The role of vancomycin has been challenged by the availability of alternative antibiotics, increased reports of vancomycin failure, and uncertainties in dosing. This manuscript considers the optimal treatment of methicillin-resistant Staphylococcus aureus infections.
Keywords: Staphylococcus aureus; MRSA, hVISA, VISA; vancomycin; AUC/MIC targets; minimum inhibitory concentration (MIC)
Abstract
For more than 4 decades, vancomycin has been the antibiotic of choice for methicillin-resistant Staphylococcus aureus (MRSA) infections. Recently, infections due to isolates with high but susceptible vancomycin minimum inhibitory concentrations have been associated with additional treatment failures and patient mortality. These poorer outcomes may in part be explained by the inability of attaining appropriate vancomycin levels in these patients. However, assumptions that these poor outcomes are solely due to failure to achieve optimal serum levels of vancomycin are premature. The availability of effective alternatives further erodes the position of vancomycin as first-line therapy. The emergence of resistance and cost considerations, however, favor a more measured approach when using alternative antimicrobials. Collectively, the current available data suggest that the optimal therapy for MRSA infections remains unclear. In the absence of further data, the Infectious Diseases Society of America guidelines remain relevant and inform clinicians of best practice for treating patients with MRSA infections.
For more than 4 decades, vancomycin has been the antibiotic of choice for infections caused by methicillin-resistant Staphylococcus aureus (MRSA). Despite substantial vancomycin usage during this period, the first vancomycin-intermediate S. aureus (VISA) isolate was only recently described in 1996 [1]. Shortly thereafter, the first heteroresistant VISA (hVISA) and vancomycin-resistant S. aureus (VRSA) isolates emerged [2, 3]. Subsequently, increased reports of vancomycin failure and poorer outcomes with these infections started to appear in the literature.
In 2006, partly because of concerns of reduced vancomycin efficacy, the Clinical Laboratory and Standards Institute reduced the vancomycin-susceptible minimum inhibitory concentration (MIC) breakpoint for S. aureus from 4 μg/mL to 2 μg/mL [4]. However, since then, multiple experts have debated whether these breakpoints should be lowered further with increased reports of antibiotic failures in vancomycin-susceptible isolates [5–7].
It was in this context that the Infectious Diseases Society of America (IDSA) published its MRSA treatment guidelines in 2011. Following review of the data at the time, the guidelines recommended vancomycin as first-line therapy for severe MRSA infections and thus effectively endorsed the current breakpoints [8]. In addition, experts in the field advocated more aggressive vancomycin dosing, recommending a target ratio of the area under the serum drug concentration curve to MIC (AUC/MIC) ≥400 [9].
In light of increased reports of vancomycin failures, the purpose of this review is to critically evaluate the evidence for and against substituting vancomycin as first-line therapy for invasive MRSA infections.
THE CASE FOR VANCOMYCIN SUBSTITUTION
Failure With Susceptible High-Vancomycin-MIC Isolates
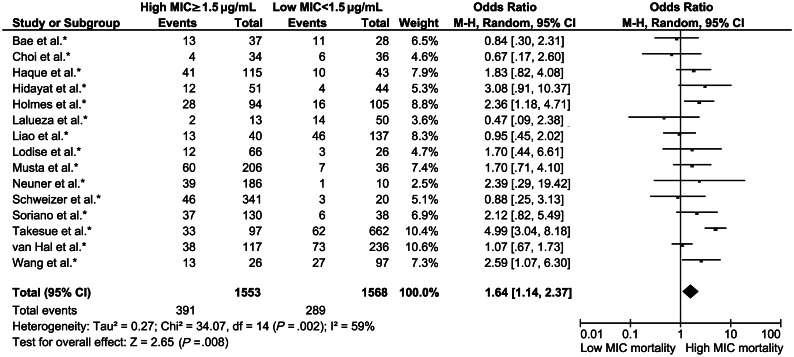
At the time of writing, no fewer than 50 studies, summarized in a meta-analysis [10], have examined the impact of high vancomycin MIC (≥1.5 mg/L) on outcomes of patients with MRSA infections. Although conclusions of the meta-analysis were limited by shortcomings of the original studies, including predominantly retrospective design, varying definitions of treatment failure, and differing MIC testing methodologies, most studies showed an association with high-MIC vancomycin-susceptible S. aureus (VSSA) infections and poorer outcomes. Vancomycin treatment failure (usually defined as persistent bacteremia) occurred more frequently in patients with high-MIC VSSA infections, independent of MIC methodology or site of infection. In contrast, the increased all-cause 30-day mortality associated with high-MIC VSSA infections (Figure 1) was predominantly driven by bloodstream isolates with an MIC of 2 μg/mL (determined by Etest), as no mortality difference was detected between infections caused by isolates with an MIC of 1 μg/mL and 1.5 μg/mL. Subsequent to this meta-analysis, several other groups have examined this association with conflicting results [11–16]. Collectively, current data suggest an association between poorer patient outcomes and infection with high-vancomycin-MIC VSSA. These findings suggest that substituting newer agents for vancomycin in the treatment of such infections could potentially result in better patient outcomes. As described below, however, the biological basis of the association between high-vancomycin-MIC S. aureus infections and worse patient outcome is simply not understood.
Figure 1.
Forest plot of all-cause 30-day mortality for Staphylococcus aureus infections stratified by high (≥1.5 mg/L) and low (<1.5 mg/L) vancomycin minimum inhibitory concentrations. Figure adapted from van Hal et al [10] with permission (Oxford University Press). Readers are encouraged to consult the meta-analysis for specific details of the original reviewed studies. Abbreviations: CI, confidence interval; M-H, Mantel-Haenszel; MIC, minimum inhibitory concentration.
