Abstract
In order to improve the therapeutic efficacy and minimize the side effects of lung cancer chemotherapy, the formulation of paclitaxel-loaded poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate nanoparticles (PTX-loaded [PGA-co-PCL]-b-TPGS2k NPs) was prepared. The novel amphiphilic copolymer (PGA-co-PCL)-b-TPGS2k was synthesized by ring-opening polymerization and characterized by proton nuclear magnetic resonance spectroscopy and gel permeation chromatography. The PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs were characterized in terms of size, size distribution, zeta potential, drug encapsulation, surface morphology, and drug release. In vitro cellular uptakes of NPs were investigated with confocal laser scanning microscopy, indicating the coumarin 6-loaded (PGA-co-PCL)-b-TPGS2k NPs could be internalized by human lung cancer A-549 cells. The antitumor effect of PTX-loaded NPs was evaluated, both in vitro and in vivo, on an A-549 cell tumor-bearing mouse model via intratumoral injection. The commercial PTX formulation Taxol was chosen as the reference. Experimental results showed that the PTX-loaded NPs possessed higher cytotoxicity and could effectively inhibit the growth of tumor. All the results suggested that amphiphilic copolymer (PGA-co-PCL)-b-TPGS2k could act as a potential biological material for nanoformulation in the treatment of lung cancer.
Keywords: (PGA-co-PCL)-b-TPGS2k, paclitaxel, nanoparticles, drug delivery, lung cancer
Introduction
Lung cancer is a major cause of malignancy-related death in most developed countries, and lung cancer incidence in developing countries is increasing quickly.1 The lungs are a frequent site of metastasis, and more than 80% of deaths from lung cancer are attributed to the metastatic process.2 There are two main types of lung carcinoma: non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). NSCLC is a very invasive type of lung cancer and is more common.3 However, in clinics, current approaches for NSCLC therapy are still limited to surgical resection, radiotherapy, chemotherapy, or their combinations.4,5 These are highly aggressive or nonspecific, and are often accompanied by undesirable side effects and toxicity because the anticancer agents show conspicuous cytotoxicity to normal cells and tissues.6,7 The promises of nanotechnology in cancer research lie in the potential to solve these problems.8
During past decades, a large number of anticancer drugs were identified in drug-discovery programs, but most of them are hydrophobic and poorly soluble. The challenges of drug-delivery systems have impelled industries to develop novel techniques for achieving high bioavailability. Paclitaxel (PTX) is one of the most potent anticancer drugs, and is widely used in the therapy of NSCLC.9,10 Nevertheless, the clinical use of PTX is limited by poor aqueous solubility, high toxicity, and low bioavailability.11 Taxol is one of the formulations of PTX that can be used in the therapy of NSCLS.12,13 However, Cremophor-EL, an adjuvant used in Taxol, has been found to have serious side effects, including hypersensitivity reactions, neurotoxicity, cumulative fluid retention, cardiotoxicity, and nephrotoxicity.14 Moreover, for PTX, it has been found that the concentration of drug in the lung is rather low, which is the reason for unsatisfactory therapeutic efficacy for NSCLC.15
In order to reduce the severe side effects and increase the therapeutic efficacy of PTX, the development of drug-delivery systems facilitating lung cancer treatment has shown significant progress. Nontoxic and safe nanoparticles (NPs) for delivering anticancer drugs to tumor cells and tissues are the hot spot in the field of medicine.16 In the last decade, several drug-delivery technologies based on biodegradable polymeric NPs were able to be easily controlled to achieve both active and passive drug targeting after parenteral administration.17 Increased vascular permeability coupled with damaged lymphatic drainage in tumor tissues allows an enhanced permeability-and-retention effect of the NPs at the site of tumors.18 NPs used as drug carriers have many important advantages, such as high encapsulation efficiency and drug-loading capacity, high stability, sustained release, and excellent feasibility of routes of administration like parenteral and dermal.19 A large number of anticancer drugs can be delivered by using NPs, such as docetaxel,20 puerarin,21 PTX, and doxorubicin.22,23 Furthermore, NPs can also be used as targeted administration to specific cells and organs.24
Recently, some attention has been paid to biodegradable aliphatic polyesters, such as polylactide (PLA), polyglycolide (PGA), and poly(ε-caprolactone) (PCL), for applications in the field of drug delivery and tissue engineering.25,26 PCL has attracted considerable interest due to its excellent permeability to drugs, thermal properties, and biocompatibility. However, the rather high crystallinity of PCL decreases its compatibility with soft tissues and biodegradability. Moreover, the degradable speed of PCL is very slow.27,28 These drawbacks could hamper its application in drug-delivery systems. These problems can be surmounted by copolymerization of ε-caprolactone with other monomers. Also, PGA is not a perfect biomaterial for use in drug-delivery systems. The reason is that PGA has a very high melting temperature (about 220°C), and it is insoluble in common solvent.27 Copolymerization with other monomers, eg, lactide, ε-caprolactone, has been carried out to improve its physical properties.29 However, PGA-co-PCL has a high degree of hydrophobicity.30 PGA-co-PCL NPs can be rapidly removed by the reticuloendothelial system when they are intravenously injected into the body31,32 These drawbacks can be overcome by introduced D-α-tocopheryl polyethylene glycol (PEG) 2000 succinate (TPGS2k) to PGA, PCL, and PGA-co-PCL.13,33 TPGS, a water-soluble derivative of 2k natural vitamin E, is formed by esterification of vitamin E succinate with PEG 2000. The chemical structure of TPGS is hydrophilic polar head group and lipophilic alkyl tail. It is an excellent emulsifier and bioavailability enhancer of hydrophobic drugs. It has been reported that TPGS was able to enhance drug permeability through cell membranes by inhibition of P-glycoprotein activity34 Moreover, TPGS could significantly inhibit the growth of human lung cancer cells in vitro and in vivo.35
In this paper, in order to combine the permeability of PCL with the faster degradation of PGA and the good antitumor effects of TPGS2k, we prepared a novel biodegradable diblock copolymer poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl PEG 2000 succinate ([PGA-co-PCL]-b-TPGS2k) as drug carrier for lung cancer chemotherapy. PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs were characterized by drug-loading capacity, encapsulation efficiency, morphology, in vitro drug release, cellular uptake, and cytotoxicity. In addition, the antitumor effect of PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs was studied on an A-549 xenograft tumor model on severe combined immunodeficient mice. Commercial Taxol was chosen as the reference.
