Abstract
Background:
Sex steroid hormone fluctuations during the menstrual cycle are considered a risk factor for noncontact anterior cruciate ligament injuries.
Objective:
To determine whether self-reported menstrual history data can be used to accurately categorize menstrual cycle events using calendar-based counting methods.
Study Design:
Descriptive laboratory study.
Methods:
Seventy-three women completed a menstrual history questionnaire and submitted to blood sampling for the first 6 days of menses and 8 to 10 days after a positive ovulation test over 2 consecutive months. Frequency counts determined whether appropriate criterion hormone (progesterone) levels were achieved at predefined calendar days.
Results:
For the criterion of progesterone >2 ng/mL, 18% and 59% of women attained it when counting forward 10 to 14 days after the onset of menses and counting back 12 to 14 days from the end of the cycle, respectively. Most women (76%) attained the criterion for ovulation 1 to 3 days after a positive urinary ovulation test. Regardless of the counting method employed, the criterion of progesterone >4.5 ng/mL for identifying midluteal phase was attained in 67% of cases. Serial blood sampling for 3 to 5 days after the positive urinary ovulation test captured 68% to 81% of the hormone values indicative of ovulation and 58% to 75% indicative of the luteal phase.
Conclusion:
These data suggest that self-reported menstrual history and calendar-based counting methods should not be used alone if accurate identification of ovulation is essential. A urinary ovulation test and serial blood samples for verification of progesterone postovulation enhance the proper identification of menstrual cycle events.
Clinical Relevance:
Given the cost of serial blood sampling on numerous days, the use of urinary ovulation kits and strategically selected serial blood sampling could significantly reduce participant burden and provide cost-effective measures for clinical studies related to anterior cruciate ligament injury epidemiology.
Keywords: progesterone, self-reported menstrual history, positive urinary ovulation test
Sex steroid hormone fluctuations during the menstrual cycle are considered risk factors for noncontact anterior cruciate ligament (ACL) injuries.13,25 Self-reported menstrual cycle history has been used extensively, often as the sole method of determining information about menstrual cycle characteristics and menstrual cycle phase. However, information obtained from self-reported menstrual cycle questionnaires does not reveal the length of specific menstrual cycle phases or the timing of ovulation, nor does it distinguish ovulatory from anovulatory cycles.20,36 It also does not provide information about subtle menstrual cycle disturbances, such as luteal phase defects, since normal menstruation still occurs.36 Thus, as noted in a recent review about noncontact ACL injury risk and the menstrual cycle,36 self-reported menstrual history is not sufficiently accurate for verifying current cycle length,6,33 and methods are needed to prospectively evaluate and accurately characterize the menstrual cycle to link hormonal fluctuations resulting from the menstrual cycle and ACL injury risk.36
In the fertility literature, the preferred method for capturing phase of the menstrual cycle is frequent or daily measures of sex hormone levels combined with vaginal ultrasound for follicular development.9,34 However, these studies are labor intensive and expensive, and they cause significant participant burden. Many methodologies have been employed in menstrual cycle–related research to circumvent these problems, but the best way to determine menstrual cycle phase with minimal expense and subject burden remains unknown. Identifying a cost-effective, accurate method for identifying menstrual cycle phase is thus imperative for research in the area of ACL injury epidemiology.36
Calendar-based counting methods used to assign menstrual cycle phase vary greatly, as do methods for defining cycle phase. Self-reported onset of menses is commonly used as a starting point, and cycle phase is determined by counting a given number of days forward4,5,11,12,15,17,19,23 or counting back from the self-reported predicted start date of the next cycle.10 Based on a normal 28-day cycle, counting forward 10 to 14 days from the start of the cycle4,5,15,19 and counting back 12 to 14 days from the end of the cycle or the anticipated start of the next cycle10 is often used to represent ovulatory events (near peak estradiol levels, prior to a significant progesterone rise). To capture midluteal hormone levels, days 17 to 21 are often used (by counting forward 7 additional days from days 10 to 145,19 or counting back 7 to 9 days from the end of the cycle10). But while ovulatory events on average occur between days 10 and 14, the actual timing of ovulation can vary significantly from this window.27,32 Thus, many studies also use a positive urinary ovulation test that detects the luteinizing hormone surge as the alignment point, counting forward a given number of days to represent “ovulation” or different phases, such as midluteal events.3,12,14,23,24,35 However, the extent to which these methods actually capture ovulatory and midluteal events is unknown. This information is critical if we are to appropriately interpret findings using calendar-based counting methods.