Failure With hVISA Infections
A direct relationship exists between the likelihood of detecting the hVISA phenotype and increasing vancomycin MIC, as most heteroresistant subpopulations are identified in VSSA isolates with an MIC of 2 μg/mL [17]. Detection of hVISA remains problematic. The gold standard, population analysis profiling, is labor intensive and costly. Testing is generally batched, resulting in long turn-around times that prevent its use in real-time patient management [18]. Alternative testing methods are suboptimal, especially when applied to unselected (nonpersistently bacteremic) MRSA isolates (Table 1) [18]. The clinical significance of hVISA is also unresolved. For example, although hVISA infections have been clearly associated with high-inoculum infections, persistent bacteremia, metastatic complications, and vancomycin treatment failure [19], most studies to date have not found an association between hVISA MRSA infections and higher mortality in these patients [19].
Table 1.
Summary of Testing Methodologies for Vancomycin Minimum Inhibitory Concentration and Heteroresistant Vancomycin-Intermediate Staphylococcus aureus Detection
| Method | Characteristics of Various Testing Methodologies |
|---|---|
| Vancomycin MIC testing | |
| Broth microdilution | Considered the reference standard |
| MIC is the lowest antibiotic concentration that completely inhibits visible bacterial growth at 24 h in microtiter plates | |
| Antibiotic concentrations increase by 2-fold dilutions by convention | |
| Labor intensive, requires batching, generally not used for diagnostic purposes | |
| Gradient diffusion (eg, Etest) | Easy to perform, greater precision, measures actual MIC |
| Correlation with BMD moderate with MIC results approximately 1–2 dilutions higher than BMD | |
| Storage affects MIC: an inverse relationship between duration of storage and MIC obtained exists | |
| Automated broth microdilution (eg, Vitek2, Phoenix) | Detection of MIC by microdilution using an automated system |
| Bacterial inoculum and incubation conditions differ between instruments and against gold standard BMD | |
| Correlation with BMD moderate with MIC results generally 1 dilution lower than BMD | |
| Registration is based on overall agreement (ie, ±1 dilution) | |
| Agar disk diffusion | No longer considered appropriate to discriminate susceptible from nonsusceptible strains as large molecules such as vancomycin diffuse too slowly in the agar |
| hVISA testing | |
| Population analysis profiling | Considered the gold standard as initial test or as confirmation |
| Labor intensive, time consuming (3–5 d) and costly | |
| hVISA screening assays | |
| BMD vancomycin MIC | Direct relationship between increasing MIC and presence of hVISA |
| Overall poor sensitivity (hVISA can occur in VSSA isolates with an MIC as low as 0.5 mg/L) but good specificity in selecting isolates which require confirmation | |
| Similar issues as when used for vancomycin MIC testing | |
| Screening agars | Variable sensitivity and specificity dependent on agar, bacterial inoculum, glycopeptide (vancomycin or teicoplanin), and antibiotic concentration used |
| Variability in performance of in-house screening plates compared to commercial agar plates | |
| Easy to perform: growth of ≥2 colonies after 24–48 h indicative of possible hVISA | |
| Macromethod Etest | Increased sensitivity and specificity compared to screening agars |
| Positive results based on MIC values obtained for teicoplanin and/or vancomycin following 48 h incubation | |
| Higher inoculum used than standard testing | |
| Easy to perform and accurate | |
| Delay in results and expensive when used as screening test | |
| Etest glycopeptide resistance detection | Increased sensitivity and specificity compared to screening agars |
| Positive result is based on MIC values obtained for teicoplanin and/or vancomycin following 24–48 h incubation | |
| Uses standard bacterial inoculum | |
| Improvement on Etest as method uses double-ended Etest (less expensive), allows for an initial read at 24 h | |
Abbreviations: BMD, broth microdilution; hVISA, heteroresistant vancomycin-intermediate Staphylococcus aureus; MIC, minimum inhibitory concentration; VSSA, vancomycin-susceptible Staphylococcus aureus.
MIC “Creep”
In any given S. aureus population, the central vancomycin MIC tendency can change over time with increases termed MIC “creep.” This phenomenon may result from multiple factors, including clonal replacement and antibiotic exposure. Although its clinical significance is not established, one possible consequence of MIC “creep” would be increased rates of treatment failures and mortality in vancomycin-treated patients with high-MIC infections (see previous sections). Evidence for the widespread existence of MIC “creep” is conflicting (Table 2). Most positive association studies have arisen from single institutions. In contrast, large multicenter studies have generally not identified such trends [20, 21]. One possible explanation for these conflicting results relates to the MIC methodology employed. Multicenter analyses have generally used broth microdilution, which may fail to detect small changes in vancomycin MIC values, whereas single center studies have typically used Etest assays.
Table 2.