Materials and methods
Materials
Stannous octoate (Sn[Oct]2), glycolide, and ε-caprolactone were purchased from Sigma-Aldrich (St Louis, MO, USA). D-α-TPGS2k was kindly supplied by Shanghai Yare Biotech, (Shanghai, People’s Republic of China). PTX was purchased from Meilian Pharm (Chongqing, People’s Republic of China). Acetonitrile and methanol (ChromAR, high-performance liquid chromatography [HPLC] grade) were purchased from Mallinckrodt Baker (Philipsburg, NJ, USA). All other chemicals were of the highest quality commercially available and were used as received. Human lung cancer cell line A-549 was supplied by the Shanghai Institute of Cell Biology (Shanghai, People’s Republic of China).
Synthesis of block amphiphilic copolymer (PGA-co-PCL)-b-TPGS2k
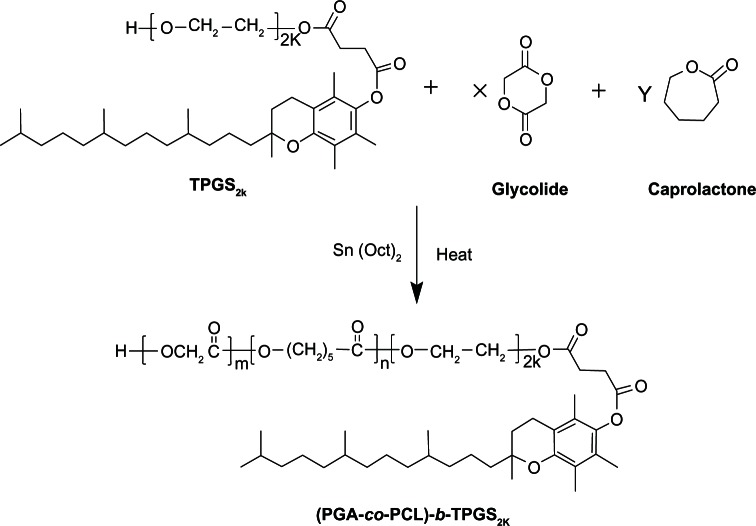
Block amphiphilic copolymer (PGA-co-PCL)-b-TPGS2k was synthesized from glycolide, ε-caprolactone, and TPGS2k in the presence of Sn(Oct)2 as a catalyst by ring-opening copolymerization, as shown in Figure 1. Briefly, weighted amounts of glycolide, ε-caprolactone, TPGS2k, and Sn(Oct)2 (0.1 mol% of monomers) were added in a glass tube that was connected to a vacuum system. Then, an exhausting–refilling with nitrogen process was repeated three times. The tube was sealed and heated to 150°C in an oil bath for 12 hours. After the reaction tube was cooled to room temperature, the resulting product was dissolved in dichloromethane and then precipitated in excess cold methanol to purify the products. The final product, named (PGA-co-PCL)-b-TPGS2k, was collected by filtration and vacuum-dried at 40°C for 2 days (90.3% yield).
Figure 1.
Schematic depiction of the synthesis of copolymer poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate ([PGA-co-PCL]-b-TPGS2k).
Characterization of copolymer (PGA-co-PCL)-b-TPGS
Proton nuclear magnetic resonance (1H NMR) imaging (AMX 500; Bruker, Billerica, MA, USA) was used to confirm the structure of synthesized (PGA-co-PCL)-b-TPGS2k, and CDCl3 was used as a solvent. Gel permeation chromatography (GPC) (150C; Waters, Milford, MA, USA) was used to obtain molecular weight and molecular weight distribution. The eluent was tetrahydrofuran with 1 mL/minute flow rate. The glass transition temperature of copolymer was detected by differential scanning calorimetry (Netzsch, Selb, Germany) with 5–10 mg samples. The temperature ranged from 0°C to 100°C at a heating rate of 5°C/minute.
Formulation of PTX-loaded nanoparticles
PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs were prepared by modified solvent extraction/evaporation.36 Briefly, a given amount of PTX and 100 mg copolymer (PGA-co-PCL)-b-TPGS2k were dissolved in 8 mL dichloromethane. The organic solution was gently poured into 100 mL of a 1% polyvinyl alcohol solution in water under stirring. The mixture was sonicated for 120 seconds at 25 W output to form an oil-in-water emulsion. The emulsion was then evaporated overnight under reduced pressure to remove dichloromethane. The resultant suspension of NPs was centrifuged at 20,000 rpm for 20 minutes and then washed three times to remove the emulsifier polyvinyl alcohol and unencapsulated drug. Particles were freeze-dried for 2 days. PTX-loaded poly(lactic-co-glycolic acid) (PLGA) NPs were prepared by the same procedure. Furthermore, the fluorescent coumarin 6-loaded (PGA-co-PCL)-b-TPGS2k NPs were prepared in the same way, except the coumarin 6 was encapsulated instead of PTX.
Characterization of nanoparticles
Size and zeta potential
The particle size and size distribution were measured by a Mastersizer 2000 (Malvern Instruments, Malvern, UK). Before measurement, the freshly prepared particles were appropriately diluted. Data were obtained by the average of three measurements.
Surface morphology
The surface morphology of NPs was examined by field-emission scanning electron microscopy (FESEM) using a JEOL (Tokyo, Japan) JSM-6700F system operated at a 5.0 kV accelerating voltage. To prepare samples for FESEM, the particles were fixed on the stub by double-sided sticky tape and then coated with a platinum layer by a JFC-1300 automatic fine platinum coater (JEOL) for 60 seconds.
Drug-loading and encapsulation efficiency
The drug-loading content and encapsulation efficiency of NPs were assayed by HPLC (LC 1200; Agilent Technologies, Santa Clara, CA, USA), in accordance with the literature.37 In summary, 5 mg NPs were dissolved in 1 mL dichloromethane under vigorous vortexing. This solution was transferred to 5 mL mobile phase consisting of deionized water and acetonitrile (50:50, v/v). A nitrogen stream was introduced to evaporate the dichloromethane for about 15 minutes, and then a clear solution was obtained for HPLC analysis. A reverse-phase C18 column (250 mm × 4.6 mm; GL Science, Tokyo, Japan) was used. The flow rate of the mobile phase was 1 mL/minute. The column effluent was detected using an ultrviolet detector at λmax of 227 nm. The drug-encapsulation efficiency was calculated as the ratio between the amount of PTX encapsulated in the NPs and that feeding into the process. Each batch was performed in triplicate.