While studies have tested the accuracy and validity of self-reported menstrual cycle length by comparison to menstrual cycle length calculated from prospective daily menstrual diaries,6,18,33 these types of studies do not address the critical issue of whether a self-reported menstrual history collected at a single time point can correctly identify menstrual cycle phase or discern menstrual cycle characteristics over longer time frames. For instance, changes in strength across the menstrual cycle3,11,12,17 and menstrual cycle–based categorization for risk of ACL injury have been largely calculated from self-reported cycle phase determinations.1,37,39 Some studies have used only self-reported menstrual cycle day to estimate phase,4,11,15,17,19 while others have aligned days on the basis of predicted ovulation using a questionnaire, basal temperature, or the previous month of data.10,14 Some investigators have improved menstrual cycle phase determination by retrospectively analyzing hormone concentrations to confirm cycle phase5,10,14 or identified ovulation with a positive urinary ovulation test3,12; 2 studies combined retrospective examination of hormone concentration and a positive urinary ovulation test.24,35 Only 2 studies have determined the timing of menstrual phase using actual hormone concentrations.7,28
The current study was designed to determine whether self-reported menstrual history data can be used to accurately categorize women into the periovulatory and midluteal menstrual cycle phases using calendar-based counting methods.
Methods
Intake Session
As part of a larger study of hormone-mediated changes in knee laxity,29,30 prospective participants individually reported to the research laboratory to meet with an investigator. After a detailed explanation of the study and the consent form, the investigator confirmed that the participant met all inclusion criteria: age between 18 and 30 years, body mass index ≤ 30, recreationally active (self-reported engagement in mild-, moderate-, or high-intensity physical activity for at least 2.5 hours per week but not more than 10 hours) for the past 3 months, reported consistent menstrual cycles (26-32 days) and no use of exogenous hormones for the past 6 months, never pregnant, nonsmoking, and no history of ligament or cartilage injury to the knee. All participants read and signed the consent form, which was approved by the university’s Institutional Review Board for the Protection of Human Subjects. Participants then filled out several additional forms, including a previously published one-page self-report menstrual history questionnaire (modified and used with permission).38
Since the menstrual history questionnaire asks the specific dates of onset of previous and upcoming menstrual cycles, the investigator provided participants with a standard monthly calendar. Once a participant filled out the questionnaire, the investigator reviewed the responses. If there were any inconsistencies in reporting, then the investigator asked the participant to review her responses. The most common mistakes were related to calculation of cycle length (ie, participants miscounted how many days were in their cycle) and the date when they anticipated the start of their next cycle (ie, participants would report a consistent 28-day cycle, but the date when they anticipated their next cycle was not 28 days from the self-reported onset of their last cycle).
Testing Schedule
After all necessary paperwork was completed, the participant contacted the investigator at the onset of her next menstrual period (first full day of bleeding) and reported to the laboratory the following morning. All data collections occurred between the hours of 6:30 and 9:00 am, within 1 hour of the original start time for a given individual, to control for diurnal hormone fluctuations and to maintain consistency.
Blood sampling procedures for the larger study were based on timing of minimal and maximal values for knee laxity; thus, blood was obtained on 6 consecutive mornings following the onset of menses and 8 to 10 consecutive mornings following a positive ovulation test.29 To determine the day of ovulation, participants began using urinary home ovulation detection kits (CVS One Step Ovulation Predictor, CVS Corp, Woonsocket, Rhode Island) on day 8 of their cycle and continued to test at the same time each day until they interpreted the test as being positive. If the participant never received a positive ovulation test (up to 25 days postmenses onset), then no ovulation date was recorded, and a new cycle of testing began at the onset of the next menses. Identical testing procedures were repeated during the participant’s next menstrual cycle.
Hormone Data Collection Procedures
Each morning of data collection, participants arrived at the laboratory and filled out a data sheet form on their compliance with study requirements (no alcohol in past 24 hours and no vigorous exercise prior to testing).
Hormone Assays
Progesterone concentrations were analyzed with Coat-A-Count RIA Assays (TKPG-2, Siemens Medical Solutions Diagnostics, Los Angeles, California). The detection sensitivity and mean intra- and interassay coefficients of variation for progesterone were 0.1 ng/mL, 4.1%, and 6.4%, respectively.
