Abstract
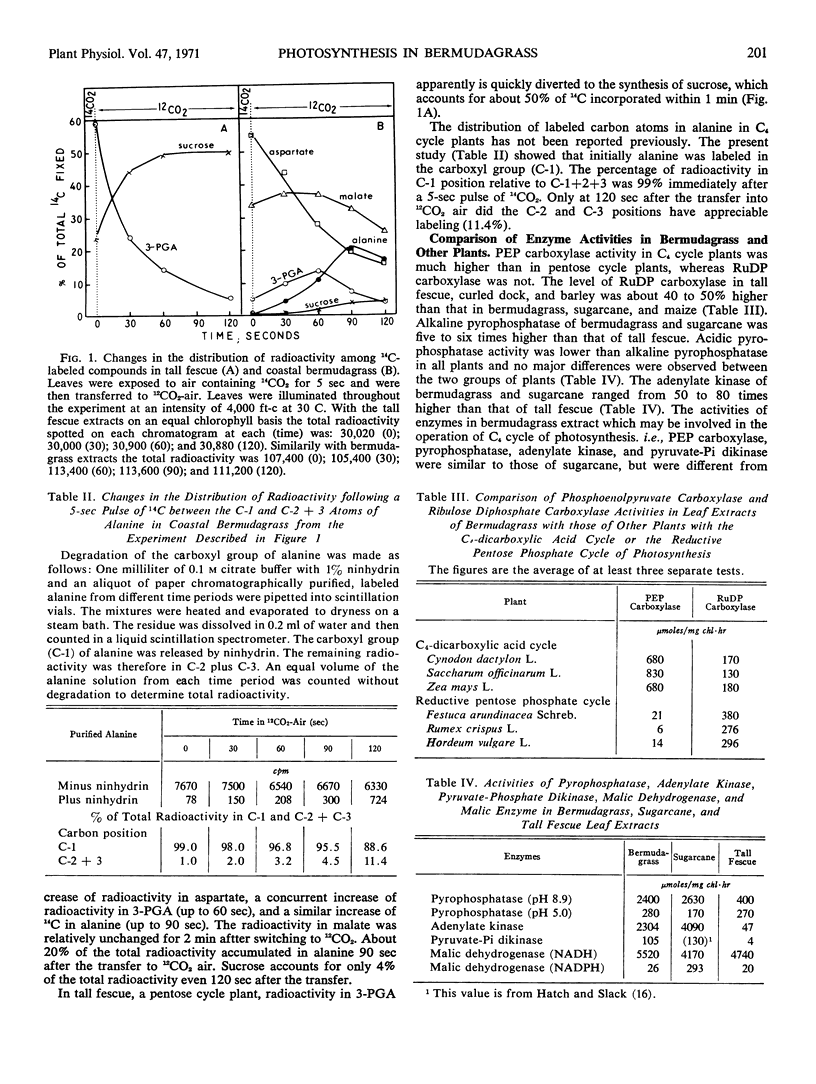
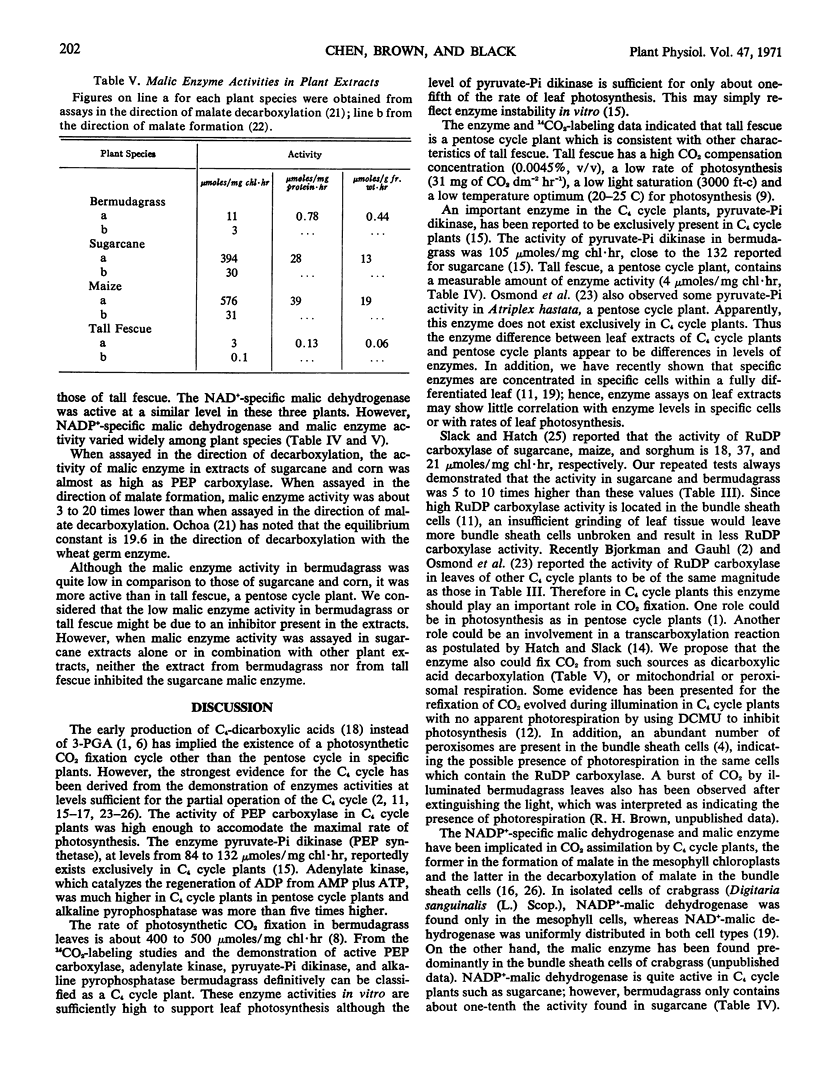
After a 5-second exposure of illuminated bermudagrass (Cynodon dactylon L. var. `Coastal') leaves to 14CO2, 84% of the incorporated 14C was recovered as aspartate and malate. After transfer from 14CO2-air to 12CO2-air under continuous illumination, total radioactivity decreased in aspartate, increased in 3-phosphoglyceric acid and alanine, and remained relatively constant in malate. Carbon atom 1 of alanine was labeled predominantly, which was interpreted to indicate that alanine was derived from 3-phosphoglyceric acid. The activity of phosphoenolpyruvate carboxylase, alkaline pyrophosphatase, adenylate kinase, pyruvate-phosphate dikinase, and malic enzyme in bermudagrass leaf extracts was distinctly higher than those in fescue (Festuca arundinacea Schreb.), a reductive pentose phosphate cycle plant. Assays of malic enzyme activity indicated that the decarboxylation of malate was favored. Both malic enzyme and NADP+-specific malic dehydrogenase activity were low in bermudagrass compared to sugarcane (Saccharum officinarum L.). The activities of NAD+-specific malic dehydrogenase and acidic pyrophosphatase in leaf extracts were similar among the plant species examined, irrespective of the predominant cycle of photosynthesis. Ribulose-1, 5-diphosphate carboxylase in C4-dicarboxylic acid cycle plant leaf extracts was about 60%, on a chlorophyll basis, of that in reductive pentose phosphate cycle plants.
We conclude from the enzyme and 14C-labeling studies that bermudagrass contains the C4-dicarboxylic acid cycle and that pyruvate-phosphate dikinase does not exist exclusively in C4-dicarboxylic acid cycle plants, and we propose that in C4-dicarboxylic acid cycle plants the transfer of carbon from a dicarboxylic acid to 3-phosphoglyceric acid involves a decarboxylation reaction and then a refixation of carbon dioxide by ribulose-1, 5-diphosphate carboxylase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black C. C., Mollenhauer H. H. Structure and distribution of chloroplasts and other organelles in leaves with various rates of photosynthesis. Plant Physiol. 