Abstract
BACKGROUND & AIMS
Helicobacter pylori infection is a risk factor for gastric cancer. Ammonia/ammonium (A/A) is a cytotoxin generated by H pylori that kills gastric epithelial cells. We investigated whether A/A cytotoxicity occurs by activating N-methyl D-aspartate (NMDA) channels, which results in Ca2+ permeation and epithelial cell death.
METHODS
Gastric epithelial cells were cultured to confluence and then incubated with A/A and NMDA channel or cell signaling antagonists. Cells were incubated with wild-type H pylori or mutant strains that do not produce A/A. Changes in intracellular Ca2+ were examined in living cells by confocal microscopy. Biochemical and histochemical techniques were used to examine the relationship between A/A-induced cell death and intracellular levels of Ca2+.
RESULTS
A/A increased Ca2+ permeation in gastric epithelial cells; the increase was blocked by NMDA receptor and cell signaling antagonists. Wild-type, but not mutant H pylori, also caused extensive Ca2+ permeation of gastric epithelial cells, which was blocked when NMDA receptor expression was repressed. Ca2+ that entered cells was initially cytoplasmic and activated proteases. Later, the Ca2+ was sequestered to cytoplasmic vacuoles that are dilatations of the endoplasmic reticulum (ER). Inositol -3-phosphate–dependent release of Ca2+ from ER and protease activity damaged mitochondria, reduced levels of ATP, and transcriptionally up-regulated cell death effectors. Expression of the NMDA receptor was altered in stomachs of mice infected with H pylori.
CONCLUSIONS
A/A affects gastric epithelial cell viability by allowing excessive Ca2+ permeation through NMDA channels. NMDA channels might thereby regulate cell survival and death pathways during development of gastric cancers associated with H pylori infection.
Keywords: stomach cancer, apoptosis, tumorigenesis, calcium IP3 signaling
BACKGROUND & AIMS
Infection with HP occurs in 50% of the world’s population and is a major risk factor for gastric cancer development. Gastric cancer is 5.5% of the global cancer burden and is the 2nd most common cause of cancer deaths worldwide 1, 2. HP express a number of virulence factors including members of the cag pathogenicity island, vacA producing vacuolating cytotoxin, lipopolysaccharide, and A/A which individually or together are thought to facilitate cancer development. The ure genes of HP are required for expression of urease, which facilitates the production of A/A from urea in the gastric juice 3. HP also express enzymes that hydrolyze asparagine and glutamine, further increasing A/A production 4, 5. A/A production is essential for HP colonization and persistence of infection 6, but is cytotoxic to gastric epithelial cells.
The concentration of A/A in gastric juice is normally 0.5–0.7 mM but can increase to more than 20 mM in patients infected with HP 7, 8. When cultured human gastric cells are incubated with urease positive HP and urea, about 20 mM A/A is generated in 2 hr and 40 mM in 24 hr and the viability of cells is reduced as the A/A concentration increases 9. In vivo 10, 11 and in vitro 12–14 studies confirmed that A/A, per se, also reduces the viability of gastric epithelial cells. Two hypotheses have been suggested to explain the effects of A/A on gastric epithelial cells. First, A/A generated by urease in the presence of urea rapidly increases intracellular pH, which elicits an associated brief spike of intracellular Ca2+ that was shown to be derived from intracellular stores 15. The lipid solubility of ammonia allows it to enter cells, resulting in an increase in intracellular pH 16. Alternatively, it was suggested that A/A accelerates the conversion of glutamine to glutamate by glutamine synthetase, which depletes cells of ATP and reduces cell viability 17. It is not fully understood why the viability of many cancer-derived epithelial cells is not affected by A/A when compared to non-transformed cells incubated with the same dose and time 18–20. Thus, we asked whether specific mechanisms exist to render non-transformed epithelial cells susceptible to ammonia cytotoxicity that are modified in gastric cancer.
NMDA channels in neurons are heterotetramers consisting of NR1 and NR2 (2A–2D) subunits, which serve as glutamate-gated ion channels that transport Ca2+ 21, 22. A/A is thought to activate NMDA channels in neurons by depolarizing the cell membrane, which increases NMDA channel-mediated Ca2+ permeation in the absence of receptor agonists, like glutamate and glycine 23. Neuronal cell death occurs when A/A is in high concentration because Ca2+ permeation through NMDA channels occurs at non-physiological rates 23. Because gastric cells are exposed to high A/A concentrations in HP infection, our aim was to determine if A/A-induced NMDA channel activation regulates Ca2+ permeation and epithelial cell death in gastric cells that can be blocked by NMDA channel antagonists. NMDA channel subunits are transcriptionally down-regulated in gastric cancer and in many gastric cancer cell lines 24, suggesting that transformed cells would be less responsive to A/A while it rapidly kills non-transformed cells in a dose-dependent manner. This work may thus provide important insights into how HP-infection affects apoptosis rates to support cancer development in vivo.
MATERIALS AND METHODS
Cell Culture, Transfection, and Incubation with HP
RGM1 cells were cultured and experiments were performed in STD buffer as described previously 13. MKN28 cells were cultured and then incubated with the cag+toxigenic HP strain 60190 or its isogenic ureB mutant as described by Wroblewski et al 19. Cultured cells were plated on 35 mm culture dishes with an affixed cover glass (Matteck, Ashland, MA) for live cell microscopy studies.
MKN28 cells were transfected with a control or NMDAR2B-pDP3 expression plasmid (kindly provided by Dr. John J. Woodward, Medical University of South Carolina) with FuGENE 6 as described by Liu et al 24. Transfection efficiency was 53.9 ± 0.3% (n=3). Cells were used 2 days after transfection. Experiments with MKN28 cells and HP were done for 48 hr in RPMI media without antibiotics but containing 10% serum.