Summary of Studies Examining Vancomycin Minimum Inhibitory Concentration “Creep”
| Study [Reference] | Study Features | MIC “Creep” | Comments |
|---|---|---|---|
| Wang et al, 2006 [58] | Single US center, 6003 isolates from 2000 to 2004 | Yes—proportion of isolates with BMD >1 μg/mL | Proportion increased from 20% to 70% |
| No typing performed | |||
| Robert et al, 2006 [59] | Single French center, 1445 isolates from 1983 to 2001 | Yes—Etest geometric mean | 1.54-fold increase in mean MIC |
| No typing performed | |||
| Jones et al, 2006 [20] | Multicenter worldwide study (SENTRY data), 35 485 isolates from 1998 to 2003 | No—proportion of isolates with BMD >2 μg/mL | No typing performed |
| Steinkraus et al, 2007 [60] | Single US center, 662 isolates from 2001 to 2005 | Yes—Etest geometric mean | 1.5-fold increase in mean MIC |
| No typing performed | |||
| Alos et al, 2008 [61] | Single Spanish center, 3141 isolates from 2002 to 2006 | No—proportion of isolates with BMD >2 μg/mL | No typing performed |
| Holmes et al, 2008 [62] | Single US center, 240 isolates from 1999 to 2006 | No—BMD MIC90 | No typing performed |
| Rybak et al, 2008 [63] | Single US center, 1499 isolates from 1986 to 2007 | Yes—proportion of isolates with BMD >1 μg/mL | Proportion increased from 81% to 93% |
| No typing performed | |||
| Sader et al, 2009 [64] | Multicenter US study, 1800 isolates from 2002 to 2006 | No—BMD modal MIC | 3 sites showed low-level geometric mean creep |
| No typing performed | |||
| Musta et al, 2009 [17] | Single-center US study, 489 isolates from 1996 to 2006 | No—proportion of isolates with Etest >1, 1.5, or 2 μg/mL | No typing performed |
| Ho et al, 2010 [65] | Multicenter Hong Kong study, 247 isolates from 1997 to 2008 | Yes—proportion of isolates with Etest >1 μg/mL | Proportion increased from 11% to 38% |
| Typing suggestive of clonal MIC “creep” | |||
| Adam et al, 2010 [66] | Multicenter Canadian study, 6397 isolates from 1995 to 2006 | No—BMD modal or proportion of isolates >2 μg/mL | No typing performed |
| Pitz et al, 2011 [67] | Single-center US study, 167 isolates from 2000 to 2008 | No—proportion of isolates with Etest >1 μg/mL | No typing performed |
| Gasch et al, 2011 [50] | Single-center Spanish study, 524 isolates from 1990 to 2008 | No—decline in geometric mean MIC by Etest | Typing confirmed clonal replacement with ST125 and ST228 |
| van Hal et al, 2011 [25] | Single-center Australian study, 417 isolates from 1997 to 2008 | No—creep dependent on MIC methodology and measurement used for analysis | Typing performed |
| Edwards et al, 2012 [49] | Multicenter Scottish study, 208 isolates from 2006 to 2010 | No—significant effect on MIC of storage most pronounced for Etest | Typing performed |
| Yeh et al, 2012 [15] | Single-center Taiwanese study, 140 isolates during 2001, 2005, & 2009 | Yes—Etest geometric mean | No typing performed |
Abbreviations: BMD, broth microdilution; MIC, minimum inhibitory concentration.
Potential Pitfalls in Vancomycin Target Attainment
Vancomycin exhibits time-dependent killing [22]. The optimal pharmacodynamic parameter associated with vancomycin efficacy based on animal neutropenic models is thought to be the AUC/MIC ratio. The first study to clinically address this question found that achieving an AUC/MIC ≥350 (MIC determined by broth microdilution) was associated with a 7-fold higher odds of clinical success in patients treated with vancomycin for S. aureus pneumonia. Time to bacterial eradication was significantly shorter (median 10 days) when the AUC/MIC achieved was ≥400 [23]. Based in part on these results, an AUC/MIC ratio ≥400 was identified as the optimal target for clinical effectiveness, and more aggressive vancomycin dosing, aiming for trough concentrations of 15–20 mg/L, was recommended in practice guidelines to achieve this target [9]. This aggressive vancomycin dosing strategy would achieve target concentrations in the majority of complicated infections due to MRSA with lower vancomycin MIC values, but may be unachievable in other clinical settings. Using Monte Carlo simulations, Patel et al [24] demonstrated that the likelihood of achieving target AUC/MIC values was inversely related to creatinine clearance and vancomycin MIC across all dosing schedules. For example, when administering 1500 mg twice daily, target attainment occurred 97% of the time for MRSA infections with low vancomycin MIC (≤0.5 mg/L), but declined to 38% for infections with a vancomycin MIC of 2 mg/L [24]. Similarly, target attainment decreased with increasing renal function. As a result, vancomycin target attainment is least likely to be obtained in the very patient populations (eg, critically ill subjects with supranormal clearance) in which appropriate drug exposure is essential.
Impact of MIC Methodology on Achieving AUC/MIC
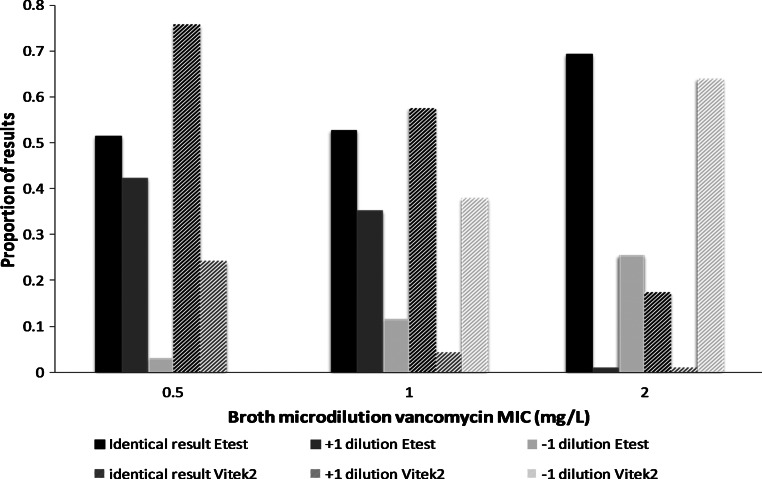
The vancomycin MIC represents the denominator of the AUC/MIC target attainment equation and is influenced by the choice of MIC methodology (Table 1). For example, Etest generally yields MIC results that are between 1 and 2 dilutions higher than broth microdilution, whereas some automated assays generate values that are between 1 and 2 dilutions lower than broth microdilution (Figure 2) [25]. The net effect is that the likelihood of AUC/MIC target attainment is influenced by MIC methodology [26]. Although appropriate adjustments could be made to MIC values to reflect broth microdilution values, these adjustments remain unpredictable and uncertain. Furthermore, controversy remains about which MIC methodology is the most relevant measure; available data suggest that the Etest may be, as its results best correlate with outcome [10, 27].
Figure 2.
Performance of Etest and Vitek2 compared to broth microdilution for vancomycin minimum inhibitory concentration (MIC). Data adapted from van Hal et al [25] with permission (Oxford University Press). Test results are interpreted compared to broth microdilution as the gold standard. Of note, although the correlation between MIC results differs significantly between methodologies, both these assays perform adequately with respect to regulatory agencies as the overall agreement is >95% (ie, an accepted variance of ±1 dilution). Abbreviation: MIC, minimum inhibitory concentration.