1971 Jan;47(1):15–23. doi: 10.1104/pp.47.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. M., Brown R. H., Black C. C. Photosynthetic Activity of Chloroplasts Isolated From Bermudagrass (Cynodon dactylon L.), a Species With a High Photosynthetic Capacity. Plant Physiol. 1969 May;44(5):649–654. doi: 10.1104/pp.44.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Lee S. S., Chen T. M., Black C. C. Carboxylation reactions and photosynthesis of carbon compounds in isolated mesophyll and bundle sheath cells of Digitaria sanguinalis (L.) Scop. Biochem Biophys Res Commun. 1970 May 11;39(3):389–395. doi: 10.1016/0006-291x(70)90589-9. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. A new enzyme for the interconversion of pyruvate and phosphopyruvate and its role in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J. 1968 Jan;106(1):141–146. doi: 10.1042/bj1060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. NADP-specific malate dehydrogenase and glycerate kinase in leaves and evidence for their location in chloroplasts. Biochem Biophys Res Commun. 1969 Mar 10;34(5):589–593. doi: 10.1016/0006-291x(69)90778-5. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortschak H. P., Hartt C. E., Burr G. O. Carbon Dioxide Fixation in Sugarcane Leaves. Plant Physiol. 1965 Mar;40(2):209–213. doi: 10.1104/pp.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J. 1967 Jun;103(3):660–665. doi: 10.1042/bj1030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D., Goodchild D. J. Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 Sep;114(3):489–498. doi: 10.1042/bj1140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]



