-of-care tests. These could transform the ability to quickly diagnose and treat syphilis globally, even in rural areas with limited healthcare penetration.
Additional studies on the likelihood of treatment response among coinfected patients with low CD4 counts would be useful, as well as further consideration of the role of additional therapy among those who are serologically serofast. Because penicillin therapy is inexpensive, available, and highly effective in both HIV-infected and uninfected populations, additional screening for syphilis in high-risk settings is warranted. Based on our findings, additional doses of penicillin for coinfected patients are not indicated.
Notes
Acknowledgments. The authors thank Mandie Selin and Julie Rufagari for data management, and Dhong-Jin Kim and Ilene Brill for assistance with statistical analyses. Special thanks go to all the couples and staff at the Rwanda Zambia HIV Research Group.
Financial support. This work was supported by the National Institutes of Health (NIH) Fogarty International Center through the International Clinical Research Fellows Program (5R24 TW007988) and the Emory AIDS International Training and Research Program (2D43 TW001042). Support was also provided by the International AIDS Vaccine Initiative, the NIH, National Institute of Mental Health (MH RO1 66767), the National Institutes of Child Health and Human Development (NICHD RO1 40125), and the National Institute of Allergy and Infectious Diseases (Emory CFAR P30 AI050409).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Chen Z-Q, Zhang G-C, Gong X-D, et al. Syphilis in China: results of a national surveillance programme. Lancet. 2007;369:132–8. doi: 10.1016/S0140-6736(07)60074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen C, Winston A, Asboe D, et al. Increasing detection of asymptomatic syphilis in HIV patients. Sex Transm Infect. 2005;81:217–9. doi: 10.1136/sti.2004.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erbelding E, Rompalo A. Changing epidemiology of syphilis and its persistent relationship with HIV. Curr Infect Dis Rep. 2004;6:135–40. doi: 10.1007/s11908-996-0010-z. [DOI] [PubMed] [Google Scholar]
- 4.Heffelfinger JD, Swint EB, Berman SM, Weinstock HS. Trends in primary and secondary syphilis among men who have sex with men in the United States. Am J Public Health. 2007;97:1076–83. doi: 10.2105/AJPH.2005.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds SJ, Risbud AR, Shepherd ME, et al. High rates of syphilis among STI patients are contributing to the spread of HIV-1 in India. Sex Transm Infect. 2006;82:121–6. doi: 10.1136/sti.2005.015040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis. 2007;44:1222–8. doi: 10.1086/513427. [DOI] [PubMed] [Google Scholar]
- 7.United Nations Joint Programme on HIV/AIDS. Country situation report: Zambia. 2009 Available at: http://www.unaids.org/ctrysa/AFRZMB_en.pdf . Accessed June 2012. [Google Scholar]
- 8.Chomba E, Allen S, Kanweka W, et al. Evolution of couples' voluntary counseling and testing for HIV in Lusaka, Zambia. JAIDS. 2008;47:108–15. doi: 10.1097/QAI.0b013e31815b2d67. [DOI] [PubMed] [Google Scholar]
- 9.Dzekedzeke K Zambian Central Statistics Office, Zambian Central Board of Health. Macro O. Zambia Demographic and Health Survey 2001–2002. 2003 Available at: http://www.measuredhs.com/pubs/pdf/FR136/FR136.pdf . Accessed 15 March 2013. [Google Scholar]
- 10.Binagwaho A, Kramer M, Asiimwe A, et al. HIV-AIDS in Rwanda: 2008 epidemic update. 2008 Available at: http://www.healthpolicyinitiative.com/Publications/Documents/385_1_Rwanda_Epi_Update_2008_FINAL.pdf . Accessed 15 March 2013. [Google Scholar]