Vancomycin-Associated Toxicity
Dosing vancomycin to achieve a trough of 15–20 mg/L is associated with an increased risk for nephrotoxicity [24, 28, 29]. Uncertainty remains, however, as to whether vancomycin actually causes this nephrotoxicity or simply reflects the fact that patients with MRSA infections are also at risk for acquired renal dysfunction. This is especially true in critically ill patients, as they have multiple other risk factors for renal insufficiency. Even after correcting for these confounders, however, vancomycin troughs >15 mg/L remain an independent predictor of nephrotoxicity [30].
Alternative Antibiotics
The role of alternative MRSA antimicrobials was reviewed in the IDSA treatment guidelines [8]. Most alternative anti-MRSA drugs are licensed for pneumonia and soft tissue infections. One exception is daptomycin, which is approved by the US Food and Drug Administration (FDA) for S. aureus bacteremia and right-sided endocarditis. Although the registrational study was not designed to answer a superiority endpoint, it performed as well as standard therapy even in methicillin-susceptible Staphylococcus aureus (MSSA) bacteremic infections [31]. In contrast, vancomycin has never been able to show equivalence to β-lactam therapy in the treatment of MSSA. On the contrary, vancomycin is considered inferior to β-lactams in MSSA [32, 33]. Furthermore, in a recent retrospective case-control study, an overall survival benefit was observed with daptomycin salvage compared to vancomycin continuation when treating high-MIC VSSA isolates [34]. Importantly, this study was limited by a significant selection/indication bias, as 98% of daptomycin patients were switched from an initial vancomycin regimen. Thus, these conclusions require confirmation.
Since publication of the IDSA guidelines, additional data have emerged to challenge the continuing preeminence of vancomycin. First, in a recent prospective double-blind multicenter trial (Zephyr), linezolid was found to be superior to vancomycin for the treatment of MRSA nosocomial pneumonia with respect to the clinical and microbiological responses at the end of therapy [35]. Toxicity, including hematological adverse events, was similar between the 2 treatment groups. Despite the multiple concerns raised following the study publication regarding study design, diagnosis, subjective endpoints, suboptimal vancomycin dosing, and lack of a mortality benefit [36, 37], the Zephyr study succeeded in demonstrating higher clinical success rates in patients with MRSA pneumonia who received linezolid as compared to vancomycin. For this reason, the study represents a landmark clinical trial in the area of MRSA infections. Nevertheless, controversy still remains with respect to the optimal empiric therapy for pneumonia and how best to apply these results in definitive MRSA pneumonia treatment decisions.
Ceftaroline received FDA registration for acute bacterial skin and soft tissue infection and community-acquired pneumonia and thus currently would not be considered as a first line-treatment option for other severe MRSA infections. However, several small case series suggest that it may have utility in these clinical settings [38, 39]. For example, ceftaroline was an effective salvage therapy in 6 patients with endocarditis and/or persistent MRSA bacteremia. All 6 patients failed vancomycin and/or daptomycin but rapidly cleared their bloodstream following ceftaroline substitution [38]. This promising observation should be verified with appropriately designed clinical studies before ceftaroline can be recommended for widespread use in such off-label settings. Collectively, these data and the availability of effective alternative agents further erode the position of vancomycin as the first-line agent in MRSA treatment.
THE CASE AGAINST VANCOMYCIN SUBSTITUTION
Do We Understand the Natural History of S. aureus Bacteremia?
In S. aureus, persistent bacteremia is often used as a surrogate for antibiotic failure. However, when daily blood cultures are performed, the median time to clearance of MRSA bacteremia is 7–9 days [31, 40]. For example, in the daptomycin-complicated S. aureus bacteremia trial, the duration of MRSA bacteremia for vancomycin-treated and daptomycin-treated patients was similar (9 vs 8 days). Thus, persistent bacteremia may sometimes not represent antibiotic failure at all, but rather reflect the natural course of MRSA bacteremia. Alternatively, persistent bacteremia often reflects a residual source of infection (eg, line-related infections vs infective endocarditis) and lack of source control. It is these factors that are likely to have the largest impact on patient outcomes [41]. A recent study supports this assertion, as the source of MRSA bacteremia and not vancomycin MIC determined antimicrobial failures [42]. Thus in settings where the source is effectively addressed (eg, line removal), vancomycin may remain an effective therapy irrespective of the isolate's vancomycin MIC [8].
High Vancomycin MIC: Altered Virulence and Not Antibiotic Failure
Conventionally, the medical community has assumed a causal relationship between high susceptible vancomycin MIC values (>1 µg/mL) and an increased mortality risk. Based on this reasoning, substituting vancomycin with alternative agents in the treatment of these high vancomycin MICs should lead to better patient outcomes. However, recent data challenge this interpretation. In the clinic, infections caused by high vancomycin MIC S. aureus strains are less likely to be associated with shock and proinflammatory responses, suggesting pathogen alterations [43]. However, the most convincing evidence to date that outcomes with high-MIC VSSA infections are not due to antibiotic failure per se was demonstrated in a multicenter observational cohort study of 532 S. aureus bacteremic patients [44]. As previously observed, the authors found that vancomycin MIC was associated with mortality in vancomycin-treated MSSA and MRSA infections. However, the same association was also present among patients who never received vancomycin, as increased mortality was also detected in high-vancomycin-MIC MSSA episodes treated exclusively with flucloxacillin. These data imply that pathogenic factors rather than antibiotic choice in S. aureus (and especially MRSA) bacteremia is the primary determinant of patient outcome.