Criterion and Generalized Methods for Assessing Menstrual Cycle Phase
A serum progesterone concentration of 2.0 ng/mL or greater is widely accepted as an indicator that ovulation has occurred.2,16,26 This criterion was compared with the progesterone levels obtained when 3 common generalized calendar-based counting methods were used to indicate ovulatory phase: counting forward 10 to 14 days from the first day of menses,4,5,15,19 counting back 12 to 14 days from the start of the next menstrual cycle,10 and counting 1 to 3 days forward from the positive urinary ovulation test.14,23,35 For the midluteal hormone phase, the criterion serum progesterone level of greater than 4.5 ng/mL was used, based on reference ranges for midluteal values (4.5-20.0 ng/mL) from the laboratory analyzing the progesterone levels in this study (Reproductive Core Laboratory, University of Virginia). This criterion was then compared with the 3 common generalized calendar-based counting methods used to assign the midluteal phase: counting forward 7 days from the ovulation window of days 10 to 14,5,19 counting back 7 to 9 days from the start of the next cycle,10 and counting 7 to 9 days forward from the positive urinary ovulation test.14,23,24 The comparison of attainment of criterion progesterone values based on the generalized calendar-based counting methods was completed for cycle 1 only, since any calendar-based counting that employed a counting-back method would have required a third month of data collection.
Statistical Analysis
All statistical analyses were run using SAS 9.13 (SAS Institute, Cary, North Carolina). Frequency counts were used to examine whether appropriate criterion hormone levels were achieved at the predefined calendar days used to define periovulatory and midluteal hormone events.
Results
The participant characteristics of women who completed all aspects of the study and those who dropped out prior to hormonal measurements are shown (Table 1). For women who completed all aspects of the study, the mean number of days in the menstrual cycle was 30 days for cycles 1 and 2 (range for cycle 1, 23-36+ days; range for cycle 2, 21-36+ days).
Table 1.
Characteristics for women who completed 2 months of hormone assessment and for those who dropped out.
| Completed (n = 73) | Dropped Out (n = 18) | |
|---|---|---|
| Age, ya | 21.4 ± 2.6 | 20.6 ± 2.1 |
| Height, cma | 164.2 ± 6.6 | 160.6 ± 6.9 |
| Weight, kga | 61.2 ± 8.8 | 63.6 ± 9.2 |
| Race, % | ||
| Black | 20.5 | 33.3 |
| Asian | 6.8 | 16.7 |
| Native American | 1.4 | 0.0 |
| White | 70.0 | 50.0 |
Mean ± SD.
Since a positive urinary ovulation test is used as a positive indicator of ovulation in the absence of sequential progesterone values, the calendar-based counting methods for capturing ovulation were compared to the urinary ovulation test. When counting 10 to 14 days forward from the first day of menses to determine ovulation, only 35% and 42% of women had a positive urinary ovulation test between days 10 and 14 after menses in cycles 1 and 2, respectively. Counting back 12 to 14 days from the start date of the next cycle to capture ovulation, only 32% and 23% of women had a positive urinary ovulation test during this window for cycles 1 and 2, respectively (Figure 1). The mean day of ovulation counted from the onset of menses was 15 for cycle 1 and 16 for cycle 2.
Figure 1.
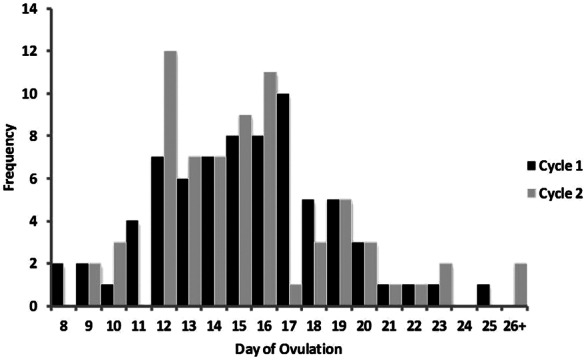
Frequency distribution for the menstrual cycle day that ovulation occurred, as counted from the onset of menses in cycle 1 and cycle 2 for each participant. All women who did not ovulate by day 25 are included in the 26+ category.
When the criterion serum progesterone level of >2.0 ng/mL was applied as the indicator that ovulation had occurred during cycle 1, only 18% of women attained this value during the 10 to 14 days after the onset of menses (Table 2). Interestingly, 71% of women did not have a positive urinary ovulation test until >14 days after the onset of menses in cycle 1. When the generalized method of counting back 12 to 14 days from the onset of menses in cycle 2 was utilized, 59% of women attained the 2.0 ng/mL level of progesterone in this window, and only 29% of the women had completed all of their luteal phase hormone collection prior to the end of this window. Finally, during the 1 to 3 days after the positive urinary ovulation test, 76% of women attained the serum progesterone level of 2.0 ng/mL.