Live-Cell Microscopy
Studies in living cells were done using a ZEISS LSM510 META laser scanning confocal system outfitted with a Life Imaging Services Cube and Box incubator chamber and O2 and CO2 control using specific probes. The details of the laser configurations, probes, and post-acquisition image processing are reported in Supplementary Materials and Methods.
Western Blots
After experiments, Western blots of RGM1 cells were prepared as described previously 25. Filters were incubated with anti-NR2B (Alomone Labs, Jerusalem, Israel), anti-BAX (BD Pharmingen, San Jose, CA), or anti-BAK (Santa Cruz, Santa Cruz, CA). The filter was stripped and re-probed with a β–actin antibody (Sigma-Aldrich). The resulting bands were quantified using ImageJ with the β–actin band used to normalize loading.
Immunohistochemistry
RGM 1 cells were fixed in 4% formaldehyde and then stained with anti-cytochrome c oxidase subunit 1 (Invitrogen) or with anti-NR2B antibody (Abcam, Cambridge,MA) and the resulting images were quantified as reported in Supplementary Materials and Methods.
Paraffin sections from control or HP-infected mice were stained using an anti-NR2B antibody (Abcam). For co-localization studies, anti-intrinsic factor (a kind gift from Dr. David Alpers, Washington University, St. Louis, MO) staining was done to localize chief cells and Dolicho biflorus agglutinin (Invitrogen) was used to localize parietal cells. Archived tissues were used at 6, 12, or 20 wkPI from C57BL/6 mice (female) infected with HP as described previously 25,26.
Cell Viability
The viability of RGM 1 cells was evaluated by a colorimetric assay using crystal violet as described previously 13.
ATP and Proteases
ATP was measured with the CellTiter-Glo kit (Promega, Madison, WI) in cells incubated for 120 min with 20 mM NH4Cl at pH 7.4 with or without inhibitors. Calpain and cathepsin B activation was measured in cells at 0, 10, 30, 60 and 120 min after the addition of 20 mM NH4Cl at pH 7.4 with or without E64d (10 µM-Biomol, Plymouth Meeting, PA), a calpain and cathepsin B inhibitor, using specific kits (Invitrogen).
Statistics
Results are expressed as mean ± SEM and were analyzed using SigmaStat software (SPSS, Inc., Chicago, IL). Data with a single treatment were analyzed by one-way ANOVA. Data to compare differences in multiple treatments over time were analyzed by 2-way ANOVA. The Student-Neuman-Keuls post-hoc test was used to determine differences between means. If variances were not normally distributed, the analysis was done on ranks.
RESULTS
NMDA Channel Activation occurs after Exposure to NH4Cl or HP-generated AA to Induce Ca2+-Permeation in Gastric Epithelial Cells
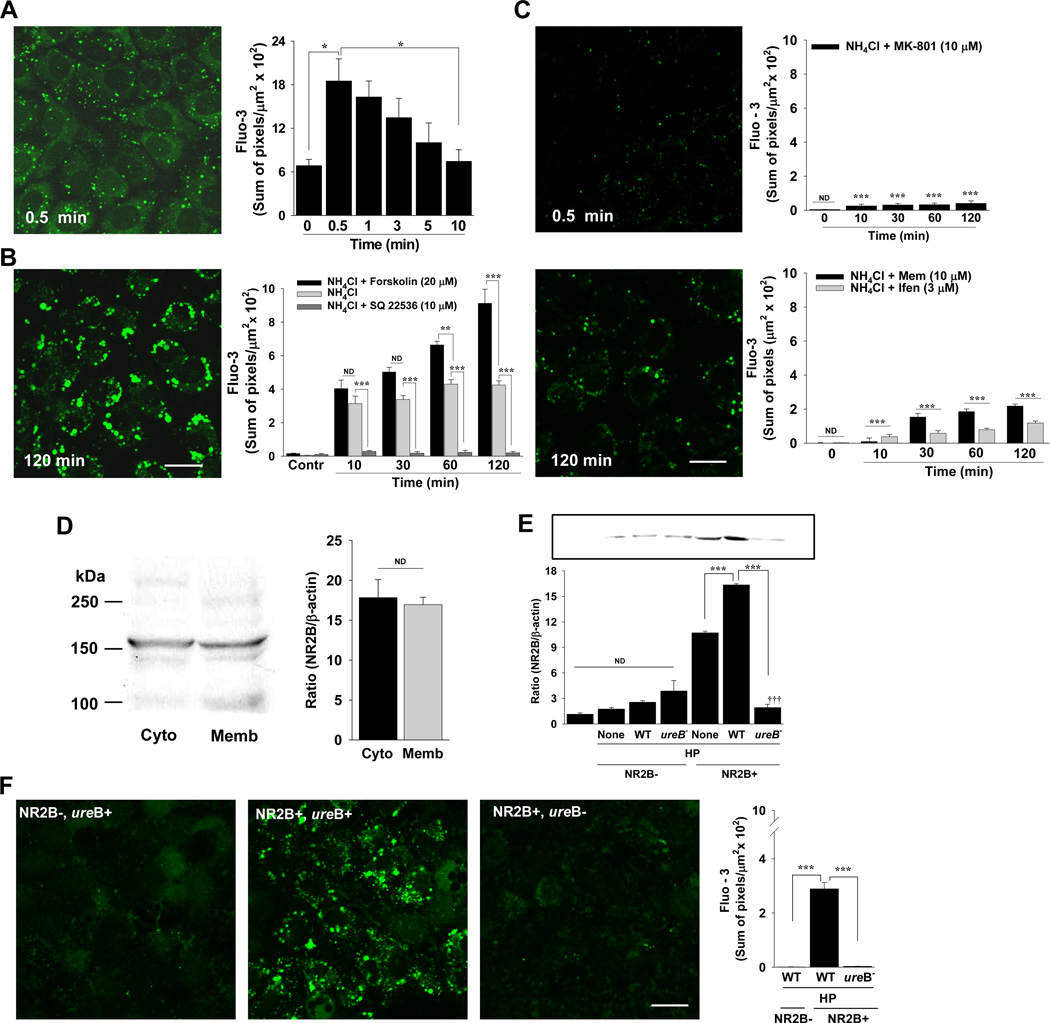
Immediately after the addition of A/A to RGM 1 cells, there was a weak and diffuse fluo-3 fluorescence signal in the cytoplasm that peaked and then decreased to control levels by 10 min (Figure 1A). The fluo-3 signal was also detected in a vacuolar compartment that expanded significantly (P < 0.001) in size (perimeter of 3.56 ± 0.73 µm at 0.5 min to 6.67 ± 0.73 µm at 120 min, n=7), and intensity (Figure 1A and B) over time. The sum of fluo-3 signal in vacuoles increased from 10–120 min in the presence of A/A alone (Figure 1B), but could be amplified significantly in the presence of forskolin, which increases cAMP (Figure 1B). Likewise, fluo-3 signal in vacuoles with A/A exposure (no forskolin) was attenuated to near control levels when cAMP was blocked with SQ 22536 (Figure 1B).