In vitro drug release
Dialysis was used to examine the drug release in vitro.38 In brief, 15 mg of NPs was dispersed in 5 mL of phosphate-buffered saline (PBS; containing 0.1% w/v Tween 80), pH + 7.4. The suspension was put into a standard-grade regenerated cellulose dialysis membrane (Spectra/Por6, molecular weight cutoff + 3500; Spectrum, Houston, TX, USA). The closed bag was then put into a centrifuge tube and immersed in 15 mL of release medium PBS. The tube was put into a water bath shaking at 120 rpm at 37°C. Quantitative samples were taken at various time intervals for analysis and replaced with fresh medium. The latest collected samples were extracted by 2 mL of dichloromethane and reconstituted in 5 mL of the mobile phase. The dichloromethane was removed in an N2 stream. The analysis method was similar to the measurement of drug-loading content and encapsulation efficiency. Each batch was performed in triplicate.
Cellular uptake of nanoparticles
Human lung cancer cell line A-549 was cultured in Dulbecco’s modified essential medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum and antibiotics. A-549 cells at an initial density of 1 × 104 cells/well were plated in 96-well plates. The culture was kept in 95% air humidified atmosphere containing 5% CO2 at 37°C. The cells were incubated with 250 μg/mL coumarin 6-loaded (PGA-co-PCL)-b-TPGS2k NPs at 37°C for 4 hours, washed with cold PBS three times, and then fixed with methanol for 20 minutes. Cells were stained with propidium iodide (PI) for 30 minutes and washed twice with PBS. In order to observe the cells, the chambers were mounted onto a confocal laser scanning microscope (Fluoview FV-1000; Olympus, Tokyo, Japan) with imaging software. The images of the cells were determined with differential interference contrast channel, and the images of coumarin 6-loaded NPs and the nuclei of the cells stained by PI were recorded with following channels: red channel (PI) with excitation at 430 nm and green channel (coumarin 6) with excitation at 485 nm.
In vitro cytotoxicity
A-549 cells were seeded in 96-well plates (Sigma-Aldrich) at a density of 5000 viable cells per well and incubated for 24 hours to allow cell attachment. The cells were incubated with the PTX-loaded (PGA-co-PCL)-b-TPGS2k NP suspension and Taxol at equivalent drug concentrations ranging from 0.25 to 25 mg/mL for 24, 48, and 72 hours. The drug free (PGA-co-PCL)-b-TPGS2k NPs with the same amount of NPs. At designated time intervals, the formulations were replaced with DMEM containing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5 mg/mL) and cells were then incubated for an additional 4 hours. MTT was aspirated off and dimethyl sulfoxide was added to dissolve the formazan crystals. Absorbance was measured at 570 nm using a microplate reader. Untreated cells were taken as control with 100% viability, and cells without addition of MTT were used as blanks to calibrate the spectrophotometer to zero absorbance.
In vivo antitumor efficacy and side-effect analysis
The tumor growth-inhibitory activities of PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs were assessed in a nude mice bearing A-549 cells xenograft model, which was reported by Zhang et al.39 In brief, A-549 cells (0.1 mL) in the culture medium were implanted into the subcutaneous space of the right-limb armpit of mice at a dosage of 2 × 107 cells/mouse. After inoculation of A-549 cells, the tumor growth in each mouse was closely observed. The tumor volume was monitored with a caliper every 2 days until the 16th day. Tumor volume can be calculated from the formula: (π/6) × larger diameter × (smaller diameter)2.40 When the tumor volume achieved about 100 mm3, the mice were randomly divided into three groups (each group, n + 5), which were subject to intratumoral injection of the (PGA-co-PCL)-b-TPGS2k nanoformulation, Taxol (10 mg/kg PTX dose), and saline, respectively. Mice were closely observed for clinical signs and behavior. Intratumoral injection was given at days 0, 4, 8, and 12 for four consecutive cycles. Furthermore, in order to estimate the side effects of the PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs, body weight of the mice was recorded during the treatment.
Statistical methodology
All experiments were repeated at least three times unless otherwise stated. Statistical analysis (t-test) was performed with SPSS 16.0 software (IBM, Armonk, NY, USA). P < 0.05 was considered to indicate statistical significance for all comparisons.
Results and discussion
Characterization of block copolymer (PGA-co-PCL)-b-TPGS2k
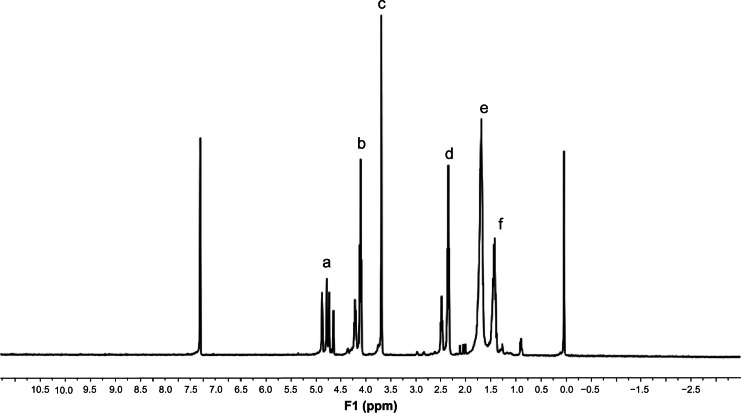
The copolymerizations of glycolide and 8-caprolactone were carried out with initiator TPGS2k and Sn(Oct)2 as a catalyst in bulk at 150°C to synthesize biodegradable block copolymer (PGA-co-PCL)-b-TPGS2k In order to demonstrate the formation of copolymer, 1H NMR in CDCl3 was recorded and is presented in Figure 2. The peak at 1.38 ppm (f), 1.65 ppm (e), 2.31 ppm (d) and 4.05 ppm (b) are assigned to ε-CL repeating unit (–CH2CH2CH2CH2CH2–), (–CH2CH2CH2CH2CH2), (COCH2) and (CH2 OCO), respectively The peak at 4.82 ppm (a) was assigned to the methylene protons (–CH2–) of PGA segment. The peak at 3.65 ppm (c) was assigned to the (–CH2–) protons of PEG part of TPGS2k.41 The molecular weight of (PGA-co-PCL)-b-TPGS2k was calculated from the peak areas’ integral ratios of 1.38 ppm, 4.82 ppm, and 3.65 ppm, and the number-averaged molecular weight of the copolymer (PGA-co-PCL)-b-TPGS2k was determined by 1H NMR to be 21,970. The molecular weight and polydispersity index (PDI) of copolymer (PGA-co-PCL)-b-TPGS2k determined by GPC were 20235 and 1.25, respectively. The molecular weight detected from GPC and 1H NMR can demonstrate each other. The glass transition temperature of copolymer (PGA-co-PCL)-b-TPGS2k was 37.6°C, which was obtained by differential scanning calorimetry analysis.