Table 2.
Women meeting the criterion hormone concentrations for ovulation in cycle 1 when utilizing previously published calendar-based counting methods for determining ovulation (in percentages).
| Method | Progesterone >2.0 ng/mL |
|---|---|
| Count forward 10-14 days from the first day of menses a | |
| Yes | 18 |
| No | 82 |
| Count back 12-14 days from the start of the next cycle b | |
| Yes | 59 |
| No | 41 |
| Count 1-3 days forward from positive urinary ovulation test | |
| Yes | 76 |
| No | 24 |
In 71% of cases, a positive ovulation test was obtained >14 days after the onset of menses.
In 29% of cases, the luteal phase hormone collection days (8-10 days after a positive ovulation test) were completed prior to window of interest.
The mean number of days to attain >2.0 ng/mL of progesterone during this postovulation sampling window was 2 days for cycles 1 and 2 (Figure 2). Further analysis revealed that serial blood sampling for a minimum of 3 days after the positive urinary ovulation test would have captured the attainment of this criterion progesterone level in 76% of participants in cycle 1 and 68% in cycle 2, while sampling for an additional 2 days (5 days total) would have captured 81% in cycle 1 and 76% in cycle 2. Women never attaining the criterion during the 10-day postovulation sampling window are included in the 11+ category (10% of women in cycle 1 and 14% of women in cycle 2).
Figure 2.
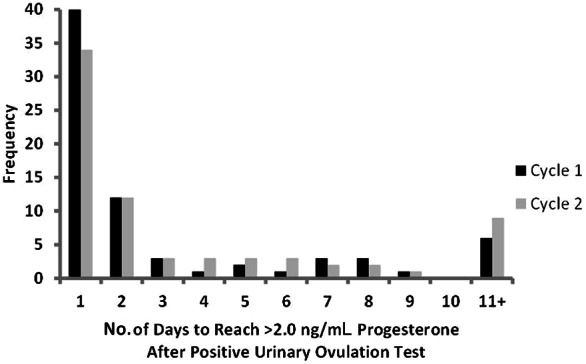
Distribution of the number of days after the positive urinary ovulation test required to reach the criterion progesterone level for ovulation of >2.0 ng/mL for each participant in cycle 1 and cycle 2. All women who did not reach the criterion by 10 days after the positive urinary ovulation test are included in the 11+ category.
In the attempt to capture the midluteal phase in cycle 1 by counting 7 days forward from the assumed 5-day ovulation window (days 10-14), 67% of women reached a criterion progesterone level >4.5 ng/mL, indicating a luteal phase hormonal milieu (Table 3). However, 25% did not reach this progesterone level during this window, and 8% of women actually had luteal days that started after day 21 (ie, a positive ovulation test did not occur until after day 21). Similarly, utilizing the generalized method of counting back 7 to 9 days from the start of the next menstrual cycle to identify the previous luteal phase, 67% of women reached a progesterone level >4.5 ng/mL during this window. A total of 8% of women did not reach this level during the window, and 25% of women had finished their luteal days before the window (ie, a positive ovulation test occurred early, and 8-10 days of blood collection was completed before the 9-day-back threshold). Finally, using the method of counting 7 to 9 days forward from a positive urinary ovulation test, 67% of women reached a progesterone level >4.5 ng/mL during this window, while 32% did not.
Table 3.
Women meeting the criterion hormone concentrations for the luteal phase in cycle 1 when utilizing previously published calendar-based counting methods for determining the luteal phase (in percentages).
| Method | Progesterone >4.5 ng/mL |
|---|---|
| Count 7 days from days 10-14 (ovulation; days 17-21) a | |
| Yes | 67 |
| No | 33 |
| Count back 7-9 days from the start of the next cycle b | |
| Yes | 67 |
| No | 33 |
| Count 7-9 days forward from positive urinary ovulation test c | |
| Yes | 67 |
| No | 32 |
In 8% of cases, the luteal phase hormone collection days were outside the window for the corresponding menstrual cycle days (completed prior to day 17 or started after day 21).
In 25% of cases, the luteal phase hormone collection days were completed prior to this window of interest.
One participant (1%) had missing data for this portion of the analysis.