Figure 1.
Ca2+ influx during A/A exposure occurs by NMDA channel activation in gastric epithelial cells. (A) Fluorescence and quantification of cytoplasmic fluo-3 signal after the addition of A/A. n=3; *P < 0.05 compared to 0.5 min. (B) Fluorescence and quantification of fluo-3 signal in vacuoles in the presence of A/A. Fluo-3 in vacuoles was also measured with forskolin, which increases cAMP, and with SQ 22536, which is a cAMP antagonist. Control (Contr) are cells imaged with vehicle, forskolin, or SQ without A/A. n ≥ 7; ** P < 0.01, *** P < 0.001, ND, not different; comparisons are as indicated by brackets; scale bar, 20 µm for A and B. (C) Fluorescence and quantification of fluo-3 signal in vacuoles with A/A and MK-801, memantine (Mem), or ifenprodil (Ifen). n = 3; *** P < 0.001; ND, not different; comparisons to time-matched data for A/A in Fig. 1B; scale bar, 20 µm. (D) Western blot and quantification of NR2B in membrane-associated (Memb) or cytosolic (Cyto) fractions of RGM 1 cells as a ratio of band density to beta-actin. (E) Western blot and quantification of NR2B in whole cell extracts from native MKN28 cells, MKN28 cells transfected with the control plasmid (NR2B−), or MKN28 cells transfected with the NMDAR2B expression plasmid (NR2B+) and incubated without (None) or with wild-type (WT) HP or its isogenic ureB mutant (ureB−). n = 2 different sets of transfected cells; *** P < 0.001; ND, not different; comparisons are as indicated by brackets; ††† P < 0.001 compared to both None and WT cells incubated with HP. (F) Fluorescence and quantification of fluo-3 signal from MKN28 cells without (NR2B−) or with (NR2B+) NR2B expression at 48 hr after incubation with WT (ureB+) or mutant (ureB−) HP as described in E. n = 2 different sets of transfected cells; *** P < 0.001; comparisons are as indicated by brackets; scale bar, 20 µm.
MK-801, a selective and high affinity NMDA receptor antagonist, has been used extensively to block NMDA channel activation in vitro and in vivo in animal models 24, 27–29. We thus used MK-801 to show that the intracellular Ca2+ signal with A/A exposure was due to extracellular Ca2+ permeation through NMDA channels. When cells were exposed to A/A in the presence of MK-801, the initial diffuse calcium signal and the bright fluorescence in vacuoles was significantly attenuated (Figure 1C). Likewise, the NMDA channel antagonist memantine and the highly specific NR2B subunit antagonist ifenprodil blocked the initial cytoplasmic signal (not shown) and bright fluorescence in vacuoles (Figure 1C). The NR2B receptor subunit of NMDA channels is found in both cytoplasmic and membrane-associated compartments in RGM 1 cells, as shown in Western blots (Figure 1D). The molecular weight of NR2B in brain is about 166 kDa and we see a strong band at that molecular weight in RGM 1 cells (Figure 1D). Immunostaining of non-permeabilized RGM1 cells showed that NR2B subunits are expressed in the apical cell cytoplasm and are localized to the apical, rather than basolateral surface (Supplementary Figure 1).
To determine if HP infection also results in AA-induced Ca2+ permeation through NMDA channels, we used human MKN28 gastric cancer epithelial cells incubated with wild-type HP strain 60190 or its isogenic ureB− mutant. Because MKN28 cells transcriptionally down-regulate NR2B but express all other NMDA receptor subunits 30, we transfected cells with an NR2B expression plasmid that significantly increased NR2B protein expression (Figure 1E). When NR2B- cells were incubated with wild-type HP (ureB+), there was virtually no fluo-3 signal (Figure 1F). In contrast, NR2B+ cells incubated with wild-type HP (ureB+) showed significant fluo-3 fluorescence in an intracellular compartment (Figure 1F), identical to the results with AA alone. Additionally, these cells also showed a significant increase in NR2B protein expression (Figure 1E). Results with NR2B+ cells incubated with the HP ureB− mutant showed a significant attenuation of Ca2+ permeation through NMDA channels (Figure 1F). This mutant also blocked the plasmid-induced expression of NR2B (Figure 1E).