Figure 2.
Typical proton nuclear magnetic resonance spectra of copolymer poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate.
Characterization of nanoparticles
The size and size distribution of the PTX-loaded NPs in this research are presented in Table 1. Physicochemical characteristics, such as particle size and surface properties, play an important role in in vitro drug release, cytotoxicity, and cellular uptake of these NPs, as well as their in vivo pharmacokinetics and biodistribution. Therefore, they affect the therapeutic efficacy of the anticancer drug.42 The size of the NPs was 200–260 nm in diameter, which is in the excellent size range for cellular uptake of nanoparticles.43 The size of the PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs was much smaller than that of the PLGA NPs; this was probably due to the self-emulsifying function of the TPGS2k component in the copolymer. The PDI of PTX-loaded PLGA NPs and (PGA-co-PCL)-b-TPGS2k NPs was 0.153 and 0.126, respectively. The PDI of (PGA-co-PCL)-b-TPGS2k NPs was lower than that of the PLGA NPs. This could be also attributed to the TPGS2k component in the copolymer. As can be seen from Table 1, the drug-loading content and encapsulation efficiency of polymer (PGA-co-PCL)-b-TPGS2k NPs were higher than PLGA NPs. Furthermore, the encapsulation efficiency of (PGA-co-PCL)-b-TPGS2k NPs could achieve around 100% at about 10% drug-loading content.
Table 1.
Characterization of paclitaxel-loaded nanoparticles (n + 3)
| Polymer | Size (nm) | PDI | ZP (mV) | LC (%) | EE (%) |
|---|---|---|---|---|---|
| PLGA | 263.5 ± 7.4 | 0.153 | −21.4 ± 1.8 | 8.17 | 82.3 |
| (PGA-co-PCL)-b-TPGS2k | 202.1 ± 5.3 | 0.126 | −31.2 ± 2.7 | 9.76 | 96.5 |
Abbreviations: PDI, polydispersity index; ZP, zeta potential; LC, loading content; EE, encapsulation efficiency; PLGA, poly(lactic-co-glycolic acid); (PGA-co-PCL)-b-TPGS2k, poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate.
The zeta potential of NPs is a crucial factor for stability in suspension through the electrostatic repulsion between the NPs, interaction with the cell membrane in vivo, and judgment of component onto the NP surface.44 The PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs showed a negative surface charge of around −31.2 mV. Compared with the polymer PLGA NPs, the zeta potential of which was around −21.4 mV, an increase in the absolute value of the zeta potential could be found for the polymer PLGA NPs, which may lead to higher dispersion stability. As is known to us, the higher absolute value of the zeta potential means a more stable suspension for NPs.45,46
The surface morphology of the PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs was investigated by FESEM. Figure 3A shows the FESEM image for PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs. The NPs seemed to be about 200 nm in diameter and have smooth surface within the resolution level. Furthermore, the FESEM images demonstrated the particle size detected by the Malvern Mastersizer, which is based on dynamic light scattering (Figure 3B).
Figure 3.
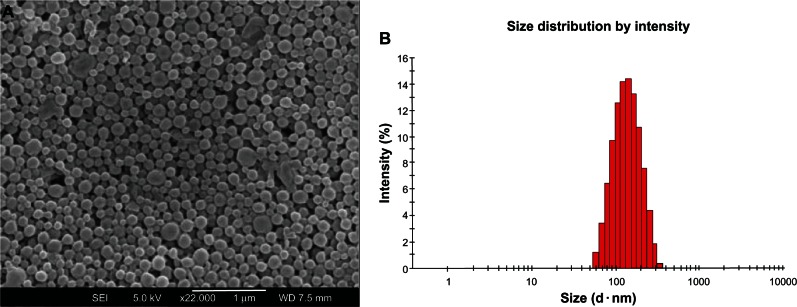
Field-emission scanning electron microscopy image (A) and dynamic light-scattering spectra (B) of paclitaxel-loaded poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate nanoparticles.
The in vitro drug-release profiles of the PTX-loaded NPs in the first 30 days are displayed in Figure 4. The drug release from the (PGA-co-PCL)-b-TPGS2k NPs was found to be 36.21% and 74.38% of the encapsulated drug in the first 5 days and after 30 days, respectively, which was much faster than that of the PLGA NPs, which was just 17.19% and 43.53% in the same times, respectively. The faster drug release of the (PGA-co-PCL)-b-TPGS2k NPs may be attributed to the higher hydrophilicity of (PGA-co-PCL)-b-TPGS2k copolymer in comparison with the PLGA NPs. It leads to the copolymer swelling and degrading more quickly.47 Moreover, the drug release from the (PGA-co-PCL)-b-TPGS2k NPs was also faster than PLGA-TPGS NPs. This could be ascribed to the higher hydrophilicity of the PGA-co-PCL core than the PLGA core.48 In comparison with the PLGA NPs, which were observed to release the PTX too slowly to meet the treatment effect for lung carcinoma, this is another reason for choosing (PGA-co-PCL)-b-TPGS2k NPs when applied to cancer therapy.
Figure 4.
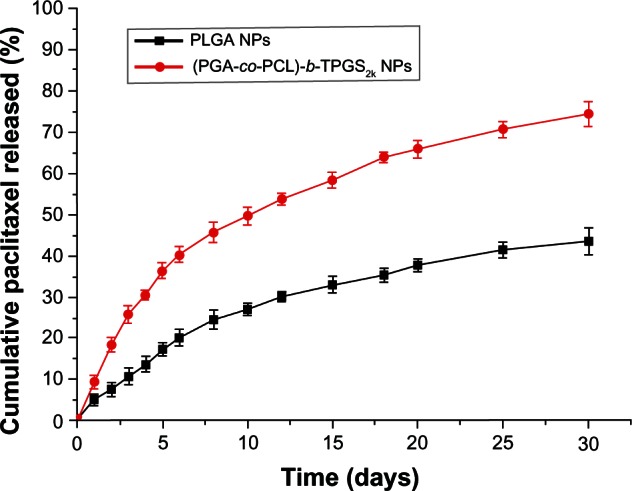
The in vitro release profile of paclitaxel-loaded poly(lactic-co-glycolic acid) nanoparticles (PLGA NPs) and poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate ([PGA-co-PCL]-b-TPGS2k) NPs.