In women that attained the criterion (midluteal phase >4.5 ng/mL progesterone) during the 10-day postovulation sampling window (after a positive urinary ovulation test), the mean number of days to attain the criterion was 3 days for cycles 1 and 2 (Figure 3). In addition, 3 days of serial blood sampling would have captured the attainment of the luteal phase criterion progesterone level in 64% and 58% of women for cycles 1 and 2, respectively. Adding 2 days of serial blood sampling (5 days total) would have captured this criterion progesterone level in 75% of women in cycle 1 and 65% of women in cycle 2. Women never attaining the criterion during the 10-day postovulation sampling window are included in the 11+ category (18% of women in cycle 1 and 21% of women in cycle 2).
Figure 3.
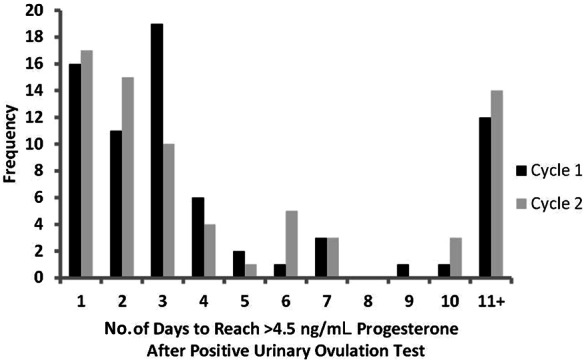
Distribution of the number of days after the positive urinary ovulation test required to reach the criterion progesterone level for luteal phase of >4.5 ng/mL for each participant in cycle 1 and cycle 2. All women who did not reach the criterion by 10 days after the positive urinary ovulation test are included in the 11+ category.
Discussion
As suggested in a recent review by Vescovi,36 the ability to properly identify menstrual cycle phase is essential for research on the epidemiology of noncontact ACL injuries. However, while daily hormone measures provide the most accurate assessment of menstrual cycle phase, it is not always feasible to obtain these measures. Furthermore, it is virtually impossible to predict when an ACL injury will occur, and it is not practical to measure menstrual cycle function over time in large cohorts of female athletes in an attempt to capture injury events. Thus, other methods are needed to identify menstrual cycle/phase characteristics.36 The results from our current investigation indicate that (1) generalized calendar-based counting methods are not advised if proper identification of menstrual cycle phase and the corresponding hormonal milieu is paramount to the research question; (2) using a positive urinary ovulation test greatly increases the likelihood of capturing postovulation hormone changes, which increases the accuracy of proper menstrual cycle phase determination; and (3) short durations of serial blood sampling after a positive urinary ovulation test capture attainment of criterion progesterone levels in a significant percentage of women, albeit not all women.
Investigators considering the use of calendar-based counting methods for identifying menstrual cycle phase must realize that the results from the current investigation represent the “best-case scenario” for many of these methods. During the current investigation, participants were asked to self-report the onset of menses and test for ovulation using a urinary ovulation kit for 2 months. Blood samples to assess hormone levels were taken 6 days after the onset of menses and 8 to 10 days after the positive urinary ovulation test. Thus, while we were able to measure postovulatory and midluteal hormone concentrations prospectively over multiple days and compare them with generalized calendar-based counting methods, we recognize that we may have missed attainment of the criterion values used for ovulation (progesterone >2.0 ng/mL) and the luteal phase (progesterone >4.5 ng/mL) in some participants since we did not collect blood every day for the entire month. Currently, the only direct assessment of ovulation is serial transvaginal ultrasound.9,34 Luteinizing hormone peak has been used extensively, but it is still an indirect index of the expected day of ovulation.9 Genuine false-positive test results are rare when commercially available urinary luteinizing hormone kits are used, as long as instructions are followed precisely. However, “borderline” or equivocal results are common and can be confusing.34 In the event that participants reported uncertainty about positive results in the current study, we began collecting hormone samples and asked the participants to continue the ovulation tests until either a strong positive or a negative result occurred. However, similar to most studies, ours still relied on the participant’s ability to correctly identify a positive ovulation test result, and we recognize that if the tests were improperly performed or interpreted, our ability to correctly identify the attainment of the criterion progesterone levels may also have been adversely affected.