Further Evidence of Altered Pathogen Virulence in hVISA Infections
Heteroresistant VISA infections have not been associated with increased overall 30-day all-cause mortality despite the majority of isolates having a high vancomycin MIC [19]. On the contrary, one study has reported a reduced mortality with hVISA compared to VSSA ST239 MRSA bacteremia [45]. Further evidence of attenuated virulence with hVISA is seen in animal infection models [46], where hVISA isolates were significantly less likely to kill infected hosts than isogenic VSSA strains [47]. Similarly, altered pathogen virulence is implied in a clinical cohort study, as hVISA was significantly more likely to be a colonizer and cause fewer infections than VSSA [48].
Vancomycin MIC “Creep”
Recent data revisit the concept of MIC “creep.” A number of external factors including analysis method [25] , storage duration of isolates prior to testing, and the MIC testing method employed [49], all impact on finding evidence for MIC “creep.” In addition, bacterial genotyping data are generally absent from MIC “creep” studies (Table 2), limiting study generalizability. This is highlighted by a recent publication that documented MIC “de-creep” secondary to clonal replacement of the predominant multiresistant hospital MRSA clone with community MRSA clones [50]. These data suggest that published MIC trends do not support substitution of vancomycin as first-line therapy in MRSA infections.
Vancomycin Target Attainment
To date, 6 retrospective clinical studies have examined potential association between optimized vancomycin AUC/MIC ratios and patient outcomes in MRSA infections, with divergent conclusions and targets (Table 3). For this reason, arguments for vancomycin substitution based on the clinical importance of vancomycin AUC/MIC ratio ≥400 attainment seem premature, as the evidence supporting this target ratio is still primarily hypothesis-generating. By contrast, there is significant evidence to suggest that the higher vancomycin doses needed to achieve the recommended therapeutic ratio may carry a higher risk of nephrotoxicity. These circumstances create the clinical “equipoise” needed to conduct a definitive clinical trial to test the risks and benefits of optimized vancomycin dosing versus more conventional dosing strategies for invasive MRSA. If the treatment effect of optimized vancomycin AUC/MIC dosing on MRSA-infected patients is indeed as large as the retrospective studies to date suggest, only modest sample sizes will be required. By contrast, if no difference in clinical outcome between optimized vancomycin dosing and standard vancomycin dosing is shown in MRSA-infected patients, this finding would also improve clinical care by providing evidence to discontinue the potentially nephrotoxic dosing practices required for optimized AUC/MIC dosing strategies.
Table 3.
Summary of Studies Comparing Area Under the Curve to Minimum Inhibitory Concentration Ratio to Clinical Outcomes in Patients With Staphylococcus aureus Infection
| Study [Reference] | Study Characteristics | MIC Method | Outcome/Comments |
|---|---|---|---|
| Moise-Broder et al [23] | Retrospective study of MRSA/MSSA hospital-acquired pneumonia (n = 50) | BMD | AUC/MIC ≥350 associated with clinical success (OR, 7.2; 95% CI, 1.9–27) |
| Jeffres et al [68] | Retrospective study of MRSA healthcare-associated pneumonia | Inferreda | None of the AUC strata (<200; 201–300; 301–400; >400) were associated with better outcomes |
| Kullar et al [69] | Retrospective study of MRSA bacteremia (n = 320) | Etest | AUC/MIC <421 was associated with increased failures (composite endpoint of 30-d mortality, persistent bacteremia, and ongoing symptoms) |
| Brown et al [70] | Retrospective study of complicated MRSA bacteremia and IE (n = 50) | Etest | AUC/MIC <211 was associated with attributable mortality (OR, 10.4; 95% CI, 3.9–16.8) |
| Neuner et al [71] | Retrospective study of MRSA bacteremia (n = 222) | Etest | AUC/MIC did not correlate with the presence of persistent bacteremia |
| Holmes et al [26] | Retrospective cohort study of patients with MRSA bacteremia | BMD | AUC/MIC> 373 was associated with reduced mortality (OR, 0.44; 95% CI, .2–.99) |
Abbreviations: AUC/MIC, area under the serum drug concentration curve to minimum inhibitory concentration ratio; BMD, broth microdilution; CI, confidence interval; IE, infective endocarditis; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; OR, odds ratio.
a Vancomycin MIC was inferred from the results obtained by disk diffusion, which is no longer considered an accepted susceptibility method (see Table 2).
Alternative Antibiotics
Resistance
Resistance in MRSA to currently available vancomycin-alternative antibiotics is of great concern. Unlike vancomycin, where resistance emerged only after 40 years of widespread use, resistance to both daptomycin and linezolid occurred soon after introduction [51]. A link between linezolid usage and resistance has been observed in outbreak settings with linezolid restriction required in addition to other measure to terminate the outbreak [52]. Of greater concern is the appearance of a novel linezolid resistance (LRSA) mechanism carried on a mobile genetic element, which in turn is able to disseminate rapidly between multiple clonal lineages [53]. For example, in the well-publicized outbreak of cfr-constitutive LRSA in Madrid, 6 isolates from 4 genetic lineages were described in a single intensive care unit over 2 months [52].
The primary concern when using daptomycin is the development of treatment-emergent resistance. This concern is highest when daptomycin used as salvage therapy following vancomycin failure or when treating hVISA/VISA [31, 54, 55]. For example, one report from St John's Hospital in Detroit reported emergence of daptomycin-resistant S. aureus isolates in 60% of daptomycin-treated patients for persistent S. aureus bacteremia refractory to vancomycin [56]. Other studies, however, have reported far more encouraging experience with treatment-emergent resistance. For example, a recent retrospective case-control study of daptomycin salvage therapy for vancomycin failure reported no emergence of resistance [34].
Thus the overall impact of vancomycin substitution could be a growing burden of resistance, which in turn would reduce the utility of alternative agents. We believe that the focused use of these agents would extend the lifespan of both these important agents and vancomycin.
Cost
In healthcare systems under increasing fiscal constraints, antimicrobial acquisition costs form part of the argument against substituting vancomycin as primary therapy. This argument is substantially reduced if patient outcomes are improved by alternative therapy or if overall patient costs decline secondary to reduced lengths of stay. Recent data suggest that this may be the case [57]. However, the observed differences between the historical vancomycin treatment arm and more recent daptomycin-treated episodes may simply reflect improved patient management. Until these data are confirmed, cost remains an important consideration and thus vancomycin remains an attractive option.