- 11.Bogaerts J, Vuylsteke B, Martinez TeIlo W, et al. Simple algorithms for the management of genital ulcers: evaluation in a primary health care centre in Kigali, Rwanda. Bull World Health Organ. 1995;73:761–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Rolfs RT, Joesoef MR, Hendershot EF, et al. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group. N Engl J Med. 1997;337:307–14. doi: 10.1056/NEJM199707313370504. [DOI] [PubMed] [Google Scholar]
- 13.Rompalo AM, Joesoef MR, O'Donnell JA, et al. Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis. 2001;28:158–65. doi: 10.1097/00007435-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Rompalo AM, Lawlor J, Seaman P, Quinn TC, Zenilman JM, Hook EW., 3rd Modification of syphilitic genital ulcer manifestations by coexistent HIV infection. Sex Transm Dis. 2001;28:448–54. doi: 10.1097/00007435-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines. 2010 Available at: http://www.cdc.gov/std/treatment/2010/STD-Treatment-2010-RR5912.pdf . Accessed 15 March 2013. [Google Scholar]
- 17.Dowell D, Polgreen PM, Beekmann SE, Workowski KA, Berman SM, Peterman TA. Dilemmas in the management of syphilis: a survey of infectious diseases experts. Clin Infect Dis. 2009;49:1526–9. doi: 10.1086/644737. [DOI] [PubMed] [Google Scholar]
- 18.Stoner BP. Current controversies in the management of adult syphilis. Clin Infect Dis. 2007;44(suppl 3):S130–46. doi: 10.1086/511426. [DOI] [PubMed] [Google Scholar]
- 19.Blank LJ, Rompalo AM, Erbelding EJ, Zenilman JM, Ghanem KG. Treatment of syphilis in HIV-infected subjects: a systematic review of the literature. Sex Transm Infect. 2011;87:9–16. doi: 10.1136/sti.2010.043893. [DOI] [PubMed] [Google Scholar]
- 20.Wall K, Karita E, Nizam A, et al. Influence network agent effectiveness in promoting couples' HIV counseling and testing in Kigali, Rwanda. AIDS. 2012;26:217–27. doi: 10.1097/QAD.0b013e32834dc593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen S, Karita E, Chomba E, et al. Promotion of couples' voluntary counselling and testing for HIV through influential networks in two African capital cities. BMC Public Health. 2007;7:349. doi: 10.1186/1471-2458-7-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenna SL, Muyinda GK, Roth D, et al. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS. 1997;11(suppl 1):S103–10. [PubMed] [Google Scholar]
- 23.Peters P, Karita E, Kayitenkore K, et al. HIV-infected Rwandan women have a high frequency of long-term survival. AIDS. 2007;21(suppl 6):s31–7. doi: 10.1097/01.aids.0000299408.52399.e1. [DOI] [PubMed] [Google Scholar]
- 24.Lambdin BH, Kanweka W, Inambao M, et al. Local residents trained as ‘influence agents’ most effective in persuading African couples on HIV counseling and testing. Health Aff (Millwood) 2011;30:1488–97. doi: 10.1377/hlthaff.2009.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conkling M, Shutes EL, Karita E, et al. Couples' voluntary counselling and testing and nevirapine use in antenatal clinics in two African capitals: a prospective cohort study. J Int AIDS Soc. 2010;13:10. doi: 10.1186/1758-2652-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen S, Meinzen-Derr J, Kautzman M, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17:733–40. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 27.Lifson AR, Allen S, Wolf W, et al. Classification of HIV infection and disease in women from Rwanda. Evaluation of the World Health Organization HIV staging system and recommended modifications. Ann Intern Med. 1995;122:262–70. doi: 10.7326/0003-4819-122-4-199502150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Central Statistics Office of the Zambian Ministry of Health. Sexual behavior survey. 2005. Available at: http://www.cpc.unc.edu/measure/publications/tr-06-33 . Accessed 15 March 2013. [Google Scholar]