Women included in the current study were physically active (range, 2.5-10 hours per week), and previous research has shown that menstrual dysfunctions, such as anovulation and luteal phase defect, are more common in physically active women.36 The prevalence of luteal phase defect in physically active women has been shown to be as high as 48%.36 Luteal phase defect is defined as either reduced progesterone production or shortening of the luteal phase (< 10 days) and reflects an ovarian system that functions at a level adequate for ovulation but inadequate to support implantation.8
Utilizing the attainment of a progesterone level of >2 ng/mL in the luteal phase as an indication that ovulation occurred, we found that 12 women had an anovulatory cycle despite having a luteinizing hormone surge large enough to produce a positive urinary ovulation test (9 women with 1 anovulatory cycle and 3 women with both cycles anovulatory). Thus, only 10% (15 of 146) of the cycles assessed in the current study were anovulatory. This is close to the documented false-positive rate of 7% for urinary ovulation kits21 and similar to the range reported for physically active women (12%-29%) by Vescovi.36 In the current study, the activity level for the women with anovulatory cycles did not differ significantly from the women with ovulatory cycles (mean Marx activity scores, 8.92 and 8.06, respectively; P = 0.67). However, further analysis showed that although body mass index was in the normal range for both groups of women, the women with anovulatory cycles had a significantly lower index than the ovulatory women (means, 21.3 kg/m2 and 22.7 kg/m2, respectively; P = 0.04). These data suggest that women with a lower body mass index, perhaps because of higher activity levels or inadequate caloric intake, may be more likely to show variability in sex hormone concentrations across cycles,32 as well as an increased likelihood of anovulation and luteal phase defect, both of which lack perceptible symptoms22 and are not detected by any type of menstrual history questionnaire.36 One key point from female athlete research is worth highlighting: menstrual cycle dysfunction is less likely to occur when women have adequate caloric intake (>30 kcal·kg-1 fat-free mass·day-1).22 Perhaps documentation of caloric intake should be considered in future ACL research. Thus, it is possible that the connection between ACL injury risk and menstrual cycle phase is tangential to low energy availability and the disruption of sex steroid hormones that accompanies this. Recent research indicates that collagen markers and insulin-like growth factor 1 predicted changes in anterior knee laxity across the cycle, and other research has clearly shown that both collagen markers and insulin-like growth factor 1 are altered by sex steroid hormone concentrations31; these hormones are clearly altered in situations of low energy availability.22
When the hormone criterion level of progesterone >2.0 ng/mL was used as the indicator of ovulation, the generalized method of counting backward from the next menstrual cycle start date was more accurate than counting forward from the onset of menses (59% vs 18%) (Figure 1). These findings are consistent with previously reported research on the use of generalized calendar-based counting methods for determining menstrual cycle phase.
Identification of midluteal phase hormone levels based on the criterion of progesterone concentration >4.5 ng/mL was similar for all generalized counting methods used in the current study. The methods of counting forward 7 days from the estimated ovulation window of 10 to 14 days, counting back 7 to 9 days from the start of the next menstrual cycle, and counting 7 to 9 days from a positive urinary ovulation test all identified midluteal phase hormone concentrations in two-thirds of women. However, none of these methods is particularly helpful if identification of the midluteal phase hormonal criterion is essential. In the current study, we found that utilizing serial sampling for 5 days after a positive urinary ovulation test identified the luteal phase progesterone criterion of >4.5 ng/mL in 75% of women in cycle 1 and 65% of women in cycle 2.
The findings of the current study suggest that if more accurate assessments of menstrual cycle phase determination are not feasible, then determination can be optimized by combining self-report questionnaire data, a positive urinary ovulation test, and 3 to 5 days of serial blood sampling after a positive urinary ovulation test. Given the cost and labor associated with daily blood samples, the use of urinary ovulation kits and strategically selected serial blood sampling could save considerable dollars and significantly reduce participant burden. We recognize that in the best-case scenario, we still did not capture 100% of the criterion progesterone values in our sample, suggesting that we need to continue to work to identify the most accurate, cost-effective algorithms for categorizing menstrual cycle hormone events.
Acknowledgments
This project was supported by grant RO1-AR053172 NIH NIAMS and by a cooperative agreement in reproductive research (NICHD/NIH U54 HD28934)
Footnotes
Laurie Wideman received funding from a grant from NIH NIAMS (RO1-AR053172); Melissa M. Montgomery received funding from a grant from NIH NIAMS (RO1-AR053172); Sandra J. Shultz received grants from NIH-NIAMS (R01-AR053172) and NFL Charities, received payment for lectures at the Iowa Sports Medicine Meeting, and received royalties from Human Kinetics.