Conclusions and the Current Role of the IDSA Guidelines
Collectively, the balance of the data summarized above supports the position that vancomycin remain the standard of care for most infections caused by MRSA. However, the optimal dosing targets for vancomycin remain elusive. Thus, pending more definitive data, we believe that the IDSA guidelines remain the preferred approach when selecting an antibiotic for MRSA treatment [8]. This includes continued vancomycin use, albeit cautiously, in many MRSA infections with vancomycin MIC of ≤2 µg/mL. We agree with the IDSA treatment guidelines to base the decision to switch from vancomycin to an alternative antibiotic on the clinical circumstances and treatment response of the particular patient in question rather than upon a specific MIC breakpoint, as long as the vancomycin MIC of the organism does not exceed 2 µg/mL.
In conclusion, it remains unclear whether reported treatment failures with vancomycin are secondary to problems with definitions of failure, pathogen alterations, source of infection, or antibiotic failure per se. Before we relegate vancomycin to second-line status, we need to carefully consider how best to extend the utility of this inexpensive antibiotic that has been the mainstay of anti-MRSA treatment for almost half a century.
Notes
Financial support. This work was supported by the National Institutes of Health (K24 AI093969 and R01 AI068804 to V. G. F.).
Potential conflicts of interest. S. J. vH. has received grant support from Novartis, Pfizer, Merck, and Gilead. V. G. F. was chair of the Merck V710 Scientific Advisory Committee; has received grant support from Merck, Cerexa, Pfizer, Novartis, Advanced Liquid Logics, MedImmune, and the National Institutes of Health; is a paid consultant for Merck, Astellas, Cubist, Cerexa, Durata, Pfizer, NovaDigm, Novartis, The Medicines Co, Biosynexus, MedImmune, Galderma, and Affinium; and has received honoraria from Merck, Astellas, Cubist, Pfizer, Theravance, and Novartis.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–6. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 2.Hiramatsu K, Aritaka N, Hanaki H, et al. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–3. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 3.Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:565–7. [PubMed] [Google Scholar]
- 4.Tenover FC, Moellering RC., Jr The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007;44:1208–15. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 5.Deresinski S. Counterpoint: Vancomycin and Staphylococcus aureus—an antibiotic enters obsolescence. Clin Infect Dis. 2007;44:1543–8. doi: 10.1086/518452. [DOI] [PubMed] [Google Scholar]
- 6.Mohr JF, Murray BE. Point: Vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2007;44:1536–42. doi: 10.1086/518451. [DOI] [PubMed] [Google Scholar]
- 7.Sakoulas G, Moellering RC., Jr Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis. 2008;46(suppl 5):S360–7. doi: 10.1086/533592. [DOI] [PubMed] [Google Scholar]
- 8.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 9.Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49:325–7. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 10.van Hal SJ, Lodise TP, Paterson DL. The clinical significance of vancomycin minimum inhibitory concentration in Staphylococcus aureus infections: a systematic review and meta-analysis. Clin Infect Dis. 2012;54:755–71. doi: 10.1093/cid/cir935. [DOI] [PubMed] [Google Scholar]
- 11.Chen SY, Liao CH, Wang JL, et al. Methicillin-resistant Staphylococcus aureus (MRSA) staphylococcal cassette chromosome mec genotype effects outcomes of patients with healthcare-associated MRSA bacteremia independently of vancomycin minimum inhibitory concentration. Clin Infect Dis. 2012;55:1329–37. doi: 10.1093/cid/cis717. [DOI] [PubMed] [Google Scholar]
- 12.Rojas L, Bunsow E, Munoz P, Cercenado E, Rodriguez-Creixems M, Bouza E. Vancomycin MICs do not predict the outcome of methicillin-resistant Staphylococcus aureus bloodstream infections in correctly treated patients. J Antimicrob Chemother. 2012;67:1760–8. doi: 10.1093/jac/dks128. [DOI] [PubMed] [Google Scholar]
- 13.Wi YM, Kim JM, Joo EJ, et al. High vancomycin minimum inhibitory concentration is a predictor of mortality in meticillin-resistant Staphylococcus aureus bacteraemia. Int J Antimicrob Agents. 2012;40:108–13. doi: 10.1016/j.ijantimicag.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Han JH, Mascitti KB, Edelstein PH, Bilker WB, Lautenbach E. Effect of reduced vancomycin susceptibility on clinical and economic outcomes in Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2012;56:5164–70. doi: 10.1128/AAC.00757-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh YC, Yeh KM, Lin TY, et al. Impact of vancomycin MIC creep on patients with methicillin-resistant Staphylococcus aureus bacteremia. J Microbiol Immunol Infect. 2012;45:214–20. doi: 10.1016/j.jmii.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Mahajan SN, Shah JN, Hachem R, et al. Characteristics and outcomes of methicillin-resistant Staphylococcus aureus bloodstream infections in patients with cancer treated with vancomycin: 9-year experience at a comprehensive cancer center. Oncologist. 2012;17:1329–36. doi: 10.1634/theoncologist.2012-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Musta AC, Riederer K, Shemes S, et al. Vancomycin MIC plus heteroresistance and outcome of methicillin-resistant Staphylococcus aureus bacteremia: trends over 11 years. J Clin Microbiol. 2009;47:1640–4. doi: 10.1128/JCM.02135-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Hal SJ, Wehrhahn MC, Barbagiannakos T, et al. Performance of various testing methodologies for detection of heteroresistant vancomycin-intermediate Staphylococcus aureus in bloodstream isolates. J Clin Microbiol. 2011;49:1489–94. doi: 10.1128/JCM.02302-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Hal SJ, Paterson DL. Systematic review and meta-analysis of the significance of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:405–10. doi: 10.1128/AAC.01133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones RN. Microbiological features of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin Infect Dis. 2006;42(suppl 1):S13–24. doi: 10.1086/491710. [DOI] [PubMed] [Google Scholar]