Cellular uptake of coumarin 6-loaded (PGA-co-PCL)-b-TPGS2k nanoparticles
As is well known, the therapeutic effects of the drug-loaded NPs depend on internalization and sustained retention of the NPs by the abnormal cells. Although in vitro and in vivo bioprocesses are much different, the in vitro investigation could provide some preliminary evidence to display advantages of drug-loaded NPs versus free drug. Coumarin 6, a fluorescence molecule, has been generally used as a probe for marking NPs in cellular uptake research. Coumarin 6 was used for replacing the PTX in the NPs to visualize and analyze cellular uptake of biodegradable NPs.41 Figure 5 displays confocal laser scanning microscopy images of the A-549 lung cancer cells after 24 hours’ incubation with the coumarin 6-loaded (PGA-co-PCL)-b-TPGS2k NP suspension in DMEM at 250 μg/mL NP concentration. The images were obtained from (A) the EGFP (green) channel; (B) the PI (red) channel; and (C) the overlay of the two channels. As can be seen from Figure 5, the coumarin 6-loaded (PGA-co-PCL)-b-TPGS2k NPs (green) were closely located around the nuclei (red, stained by PI), suggesting the NPs had been internalized into the cells. These results were in good agreement with another report.49
Figure 5.
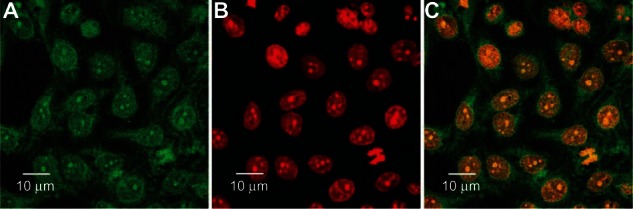
(A–C) Confocal laser scanning microscopy images of A-549 cells after 4 hours’ incubation with coumarin 6-loaded poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate nanoparticles.
Note: The coumarin 6-loaded nanoparticles were green and the cells were stained by propidium iodide (PI) (red). The cellular uptake was visualized by overlaying images obtained by enhanced green fluorescent protein (EGFP) and PI filters. (A) EGFP channel, green; (B) PI channel, red; (C) combined EGFP channel and PI channel.
Assessment of nanoparticle cytotoxicity
Human lung cancer cell line A-549 was used to research the cytotoxicity of nanoparticles, which was compared with the commercial formulation Taxol. The PTX-loaded NPs were sterilized by gamma rays to exclude any contamination effect, then were suspended and cultured with A-549 cells. Figure 6 shows the viability of A-549 cells after 24, 48, and 72 hours of cell culture with PTX formulated in the (PGA-co-PCL)-b-TPGS2k NPs, respectively, in comparison with that of the Taxol formulation at the same 0.25, 2.5, and 25 μg/mL PTX dose. As shown in Figure 6, the synthesized copolymer (PGA-co-PCL)-b-TPGS2k seems to be nontoxic in cell culture, since no significant cytotoxic activity was found for the drug-free (PGA-co-PCL)-b-TPGS2k NPs at various concentrations.
Figure 6.
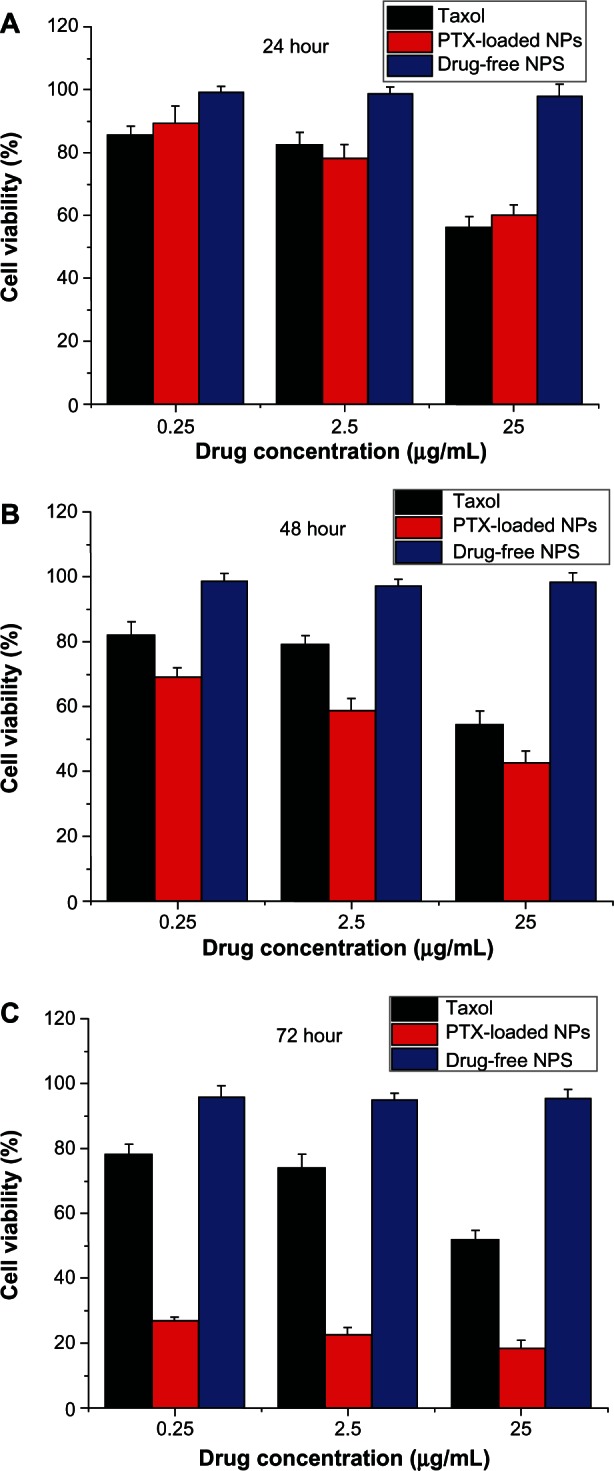
Viability of A-549 cells cultured with paclitaxel (PTX)-loaded poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate nanoparticles ([PGA-co-PCL]-b-TPGS2k NPs) in comparison with that of Taxol at the same PTX dose and drug-free (PGA-co-PCL)-b-TPGS2k NPs with the same amount of NPs. (A) 24 hours; (B) 48 hours; (C) 72 hours.