NMDA Channel Activation Reduces Cell Viability During A/A Exposure
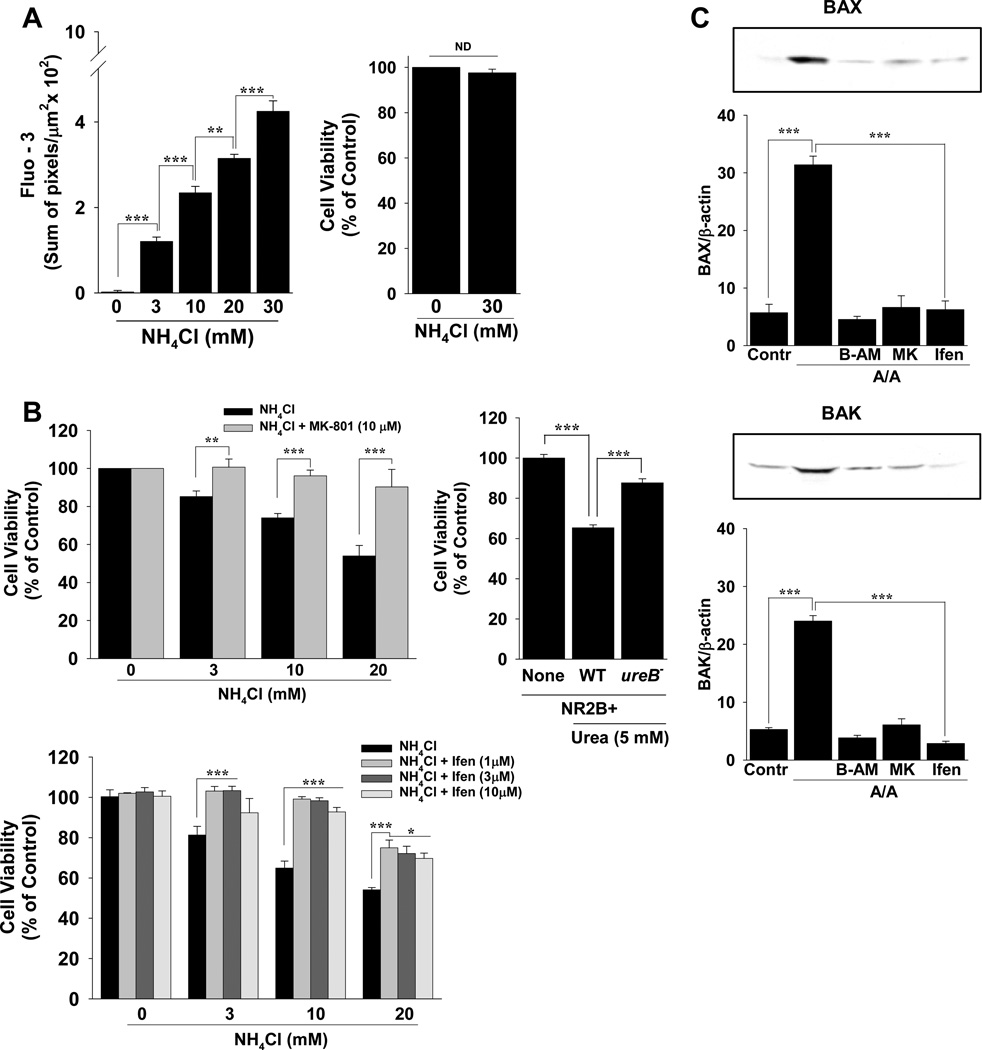
Ca2+ permeation into vacuoles increased dose-dependently from 3–30 mM NH4Cl but this rise in Ca2+ had no effect on cell viability at 120 min (Figure 2A). Long-term (24 hr), however, A/A dose-dependently reduced cell viability in RGM1 cells (Figure 2B) as did A/A produced in 48 hr from urea in the presence of wild-type HP but not ureB− mutant HP in NR2B+ MKN28 cells (Figure 2B). This reduction in cell viability with up to 20 mM NH4Cl was could be reversed with the NMDA channel antagonists MK-801 or ifenprodil (Figure 2B), or significantly improved with memantine (not shown). Because A/A reduces cell viability by activating apoptosis in RGM 1 cells 13, and BAX and BAK are the pro-apoptotic effectors in gastric surface and pit/neck cells 25, we tested whether A/A-induced NMDAR activation transcriptionally regulates BAX and BAK expression in the presence of A/A (Figure 2C). While BAX and BAK expression increased more than 6-fold with A/A exposure, this expression was attenuated to control levels or below by buffering intracellular Ca2+ with BAPTA-AM or by inhibiting NMDA receptor activation with MK-801 or ifenprodil (Figure 2C).
Figure 2.
Long-term (24 hr) A/A-induced NMDA channel activation reduced cell viability and regulated the transcription of apoptosis genes. (A) Quantification of fluo-3 signal in vacuoles at 120 min. At the end of microscopy experiments, cell viability was measured. (B) Cell viability at 24 hr with A/A and MK-801 or ifenprodil (Ifen). MKN28 cells were transfected with the NMDAR2B expression plasmid (NR2B+) and incubated without HP (None) or with 5 mM urea and wild-type (WT) HP or its isogenic ureB mutant (ureB−). (C) Cells were incubated with A/A and BAPTA-AM (B-AM), MK-801 (MK), or ifenprodil (Ifen). Band intensity for BAX or BAK was quantified as a ratio to beta-actin. n ≥ 3 experiments per condition; *P < 0.05, ** P < 0.01, *** P < 0.001, ND, not different; comparisons as indicated by brackets.