- 21.Adam HJ, DeCorby M, Rennie R, Karlowsky JA, Hoban DJ, Zhanel GG. Prevalence of antimicrobial resistant pathogens from blood cultures from Canadian hospitals: results of the CANWARD 2007-2009 study. Diagn Microbiol Infect Dis. 2011;69:307–13. doi: 10.1016/j.diagmicrobio.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Drusano GL. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug. Nat Rev Microbiol. 2004;2:289–300. doi: 10.1038/nrmicro862. [DOI] [PubMed] [Google Scholar]
- 23.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–42. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 24.Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. Vancomycin: we can't get there from here. Clin Infect Dis. 2011;52:969–74. doi: 10.1093/cid/cir078. [DOI] [PubMed] [Google Scholar]
- 25.van Hal SJ, Barbagiannakos T, Jones M, et al. Methicillin-resistant Staphylococcus aureus vancomycin susceptibility testing: methodology correlations, temporal trends and clonal patterns. J Antimicrob Chemother. 2011;66:2284–7. doi: 10.1093/jac/dkr280. [DOI] [PubMed] [Google Scholar]
- 26.Holmes NE, Turnidge JD, Munckhof WJ, et al. Vancomycin AUC/MIC and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2013;57:1654–63. doi: 10.1128/AAC.01485-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bland CM, Porr WH, Davis KA, Mansell KB. Vancomycin MIC susceptibility testing of methicillin-susceptible and methicillin-resistant Staphylococcus aureus isolates: a comparison between Etest(R) and an automated testing method. South Med J. 2010;103:1124–8. doi: 10.1097/SMJ.0b013e3181efb5b1. [DOI] [PubMed] [Google Scholar]
- 28.Wong-Beringer A, Joo J, Tse E, Beringer P. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. Int J Antimicrob Agents. 2011;37:95–101. doi: 10.1016/j.ijantimicag.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 29.van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734–44. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49:507–14. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 31.Fowler VG, Jr., Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–65. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Kim KH, Kim HB, et al. Outcome of vancomycin treatment in patients with methicillin-susceptible Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2008;52:192–7. doi: 10.1128/AAC.00700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stryjewski ME, Szczech LA, Benjamin DK, Jr, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007;44:190–6. doi: 10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 34.Moore CL, Osaki-Kiyan P, Haque NZ, Perri MB, Donabedian S, Zervos MJ. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin Infect Dis. 2012;54:51–8. doi: 10.1093/cid/cir764. [DOI] [PubMed] [Google Scholar]
- 35.Wunderink RG, Niederman MS, Kollef MH, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis. 2012;54:621–9. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 36.Wolff M, Mourvillier B. Linezolid for the treatment of nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2012;55:160–1. doi: 10.1093/cid/cis330. [DOI] [PubMed] [Google Scholar]
- 37.Lahey T. Questionable superiority of linezolid for methicillin-resistant Staphylococcus aureus nosocomial pneumonia: watch where you step. Clin Infect Dis. 2012;55:159–60. doi: 10.1093/cid/cis329. [DOI] [PubMed] [Google Scholar]
- 38.Ho TT, Cadena J, Childs LM, Gonzalez-Velez M, Lewis JS., 2nd Methicillin-resistant Staphylococcus aureus bacteraemia and endocarditis treated with ceftaroline salvage therapy. J Antimicrob Chemother. 2012;67:1267–70. doi: 10.1093/jac/dks006. [DOI] [PubMed] [Google Scholar]
- 39.Lin JC, Aung G, Thomas A, Jahng M, Johns S, Fierer J. The use of ceftaroline fosamil in methicillin-resistant Staphylococcus aureus endocarditis and deep-seated MRSA infections: a retrospective case series of 10 patients. J Infect Chemother. 2013;19:42–9. doi: 10.1007/s10156-012-0449-9. [DOI] [PubMed] [Google Scholar]
- 40.Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115:674–80. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 41.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev. 2012;25:362–86. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walraven CJ, North MS, Marr-Lyon L, Deming P, Sakoulas G, Mercier RC. Site of infection rather than vancomycin MIC predicts vancomycin treatment failure in methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2011;66:2386–92. doi: 10.1093/jac/dkr301. [DOI] [PubMed] [Google Scholar]
- 43.Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 44.Holmes NE, Turnidge JD, Munckhof WJ, et al. Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis. 2011;204:340–7. doi: 10.1093/infdis/jir270. [DOI] [PubMed] [Google Scholar]
- 45.van Hal SJ, Jones M, Gosbell IB, Paterson DL. Vancomycin heteroresistance is associated with reduced mortality in ST239 methicillin-resistant Staphylococcus aureus blood stream infections. PLoS One. 2011;6:e21217. doi: 10.1371/journal.pone.0021217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cameron DR, Howden BP, Peleg AY. The interface between antibiotic resistance and virulence in Staphylococcus aureus and its impact upon clinical outcomes. Clin Infect Dis. 2011;53:576–82. doi: 10.1093/cid/cir473. [DOI] [PubMed] [Google Scholar]