References
- 1. Adachi N, Nawata K, Maeta M, Kurozawa Y. Relationship of the menstrual cycle phase to anterior cruciate ligament injuries in teenaged female athletes. Arch Orthop Trauma Surg. 2008;128(5):473-478 [DOI] [PubMed] [Google Scholar]
- 2. Askalani H, Smuk M, Sugar J, Delvoye P, Robyn C, Schwers J. Serum progesterone in nonpregnant women: 1. Comparative study of serum progesterone concentration and urinary pregnanediol excretion. Am J Obstet Gynecol. 1974;118(8):1054-1063 [PubMed] [Google Scholar]
- 3. Bambaeichi E, Reilly T, Cable NT, Giacomoni M. The isolated and combined effects of menstrual cycle phase and time-of-day on muscle strength of eumenorrheic females. Chronobiol Int. 2004;21(4-5):645-660 [DOI] [PubMed] [Google Scholar]
- 4. Belanger MJ, Moore DC, Crisco JJ, Fadale PD, Hulstyn MJ, Ehrlich MJ. Knee laxity does not vary with the menstrual cycle, before or after exercise. Am J Sports Med. 2004;32(5):1150-1157 [DOI] [PubMed] [Google Scholar]
- 5. Beynnon BD, Bernstein IM, Belisle A, et al. The effect of estradiol and progesterone on knee and ankle joint laxity. Am J Sports Med. 2005;33(9):1298-1304 [DOI] [PubMed] [Google Scholar]
- 6. Creinin MD, Keverline S, Meyn LA. How regular is regular? An analysis of menstrual cycle regularity. Contraception. 2004;70(4):289-292 [DOI] [PubMed] [Google Scholar]
- 7. Deie M, Sakamaki Y, Sumen Y, Urabe Y, Ikuta Y. Anterior knee laxity in young women varies with their menstrual cycle. Int Orthop. 2002;26(3):154-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeSouza MJ, Williams NI. Physiological aspects and clinical sequelae of energy deficiency and hypoestrogenism in exercising women. Hum Reprod Update. 2004;10(5):443-448 [DOI] [PubMed] [Google Scholar]
- 9. Ecochard R, Boehringer H, Rabilloud M, Marret H. Chronological aspects of ultrasonic, hormonal and other indirect indices of ovulation. British J Obst Gyn. 2001;108(8):822-829 [DOI] [PubMed] [Google Scholar]
- 10. Eiling E, Bryant AL, Petersen W, Murphy A, Hohmann E. Effects of menstrual-cycle hormone fluctuations on musculotendinous stiffness and knee joint laxity. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):126-132 [DOI] [PubMed] [Google Scholar]
- 11. Elliott KJ, Cable NT, Reilly T, Diver MJ. Effect of menstrual cycle phase on the concentration of bioavailable 17-B oestradiol and testosterone and muscle strength. Clin Sci. 2003;105(6):663-669 [DOI] [PubMed] [Google Scholar]
- 12. Friden C, Hirschberg AL, Saartok T. Muscle strength and endurance do not significantly vary across 3 phases of the menstrual cycle in moderately active premenopausal women. Clin J Sports Med. 2003;13(4):238-241 [DOI] [PubMed] [Google Scholar]
- 13. Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and prevention noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34(9):1512-1532 [DOI] [PubMed] [Google Scholar]
- 14. Hertel JN, Williams NI, Olmsted-Kramer LC, Leidy HJ, Putukian M. Neuromuscular performance and knee laxity do not change across the menstrual cycle in female athletes. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):817-822 [DOI] [PubMed] [Google Scholar]
- 15. Hicks-Little CA, Thatcher JR, Hauth JM, Goldfuss AJ, Cordova ML. Menstrual cycle stage and oral contraceptive effects on anterior tibial displacement in collegiate female athletes. J Sports Med Phys Fitness. 2007;47(2):255-260 [PubMed] [Google Scholar]
- 16. Israel R, Mishell DR, Stone SC, Thorneycroft IH, Moyer DL. Single luteal phase serum progesterone assay as an indicator of ovulation. Am J Obstet Gynecol. 1972;112(8):1043-1046 [DOI] [PubMed] [Google Scholar]
- 17. Janse-de-Jonge XAK, Boot CRL, Thom JM, Ruell PA, Thompson MW. The influence of menstrual cycle phase on skeletal muscle contractile characteristics in humans. J Physiol. 2001;530(1):161-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jukic AMZ, Weinberg CR, Wilcox AJ, McCannaughey DR, Hornsby P, Baird DD. Accuracy of reporting of menstrual cycle length. Am J Epidemiol. 2008;167(1):25-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karageanes SJ, Blackburn K, Vangelos ZA. The association of the menstrual cycle with the laxity of the anterior cruciate ligament in adolescent female athletes. Clin J Sports Med. 2000;10(3):162-168 [DOI] [PubMed] [Google Scholar]