Ca2+ Permeation by A/A-induced NMDA Channel Activation Damages Mitochondria and Reduces Intracellular ATP
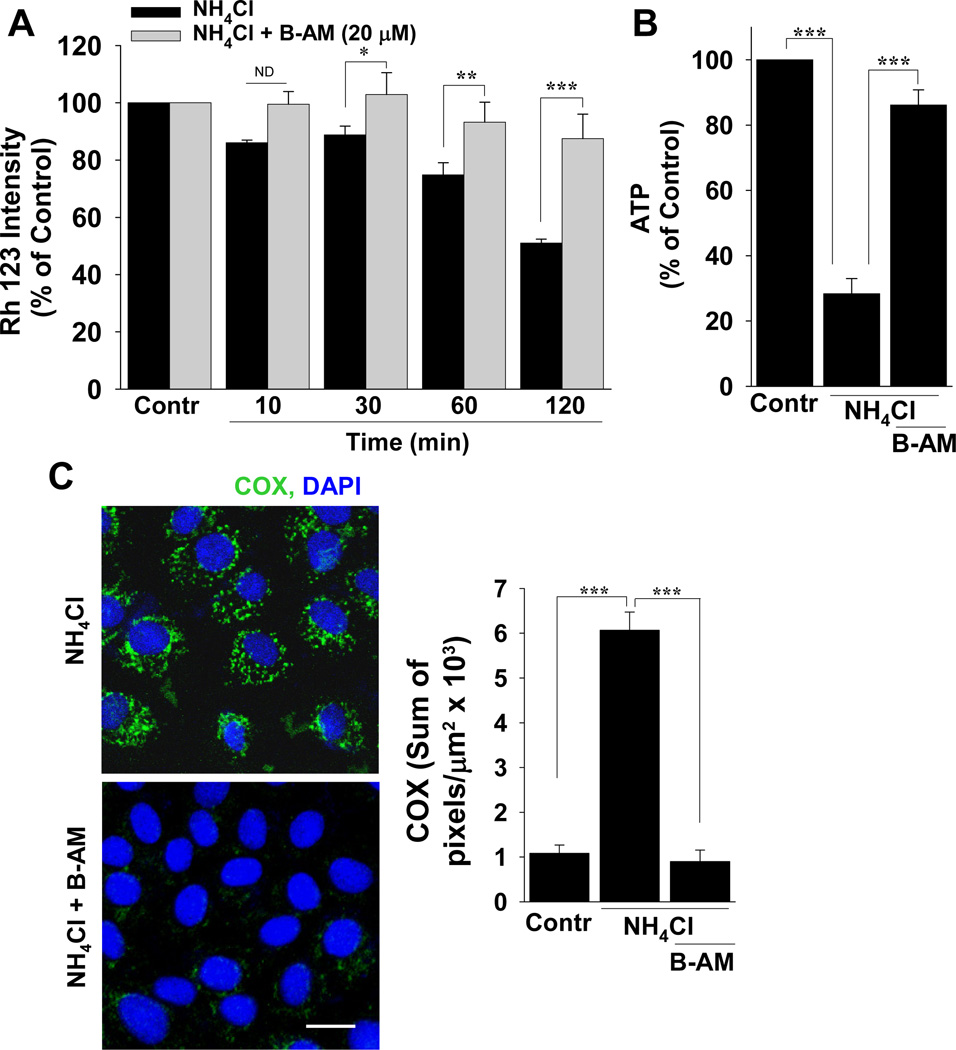
When A/A was added to live cells pre-loaded with Rh 123, the fluorescence intensity of Rh 123 was reduced significantly (Figure 3A). Concomitantly, intracellular ATP was also reduced significantly (Figure 3B). The intracellular calcium chelator BAPTA-AM was used to buffer the effects of Ca2+ permeation during A/A exposure and it not only restored mitochondrial membrane potential (Figure 3A) but improved intracellular ATP (Figure 3B). With A/A exposure, there was bright COX fluorescence in mitochondria (Figure 3C), and this fluorescence was reduced to control levels by BAPTA-AM (Figure 3C).
Figure 3.
Ca2+ from A/A-induced NMDA channel activation structurally and functionally damages mitochondria. (A) Untreated (live) RGM1 cells were incubated with Rh 123 to measure mitochondrial membrane potential (Contr). Membrane potential was also measured for 10–120 min during A/A exposure (NH4Cl) with or without BAPTA-AM (B-AM). (B) At the end of the experiment in A, ATP was measured or (C) cells were lightly fixed and incubated with anti-COX (green). n ≥ 3; *P < 0.05, ** P < 0.01, *** P < 0.001, ND, not different; comparisons as indicated by brackets; scale bar, 20 µm.