- 47.Cameron DR, Ward DV, Kostoulias X, et al. Serine/threonine phosphatase Stp1 contributes to reduced susceptibility to vancomycin and virulence in Staphylococcus aureus. J Infect Dis. 2012;205:1677–87. doi: 10.1093/infdis/jis252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horne KC, Howden BP, Grabsch EA, et al. Prospective comparison of the clinical impacts of heterogeneous vancomycin-intermediate methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-susceptible MRSA. Antimicrob Agents Chemother. 2009;53:3447–52. doi: 10.1128/AAC.01365-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edwards B, Milne K, Lawes T, Cook I, Robb A, Gould IM. Is vancomycin MIC “creep” method dependent? Analysis of methicillin-resistant Staphylococcus aureus susceptibility trends in blood isolates from north east Scotland from 2006 to 2010. J Clin Microbiol. 2012;50:318–25. doi: 10.1128/JCM.05520-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gasch O, Ayats J, Angeles Dominguez M, et al. Epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infection: secular trends over 19 years at a university hospital. Medicine (Baltimore) 2011;90:319–27. doi: 10.1097/MD.0b013e31822f0b54. [DOI] [PubMed] [Google Scholar]
- 51.Talbot GH, Bradley J, Edwards JE, Jr., Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–68. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez Garcia M, De la Torre MA, Morales G, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303:2260–4. doi: 10.1001/jama.2010.757. [DOI] [PubMed] [Google Scholar]
- 53.Gu B, Kelesidis T, Tsiodras S, Hindler J, Humphries RM. The emerging problem of linezolid-resistant Staphylococcus. J Antimicrob Chemother. 2013;68:4–11. doi: 10.1093/jac/dks354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moise PA, North D, Steenbergen JN, Sakoulas G. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions. Lancet Infect Dis. 2009;9:617–24. doi: 10.1016/S1473-3099(09)70200-2. [DOI] [PubMed] [Google Scholar]
- 55.van Hal SJ, Paterson DL, Gosbell IB. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteraemia in a daptomycin-naive patient: a review of the literature. Eur J Clin Microbiol Infect Dis. 2011;30:603–10. doi: 10.1007/s10096-010-1128-3. [DOI] [PubMed] [Google Scholar]
- 56.Sharma M, Riederer K, Chase P, Khatib R. High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis. 2008;27:433–7. doi: 10.1007/s10096-007-0455-5. [DOI] [PubMed] [Google Scholar]
- 57.Kullar R, Davis SL, Kaye KS, Levine DP, Pogue JM, Rybak MJ. Implementation of an antimicrobial stewardship pathway with daptomycin for optimal treatment of methicillin-resistant Staphylococcus aureus bacteremia. Pharmacotherapy. 2013;33:3–10. doi: 10.1002/phar.1220. [DOI] [PubMed] [Google Scholar]
- 58.Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44:3883–6. doi: 10.1128/JCM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robert J, Bismuth R, Jarlier V. Decreased susceptibility to glycopeptides in methicillin-resistant Staphylococcus aureus: a 20 year study in a large French teaching hospital, 1983-2002. J Antimicrob Chemother. 2006;57:506–10. doi: 10.1093/jac/dki486. [DOI] [PubMed] [Google Scholar]
- 60.Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001-05. J Antimicrob Chemother. 2007;60:788–94. doi: 10.1093/jac/dkm258. [DOI] [PubMed] [Google Scholar]
- 61.Alos JI, Garcia-Canas A, Garcia-Hierro P, Rodriguez-Salvanes F. Vancomycin MICs did not creep in Staphylococcus aureus isolates from 2002 to 2006 in a setting with low vancomycin usage. J Antimicrob Chemother. 2008;62:773–5. doi: 10.1093/jac/dkn246. [DOI] [PubMed] [Google Scholar]
- 62.Holmes RL, Jorgensen JH. Inhibitory activities of 11 antimicrobial agents and bactericidal activities of vancomycin and daptomycin against invasive methicillin-resistant Staphylococcus aureus isolates obtained from 1999 through 2006. Antimicrob Agents Chemother. 2008;52:757–60. doi: 10.1128/AAC.00945-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rybak MJ, Leonard SN, Rossi KL, Cheung CM, Sader HS, Jones RN. Characterization of vancomycin-heteroresistant Staphylococcus aureus from the metropolitan area of Detroit, Michigan, over a 22-year period (1986 to 2007) J Clin Microbiol. 2008;46:2950–4. doi: 10.1128/JCM.00582-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sader HS, Fey PD, Limaye AP, et al. Evaluation of vancomycin and daptomycin potency trends (MIC creep) against methicillin-resistant Staphylococcus aureus isolates collected in nine U.S. medical centers from 2002 to 2006. Antimicrob Agents Chemother. 2009;53:4127–32. doi: 10.1128/AAC.00616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ho PL, Lo PY, Chow KH, et al. Vancomycin MIC creep in MRSA isolates from 1997 to 2008 in a healthcare region in Hong Kong. J Infect. 2010;60:140–5. doi: 10.1016/j.jinf.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 66.Adam HJ, Louie L, Watt C, et al. Detection and characterization of heterogeneous vancomycin-intermediate Staphylococcus aureus isolates in Canada: results from the Canadian Nosocomial Infection Surveillance Program, 1995-2006. Antimicrob Agents Chemother. 2010;54:945–9. doi: 10.1128/AAC.01316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pitz AM, Yu F, Hermsen ED, Rupp ME, Fey PD, Olsen KM. Vancomycin susceptibility trends and prevalence of heterogeneous vancomycin-intermediate Staphylococcus aureus in clinical methicillin-resistant S. aureus isolates. J Clin Microbiol. 2011;49:269–74. doi: 10.1128/JCM.00914-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jeffres MN, Isakow W, Doherty JA, et al. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest. 2006;130:947–55. doi: 10.1378/chest.130.4.947. [DOI] [PubMed] [Google Scholar]
- 69.Kullar R, Davis SL, Levine DP, Rybak MJ. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52:975–81. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 70.Brown J, Brown K, Forrest A. Vancomycin AUC24/MIC ratio in patients with complicated bacteremia and infective endocarditis due to methicillin-resistant Staphylococcus aureus and its association with attributable mortality during hospitalization. Antimicrob Agents Chemother. 2012;56:634–8. doi: 10.1128/AAC.05609-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neuner EA, Casabar E, Reichley R, McKinnon PS. Clinical, microbiologic, and genetic determinants of persistent methicillin-resistant Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis. 2010;67:228–33. doi: 10.1016/j.diagmicrobio.2010.02.026. [DOI] [PubMed] [Google Scholar]