- 29.Ministry of Health of Rwanda. Rwanda interim demographic and health survey, 2007–2008. Available at: http://www.measuredhs.com/pubs/pdf/FR215/FR215.pdf . Accessed 15 March 2013. [Google Scholar]
- 30.Modjarrad K, Zulu I, Karita E. Predictors of HIV serostatus among HIV discordant couples in Lusaka, Zambia and female antenatal clinic attendants in Kigali, Rwanda. AIDS Res Hum Retroviruses. 2005;21:5–12. doi: 10.1089/aid.2005.21.5. [DOI] [PubMed] [Google Scholar]
- 31.Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–10. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Au JT, Kayitenkore K, Shutes E, et al. Access to adequate nutrition is a major potential obstacle to antiretroviral adherence among HIV-infected individuals in Rwanda. AIDS. 2006;20:2116–8. doi: 10.1097/01.aids.0000247580.16073.1b. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. 2005. Zambia country profile. Available at: http://www.who.int/hiv/HIVCP_ZMB.pdf . Accessed 15 March 2013. [Google Scholar]
- 34.Office of the President National AIDS Control Commission CNLS. Annual report on implementation of HIV and AIDS activities in Rwanda in 2006, 2007 Available at: http://www.rbc.gov.rw/IMG/pdf/2006_Rwanda_HIV_and_AIDS_Annual_Report.pdf . Accessed 15 March 2013. [Google Scholar]
- 35.Boeras DI, Luisi N, Karita E, et al. Indeterminate and discrepant rapid HIV test results in couples' HIV testing and counselling centres in Africa. J Int AIDS Soc. 2011;14:18. doi: 10.1186/1758-2652-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaida F, Xu R. Proportional hazards model with random effects. Stat Med. 2000;19:3309–24. doi: 10.1002/1097-0258(20001230)19:24<3309::aid-sim825>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Stephenson R, Barker J, Cramer R, et al. The demographic profile of sero-discordant couples enrolled in clinical research in Rwanda and Zambia. AIDS Care. 2008;20:395–405. doi: 10.1080/09540120701593497. [DOI] [PubMed] [Google Scholar]
- 38.Ghanem KG, Workowski KA. Management of adult syphilis. Clin Infect Dis. 2011;53(suppl 3):S110–28. doi: 10.1093/cid/cir701. [DOI] [PubMed] [Google Scholar]
- 39.Knaute DF, Graf N, Lautenschlager S, et al. Serological response to treatment of syphilis according to disease stage and HIV status. Clin Infect Dis. 2012;55:1615–22. doi: 10.1093/cid/cis757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghanem K, Erbelding E, Weiner Z, Rompalo A. Serologic response to syphilis treatment in HIV-positive and HIV-negative patients attending sexually transmitted diseases clinics. Sex Transm Infect. 2007;83:97–101. doi: 10.1136/sti.2006.021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.González-López Julio J, Guerrero Manuel LF, Luján R, Tostado Sagrario F, de Górgolas M, Requena L. Factors determining serologic response to treatment in patients with syphilis. Clin Infect Dis. 2009;49:1505–11. doi: 10.1086/644618. [DOI] [PubMed] [Google Scholar]
- 42.Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Antiretroviral therapy is associated with reduced serologic failure rates for syphilis among HIV-infected patients. Clin Infect Dis. 2008;47:258–65. doi: 10.1086/589295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agmon-Levin N, Elbirt D, Asher I, Gradestein S, Werner B, Sthoeger Z. Syphilis and HIV co-infection in an Israeli HIV clinic: incidence and outcome. Int J STD AIDS. 2010;21:249–52. doi: 10.1258/ijsa.2009.009011. [DOI] [PubMed] [Google Scholar]
- 44.Sena AC, Wolff M, Martin DH, et al. Predictors of serological cure and serofast state after treatment in HIV-negative persons with early syphilis. Clin Infect Dis. 2011;53:1092–9. doi: 10.1093/cid/cir671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh AE, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187–209. doi: 10.1128/cmr.12.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]