- 20. Liu Y, Gold EB, Lasley BL, Johnson WO. Factors affecting menstrual cycle characteristics. Am J Epidemiol. 2004;160(2):131-140 [DOI] [PubMed] [Google Scholar]
- 21. McGovern PG, Myers ER, Silva S, et al. Absence of secretory endometrium after false-positive home urine luteinizing hormone testing. Fertil Steril. 2004;82(5):1273-1277 [DOI] [PubMed] [Google Scholar]
- 22. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP. American College of Sports Medicine position stand: the female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867-1882 [DOI] [PubMed] [Google Scholar]
- 23. Park SJ, Goldsmith LT, Skurnick JH, Wojtczuk MS, Weiss G. Characteristics of the urinary luteinizing hormone surge in young ovulatory women. Fertil Steril. 2007;88(3):684-690 [DOI] [PubMed] [Google Scholar]
- 24. Pollard CD, Braun B, Hamill J. Influence of gender, estrogen and exercise on anterior knee laxity. Clin Biomech. 2006;21(10):1060-1066 [DOI] [PubMed] [Google Scholar]
- 25. Renstrom P, Ljungqvist A, Arendt EA, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shepard MK, Senturia YD. Comparison of serum progesterone and endometrial biopsy for confirmation of ovulation and evaluation of luteal function. Fertil Steril. 1977;28(5):541-548 [DOI] [PubMed] [Google Scholar]
- 27. Shultz SJ, Kirk SE, Johnson M, Sander TC, Perrin DH. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36(7):1165-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shultz SJ, Sander TC, Kirk SE, Perrin DH. Sex differences in knee laxity change across the female menstrual cycle. J Sports Med Phys Fitness. 2005;45(4):594-603 [PMC free article] [PubMed] [Google Scholar]
- 29. Shultz SJ, Schmitz RJ, Kong Y, et al. Cyclic variations in knee joint laxity profiles influence landing biomechanics. Med Sci Sports Ex. 2012;44(5):900-909 [DOI] [PubMed] [Google Scholar]
- 30. Shultz SJ, Schmitz RJ, Nguyen AD, et al. Knee joint laxity and its cyclic variation influence tibiofemoral motion during weight acceptance. Med Sci Sports Ex. 2011;43(2):287-295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shultz SJ, Wideman L, Montgomery MM, Beasley KN, Nindl BC. Changes in serum collagen markers, IGF-I and knee joint laxity across the menstrual cycle. J Orthop Res. 2012;30(9):1405-1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shultz SJ, Wideman L, Montgomery MM, Levine BJ. Some sex hormone profiles are consistent over time in normal menstruating women: implications for sports injury epidemiology. Br J Sports Med. 2011;45(9):735-742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Small CM, Manatunga AK, Marcus M. Validity of self-reported menstrual cycle length. Ann Epidemiol. 2007;17(3):163-170 [DOI] [PubMed] [Google Scholar]
- 34. Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005 [Google Scholar]
- 35. Van Lunen BL, Roberts J, Branch D, Dowling EA. Association of menstrual cycle hormone changes with anterior cruciate ligament laxity measurements. J Ath Train. 2003;38(4):298-303 [PMC free article] [PubMed] [Google Scholar]
- 36. Vescovi JD. The menstrual cycle and anterior cruciate ligament injury risk: implications of menstrual cycle variability. Sports Med. 2011;41(2):91-101 [DOI] [PubMed] [Google Scholar]
- 37. Wojtys EM, Huston L, Boynton MD, Spindler KP, Lindenfeld TN. The effect of menstrual cycle on anterior cruciate ligament in women as determined by hormone levels. Am J Sports Med. 2002;30(2):182-188 [DOI] [PubMed] [Google Scholar]
- 38. Wojtys EM, Huston LJ. Active knee stiffness differs between young men and women. Paper presented at: American Orthopaedic Society for Sports Medicine 24th Annual Meeting; July 1998; Vancouver, BC [Google Scholar]
- 39. Wojtys EM, Huston LJ, Lindenfeld TN, Hewett TE, Greenfield ML. Association between the menstrual cycle and anterior cruciate ligament injuries in female athletes. Am J Sports Med. 1998;26(5):614-619 [DOI] [PubMed] [Google Scholar]