A/A-induced Cytoplasmic “Vacuoles” are Dilatations of the ER Containing Ca2+
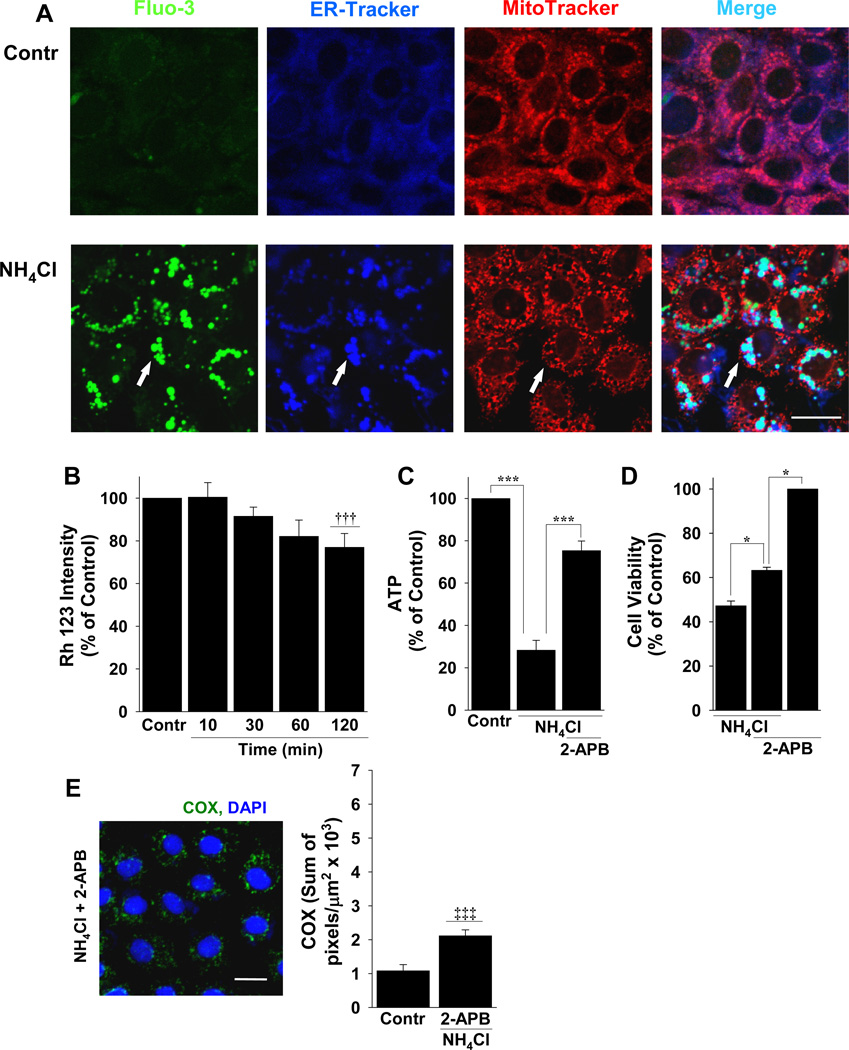
To determine if Ca2+ is taken-up by mitochondria or another intracellular compartment, we used live cell microscopy with RGM 1 cells that were incubated with fluo-3 in the presence of both ER-Tracker and MitoTracker. After the addition of A/A, the ER compartment was vesicular and in merged images from cells treated with A/A, there was co-localization of fluo-3 with ER but not mitochondria (Figure 4A). The incidence of co-localization of ER-Tracker and fluo-3 during A/A exposure was 78.53 ± 1.34% (n=3), with the remaining fluo-3 signal in small vacuoles that were not ER or mitochondria (Figure 4A). Stimulating NMDA channel activation with NMDA and glycine also resulted in fluo-3 accumulation in ER-derived vacuoles that was blocked completely with ifenprodil (Supplementary Figure 2).
Figure 4.
A/A-induced NMDA-mediated Ca2+ localizes to ER and is transferred to mitochondria, causing mitochondrial damage and cell death. (A) Untreated (live) RGM1 cells (Contr) or cells treated with A/A (NH4Cl) were incubated simultaneously with Fluo-3 (green), ER-Tracker (blue), and MitoTracker (red) and the merged images (turquoise) were used to examine the co-localization of fluo-3 with the ER or mitochondria. 2-APB, was used to block the transfer of Ca2+ from ER to mitochondria and the resulting effect this had on (B) mitochondrial membrane potential with Rh 123, (C) ATP production, (D) cell viability, and (E) mitochondrial membrane damage by staining with anti-COX (green). n ≥ 3; * P < 0.05 and *** P < 0.001; comparisons as indicated by brackets; ††† P < 0.001 compared to A/A alone in Fig. 3A; ‡‡‡ P < 0.001 compared to A/A in Fig. 3C; scale bars, 20 µm.
Because Ca2+ was sequestered to the ER, it is possible that damage to mitochondria occurs by the release of ER Ca2+ to mitochondria in the presence of A/A by IP3 receptor activation 31. To test this idea, we pre-incubated RGM 1 cells with the IP3 receptor antagonist 2-APB, incubated cells with A/A in the continued presence of 2-APB, and then measured the mitochondrial membrane potential using Rh 123 in live cells (Figure 4B). The results showed that in contrast to A/A alone (Figure 3A), 2-APB significantly improved the A/A-induced decrement in mitochondrial membrane potential (Figure 4B), reduction in ATP (Figure 4C), cell viability (Figure 4D), and mitochondrial outer membrane damage evaluated by COX immunolabeling (Figure 4E).