The A-549 cell viability after 24 hours’ incubation at a drug concentration of 2.5 μg/mL was 82.6% ± 3.8% for Taxol and 78.5% ± 4.1% for (PGA-co-PCL)-b-TPGS2k NPs. However, compared with commercial Taxol, the cytotoxicity of A-549 cell increased by 33.7% (P < 0.05, n + 5) and 160.9% (P < 0.05, n + 5) for PTX-loaded NPs after 48 and 72 hours of incubation at a drug concentration of 2.5 μg/mL, respectively. As time went by, the drug-loaded NPs showed better and more therapeutic effects for A-549 cells than commercial Taxol. This is because the (PGA-co-PCL)-b-TPGS2k NPs release only part of the drug after 24, 48, and 72 hours (Figure 4).48 Release started from 0, while Taxol quickly became 100% available for the A-549 cells in culture. In summary, the PTX-loaded NPs have excellent cytotoxicity for A-549 cells, and the copolymer (PGA-co-PCL)-b-TPGS2k that formulated NPs is safe and nontoxic.
In vivo antitumor efficacy and side-effect analysis
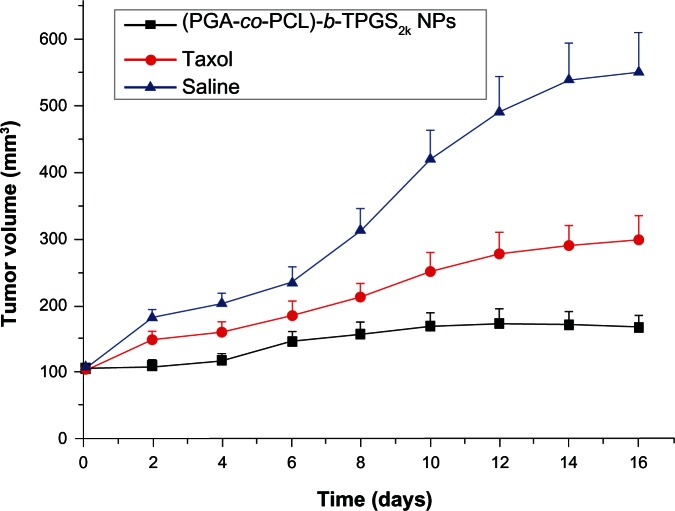
The antitumor efficacy of the PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs was investigated in A-549 cell-bearing nude mice. Figure 7 shows the tumor growth observed for 16 days in the mice injected with PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs, Taxol, and physiological saline (control). The mice were intratumorally injected with PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs and Taxol every 4 days for four consecutive cycles. The tumor size was recorded every 2 days until the 16th day. The average tumor-volume growth is shown in Figure 7. As can be seen from Figure 7, the PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs and Taxol obviously slowed down the tumor growth of mice in comparison with the saline. It can be seen that the PTX-loaded (PGA-co-PCL)-b-TPGS2k NP group presented the lowest tumor-growth rate among the three groups. The results of the antitumor-efficacy experiment indicated that PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs could be released from the NPs effectively and the released PTX maintained its bioactivity, which is important for clinical applications.
Figure 7.
Tumor-growth curve of nude mice bearing A-549 cells xenograft after intratumoral injection with paclitaxel-loaded poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate nanoparticles ([PGA-co-PCL]-b-TPGS2k NPs), Taxol, and saline (P < 0.05, n + 5).
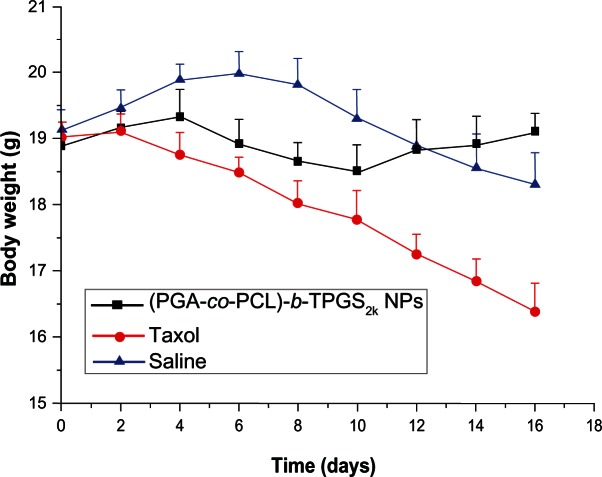
Minimization of the side effects of drug-loaded NPs is one of the main concerns when developing novel drug-delivery vehicles. The change in body weight of the nude mice is an important index to evaluate systemic adverse effects. Figure 8 presents the variation in body weight of nude mice in all groups. As shown in Figure 8, the obvious body-weight loss was observed for the Taxol and saline groups, especially the Taxol group. However, the group injected with PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs had a stable body weight. The mice treated with PTX-loaded NPs kept a vigorous and healthy appearance throughout the full experiment. Nevertheless, the mice treated with Taxol showed weakened vitality, and one of the six mice died. These observations indicated that the side effects of mice injected with PTX-loaded NPs were fewer than with Taxol. Similar results were obtained by Zhang and coworkers.39
Figure 8.
The variation in body weight of nude mice bearing A-549 cells xenograft treated with paclitaxel-loaded poly(glycolide-co-ε-caprolactone)-b-D-α-tocopheryl polyethylene glycol 2000 succinate nanoparticles ([PGA-co-PCL]-b-TPGS2k NPs), Taxol, and saline (P < 0.05, n + 5).