A/A-induced NMDA Channel Activation Increases Ca2+-Activated Proteases
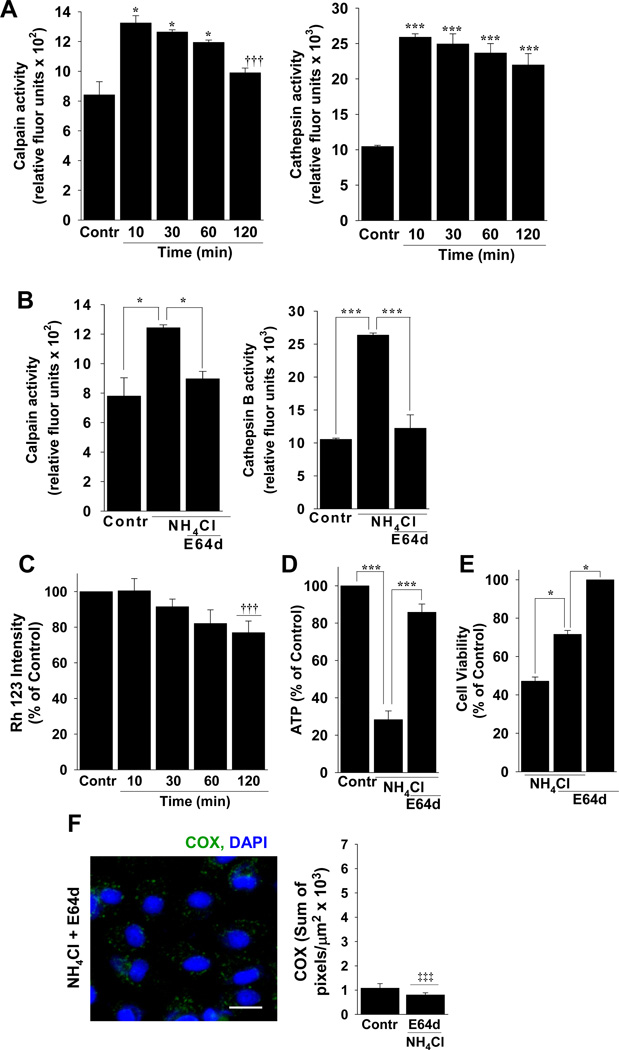
Because the initial intracellular calcium concentration increased significantly after A/A exposure, we examined the role of Ca2+-activated protease on mitochondrial damage and cell viability (Figure 5). Calpain is linked to neurotoxic NMDA receptor activation 32 and cathepsin B is released from lysosomes to affect mitochondrial function and integrity when intracellular Ca2+ increases 33. Immediately after the addition of A/A to cells, both calpain and cathepsin B were activated (Figure 5A). To test whether the level of calpain and cathepsin B activation as sufficient to affect mitochondrial function, we measured mitochondrial membrane potential using Rh 123 with A/A in the presence of E64d, a calpain and cathepsin B inhibitor that effectively reduced the activity of both enzymes to control levels in the presence of A/A (Figure 5B). With E64d, mitochondrial membrane potential improved significantly (compare Figures 5C and 3A). Intracellular ATP (Figure 5D), cell viability (Figure 5E), and mitochondrial permeability to anti-COX (Figure 5F) also improved significantly by blocking calpain and cathepsin B activity with E64d.
Figure 5.
NMDA-induced Ca2+ permeation activates proteases that contribute to mitochondrial dysfunction during A/A exposure. (A) Calpain and cathespin B activity in control (Contr) cells or at 10–120 min after exposure to 20 NH4Cl. n = 3 experiments per time point; * P < 0.05 and *** P < 0.001 compared to control, ††† P < 0.001 compared to 10 min. (B) Blockade of calpain and cathepsin B with E64d. n=3; * P < 0.05, *** P < 0.001, comparisons as indicated by brackets. Effects of E64D on (C) mitochondrial membrane potential, (D) intracellular levels of ATP, (E) cell viability, and (F) damage to the inner mitochondrial membrane evaluated by staining with anti-COX (green) and DAPI to stain nuclei (blue). n ≥ 3 experiments per condition; * P < 0.05, *** P < 0.001; comparisons as indicated by brackets; †††P < 0.001 compared to NH4Cl alone in Fig. 3A; ‡‡‡ P < 0.001 compared to NH4Cl alone in Fig. 3C, scale bar, 20 µm.
NMDA Receptor Expression is Altered in H. pylori Infection in vivo
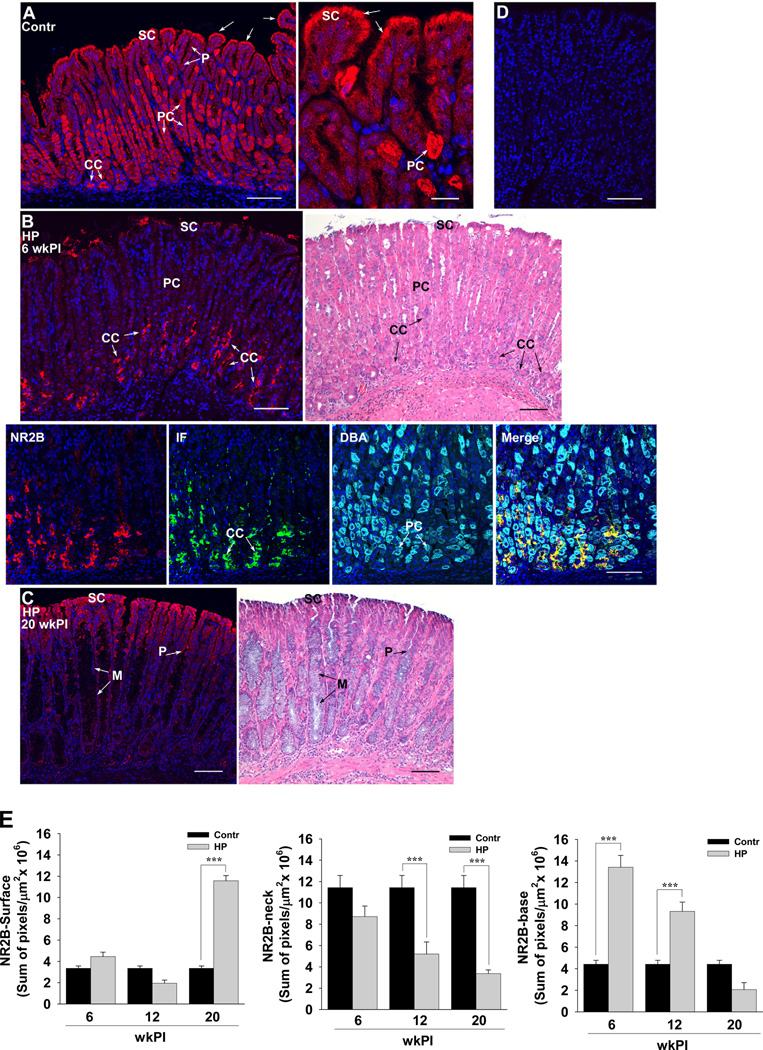
In the in vivo mouse stomach, NR2B receptor subunits of NMDA channels are highly expressed in gastric parietal cells and are moderately expressed in surface and chief cells (Figure 6A and E). While the expression of NR2B was present at 6 (Figure 6B and E) and 12 (Figure 6E) weeks post infection (wkPI) in surface cells most terminally differentiated at the luminal surface, its expression increased significantly at 20 wkPI and was localized along the surface and the length of gastric pits (Figure 6C and E). The same expression pattern was found in the antrum (Supplementary Figure 3). Parietal cells were present in gastric glands at 6 wkPI (Figure 6B, specifically identified in the DBA panel) but NR2B was only minimally expressed in these cells (Figure 6B and E). Instead, NR2B was highly expressed at the base of gastric glands in IF-positive chief cells (Figure 6B and E). At 12 (not shown) and 20 wkPI, gastric glands from HP-infected mice with metaplastic cells, which are epithelial precursor cells for gastric cancer 34, showed little to no NR2B expression (Figure 6C). The immunolocalization of NR2B in gastric tissue sections was specific, as shown by using the NR2B peptide to inhibit (block) antibody binding (Figure 6D).