Conclusion
In this paper, the novel biodegradable (PGA-co-PCL)-b-TPGS2k was successfully synthesized for NP formulation of PTX for lung carcinoma therapy. The PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs were prepared by modifiedsolvent extraction/evaporation. The size of the PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs was about 200 nm, and they had a smooth surface. The drug-loading content and encapsulation efficiency of polymer (PGA-co-PCL)-b-TPGS2k NPs were higher than PLGA NPs. The PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs could achieve much faster drug release in comparison with PLGA NPs. Assessment of cytotoxicity suggested that the PTX-loaded (PGA-co-PCL)-b-TPGS2k NPs had higher cytotoxicity against A-549 cells than Taxol. A xenograft tumor model on nude mice indicated that PTX formulated in the (PGA-co-PCL)-b-TPGS2k NPs could more effectively inhibit tumor growth and more significantly reduce side effects than could Taxol. In conclusion, the amphiphilic copolymer (PGA-co-PCL)-b-TPGS2k could provide a promising polymeric material for a PTX-delivery vehicle in lung cancer therapy.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Johnson DH. Treatment strategies for metastatic non small-cell lung cancer. Clin Lung Cancer. 1999;1:34–41. doi: 10.3816/clc.1999.n.001. [DOI] [PubMed] [Google Scholar]
- 2.Horeweg N, van Klaveren RJ, Groen HJM, et al. Blinded and uniform cause of death verification in a lung cancer CT screening trial. Lung Cancer. 2012;77:522–525. doi: 10.1016/j.lungcan.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Kaustubh AJ, Chakkumkal A, Mukesh KK, Tamishraha B, Amulya KP, Ambikanandan RM. Liposomal formulations of etoposide and docetaxel for p53 mediated enhanced cytotoxicity in lung cancer cell lines. Biomaterials. 2012;33:2492–2507. doi: 10.1016/j.biomaterials.2011.11.067. [DOI] [PubMed] [Google Scholar]
- 4.Kim I, Byeon HJ, Kim TH, et al. Doxorubicin-loaded highly porous large PLGA microparticles as a sustained-release inhalation system for the treatment of metastatic lung cancer. Biomaterials. 2012;33:5574–5583. doi: 10.1016/j.biomaterials.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Mylonakis N, Athanasiou A, Ziras N, et al. Phase II study of liposomal cisplatin (Lipoplatin) plus gemcitabine versus cisplatin plus gemcitabine as first line treatment in inoperable (stage IIIB/IV) non-small cell lung cancer. Lung Cancer. 2010;68:240–247. doi: 10.1016/j.lungcan.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Lin YJ, Liu YS, Yeh HH, Cheng TL, Wang LF. Self-assembled poly(epsilon-caprolactone)-g-chondroitin sulfate copolymers as an intracellular doxorubicin delivery carrier against lung cancer cells. Int J Nanomedicine. 2012;7:4169–4183. doi: 10.2147/IJN.S33602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 8.Esfandyari-Manesh M, Javanbakht M, Atyabi F, Dinarvand R. Synthesis and evaluation of uniformly sized carbamazepine-imprinted microspheres and nanospheres prepared with different mole ratios of methacrylic acid to methyl methacrylate for analytical and biomedical applications. J Appl Polym Sci. 2012;125:1804–1813. [Google Scholar]
- 9.Liu J, Meisner D, Kwong E, Wu XY, Johnston MR. Translymphatic chemotherapy by intrapleural placement of gelatin sponge containing biodegradable paclitaxel colloids controls lymphatic metastasis in lung cancer. Cancer Res. 2009;69:1174–1181. doi: 10.1158/0008-5472.CAN-08-1753. [DOI] [PubMed] [Google Scholar]
- 10.Tien H, Dahlberg SE, Sandler AB, Brahmer JR, Schiller JH, Johnson DH. Prognostic models to predict survival in non-small-cell lung cancer patients treated with first-line paclitaxel and carboplatin with or without bevacizumab. J Thorac Oncol. 2012;7:1361–1368. doi: 10.1097/JTO.0b013e318260e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Videira M, Almeida AJ. Preclinical evaluation of a pulmonary delivered paclitaxel-loaded lipid nanocarrier antitumor effect. Nanomedicine. 2012;8:1208–1215. doi: 10.1016/j.nano.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Yang R, Shim WS, Cui FD, et al. Enhanced electrostatic interaction between chitosan-modified PLGA nanoparticle and tumor. Int J Pharm. 2009;37:142–147. doi: 10.1016/j.ijpharm.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Mi Y, Liu YT, Feng SS. Formulation of docetaxel by folic acid-conjugated d-α-tocopheryl polyethylene glycol succinate 2000 (vitamin E TPGS2k) micelles for targeted and synergistic chemotherapy. Biomaterials. 2011;32:4058–4066. doi: 10.1016/j.biomaterials.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Mei L, Sun H, Song C. Local delivery of modified paclitaxel-loaded poly(epsilon-caprolactone)/pluronic F68 nanoparticles for long-term inhibition of hyperplasia. J Pharm Sci. 2009;98:2040–2050. doi: 10.1002/jps.21581. [DOI] [PubMed] [Google Scholar]
- 15.Yang R, Yan SG, Shim WS, et al. Lung-specific delivery of paclitaxel by chitosan-modified PLGA nanoparticles via transient formation of microaggregates. J Pharm Sci. 2008;98:970–984. doi: 10.1002/jps.21487. [DOI] [PubMed] [Google Scholar]
- 16.Fan L, Wu H, Zhang H, Li F, Yang TH. pH-sensitive podophyllotoxin carrier for cancer cells specific delivery. Polym Compos. 2010;31:51–59. [Google Scholar]
- 17.Thamake SI, Raut SL, Gryczynski Z, Ranjan AP, Vishwanatha JK. Alendronate coated poly-lactic-co-glycolic acid (PLGA) nanoparticles for active targeting of metastatic breast cancer. Biomaterials. 2012;33:7164–7173. doi: 10.1016/j.biomaterials.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 18.Sarbari A, Sanjeeb KS. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63:170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Esfandyari-Manesh M, Javanbakht M, Atyabi F, Badiei A, Dinarvand R. Effect of porogenic solvent on the morphology, recognition and release properties of carbamazepine molecularly imprinted polymer nanospheres. J Appl Polym Sci. 2011;121:1118–1126. [Google Scholar]
- 20.Sanna V, Roggio AM, Posadino AM, et al. Novel docetaxel-loaded nanoparticles based on poly(lactide-co-caprolactone) and poly(lactide-co-glycolide-co-caprolactone) for prostate cancer treatment: formulation, characterization, and cytotoxicity studies. Nanoscale Res Lett. 2011;6:260. doi: 10.1186/1556-276X-6-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng XW, Chen HB, Zheng Y, et al. Enhanced adsorption of puerarin onto a novel hydrophilic and polar modified post-crosslinked resin from aqueous solution. J Colloid Interface Sci. 2012;385:166–173. doi: 10.1016/j.jcis.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Zhao Y, Wu, et al. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials. 2011;32:8281–8290. doi: 10.1016/j.biomaterials.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 23.Esfandyari-Manesh M, Javanbakht M, Dinarvand R, Atyabi F. Molecularly imprinted nanoparticles prepared by miniemulsion polymerization as selective receptors and new carriers for the sustained release of carbamazepine. J Mater Sci Mater Med. 2012;23:963–972. doi: 10.1007/s10856-012-4565-y. [DOI] [PubMed] [Google Scholar]
- 24.Danhier F, Ansorena E, Silva JM, Coco R, Breton AL, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 25.Wu Z, Ling L, Zhou L, et al. Novel preparation of PLGA/HP55 nanoparticles for oral insulin delivery. Nanoscale Res Lett. 2012;7:299. doi: 10.1186/1556-276X-7-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma YD, Zheng Y, Zeng XW, et al. Novel docetaxel-loaded nanoparticles based on PCL-Tween 80 copolymer for cancer treatment. Int J Nanomedicine. 2011;6:2679–2688. doi: 10.2147/IJN.S25251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vert M. Degradable and bioresorbable polymers in surgery and in pharmacology: beliefs and facts. J Mater Sci Mater Med. 2009;20:437–446. doi: 10.1007/s10856-008-3581-4. [DOI] [PubMed] [Google Scholar]
- 28.Sun H, Mei L, Song C, Cui X, Wang P. The in vivo degradation, absorption and excretion of PCL-based implant. Biomaterials. 2006;27:1735–1740. doi: 10.1016/j.biomaterials.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 29.Zhou S, Xu J, Yang H, Deng X. Synthesis and characterization of biodegradable poly(ε-caprolactone)-polyglycolide-poly(ethylene glycol) monomethyl ether random copolymer. Macromol Mater Eng. 2004;289:576–580. [Google Scholar]
- 30.Tsai YM, Chang-Liao WL, Chien CF, Lin LC, Tsai TH. Effects of polymer molecular weight on relative oral bioavailability of curcumin. Int J Nanomedicine. 2012;7:2957–2966. doi: 10.2147/IJN.S32630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox ME, Szoka FC, Fréchet AMJ. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Acc Chem Res. 2009;42:1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu T, Jing X, Song X, Wang X. Doxorubicin-loaded ultrafine PEG-PLA fiber mats against hepatocarcinoma. J Appl Polym Sci. 2012;123:209–217. [Google Scholar]
- 33.Rojnik M, Kocbek P, Moret F, et al. In vitro and in vivo characterization of temoporfin-loaded PEGylated PLGA nanoparticles for use in photodynamic therapy. Nanomedicine. 2012;7:663–677. doi: 10.2217/nnm.11.130. [DOI] [PubMed] [Google Scholar]
- 34.Huang LQ, Chen HB, Zheng Y, et al. Nanoformulation of D-a-tocopheryl polyethylene glycol 1000 succinate-b-poly (ε-caprolactone-ran-glycolide) diblock copolymer for breast cancer therapy. Integr Biol (Camb) 2011;3:993–1002. doi: 10.1039/c1ib00026h. [DOI] [PubMed] [Google Scholar]
- 35.Youk HJ, Lee E, Choi MK, et al. Enhanced anticancer efficacy of alpha-tocopheryl succinate by conjugation with polyethylene glycol. J Control Release. 2005;107:43–52. doi: 10.1016/j.jconrel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Tian HY, Tang ZH, Zhuang XL, Chen XS, Jing XB. Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog Polym Sci. 2012;37:237–280. [Google Scholar]
- 37.Mei L, Zhang Y, Zheng Y, et al. A novel docetaxel-loaded poly(ε-caprolactone)/pluronic F68 nanoparticle overcoming multidrug resistance for breast cancer treatment. Nanoscale Res Lett. 2009;4:1530–1539. doi: 10.1007/s11671-009-9431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma YD, Huang LQ, Song CX, Zeng XW, Gan L, Mei L. Nanoparticle formulation of poly(ε-caprolactone-co-lactide)-d-α-tocopheryl polyethylene glycol 1000 succinate random copolymer for cervical cancer treatment. Polymer. 2010;51:5952–5959. [Google Scholar]
- 39.Zhang C, Wang W, Liu T, et al. Doxorubicin-loaded glycyrrhetinic acid-modified alginate nanoparticles for liver tumor chemotherapy. Biomaterials. 2012;33:2187–2196. doi: 10.1016/j.biomaterials.2011.11.045. [DOI] [PubMed] [Google Scholar]
- 40.Guo JW, Gao XL, Su LN, et al. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Ouyang CP, Ma GL, Zhao SX, et al. Preparation and characterization of the molecular weight controllable poly(lactide-co-glycolide) Polym Bull. 2011;67:793–803. [Google Scholar]
- 42.Sanna V, Sechi M. Nanoparticle therapeutics for prostate cancer treatment. Nanomedicine. 2012;8:S31–S36. doi: 10.1016/j.nano.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Yan F, Zhang C, Zheng Y, et al. The effect of poloxamer 188 on nanoparticle morphology, size, cancer cell uptake, and cytotoxicity. Nanomedicine. 2010;6:170–178. doi: 10.1016/j.nano.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Liu YT, Li K, Liu B, Feng SS. A strategy for precision engineering of nanoparticles of biodegradable copolymers for quantitative control of targeted drug delivery. Biomaterials. 2010;31:9145–9155. doi: 10.1016/j.biomaterials.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 45.Chen H, Zheng Y, Tian G, et al. Oral delivery of DMAB-modified docetaxel-loaded PLGA-TPGS nanoparticles for cancer chemotherapy. Nanoscale Res Lett. 2011;6:4. doi: 10.1007/s11671-010-9741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao K, Li Y, Luo J, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–3446. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mu L, Feng SS. Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for controlled release of paclitaxel (Taxol) J Control Release. 2002;80:129–144. doi: 10.1016/s0168-3659(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 48.Ma Y, Zheng Y, Liu K, et al. Nanoparticles of poly(lactide-co-glycolide)-D-a-tocopheryl polyethylene glycol 1000 succinate random copolymer for cancer treatment. Nanoscale Res Lett. 2010;5:1161–1169. doi: 10.1007/s11671-010-9620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng SS, Mei L, Anitha P, Gan CW, Zhou WY. Poly(lactide)–vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of docetaxel. Biomaterials. 2009;30:3297–3306. doi: 10.1016/j.biomaterials.2009.02.045. [DOI] [PubMed] [Google Scholar]