Figure 6.
NMDA channel subunit NR2B expression in surface, parietal, and chief cells is transcriptionally regulated in HP-infected tissues. Paraffin-embedded tissues from (A) sham- (Contr-20 wkPI) or (B) 6 and (C) 20 wkPI HP-infected mice were stained for the NR2B subunit (red) of NMDA channels, intrinsic factor (IF) to localize chief cells (green), dolichos biflorus agglutinin (DBA) to localize parietal cells (turquoise), and DAPI to identify nuclei (blue). In B-Merge, co-localization of IF and NR2B is yellow (arrows). (D) A serial section from the tissue in B (6 wkPI HP-infected) was incubated with a 100-fold excess of NR2B peptide to identify non-specific staining. In B and C, serial sections were also H&E stained and included for orientation. (E) Quantification of the sum of fluorescence pixels in tissues from control (Contr) or HP infected mice at 6, 12, and 20 weeks post infection (wkPI). n ≥ 6 mice per condition; *** P < 0.001; comparisons as indicated by brackets; CC, chief cells; P, gastric pit; PC, parietal cells; SC, surface epithelial cells; arrows, luminal expression of NR2B; scale bars, 20 µm (low-magnification images in A–D) or 100 µm (high magnification image in A).
DISCUSSION
This study shows, for the first time, that brain-specific NMDA channels are responsible for the cytotoxic effects of A/A on gastric epithelial cells. NMDA channel activity in brain has long been known to transport Ca2+ with transport rates dependent on cAMP and PKA 22. Furthermore, it is well-established that excessive A/A exposure in brain activates NMDA channel activity resulting in neurotoxicity 23, 29, 35. Our results in gastric RGM1 cells in vitro are consistent with these observations. We demonstrate that NMDA channel activation by AA, in particular NMDA channels with NR2B-containing subunits, rapidly increases intracellular Ca2+ in a cAMP-dependent manner, which is taken-up into the ER and transferred to mitochondria. This large pool of ER Ca2+ and its IP3-dependent transfer to mitochondria results in outer mitochondrial membrane damage and ATP depletion, thus reducing epithelial cell viability in the presence of A/A. Additionally, we showed that Ca2+ transcriptionally regulates the expression of cell death effectors and that Ca2+-activated calpain and cathepsin B participate in the cytotoxic effects of A/A. Overall, our results provide strong new evidence that A/A cytotoxicity in gastric epithelial cells has a distinct, channel-mediated mechanism that can be blocked with channel inhibitors and, in the continued presence of high levels of A/A, inhibition of NMDA channel activity protects gastric epithelial cells from A/A-induced cell death. Our results also suggest that the majority of “vacuoles” demonstrated in cells incubated with A/A are dilatations of the ER and not Golgi-derived vacuolar compartments. These novel results provide a new context with which to view the role of A/A in HP infection in vivo, and may provide important new insights into HP-induced gastric cancer development.
Apical surface expression of NMDA channels, which we showed to occur in surface epithelial cells from the antrum and corpus in vivo and in RGM 1 cells in vitro, would allow direct access of gastric juice A/A to NMDA channels in these cells. Because we show that wild-type HP expressing ureB significantly increases the expression of NR2B subunits in vitro and in vivo, it is likely that NMDA channel activation in HP infection is greater than in normal mucosa. This result suggests that apoptosis in non-transformed gastric surface cells, which is driven by Ca2+-induced mitochondrial dysfunction, may be related directly to the amount of A/A liberated by HP. Additionally, surface expression of NMDA channels in gastric cells may constitutively facilitate the ability of dietary amino acids like glycine and glutamate, which are the NR1 and NR2 channel agonists, respectively, to have a direct effect on the rate of Ca2+-influx and thus on cell function and viability. NMDA channel activation by glutamate may also contribute to apoptosis rates in HP infection, since gastric juice glutamate levels are increased more than 2-fold in patients with gastritis and nearly 10-fold in HP-infected patients with early gastric cancer 36.
Although A/A-induced Ca2+-influx kills gastric epithelial cells in culture, it could be argued that NMDA channel activation early in HP infection serves a protective function in vivo by increasing the rate of cell turnover by apoptosis. In primary tumors from patients with gastric cancer, the NR2B subunit of NMDA channels is not expressed due, in part, to promoter methylation 24. Determined in a large, pharmacological unmasking and microarray analysis study, methylation of NR2B was a specific event in gastric cancer, with the highest methylation status in diffuse and intestinal-type gastric cancers 24. Additionally, NR2B methylation in normal adjacent tissues was suggested to be an important risk factor and molecular marker for tumor formation 24, suggesting that NR2B normally functions as a tumor suppressor. NR2B promoter methylation, resulting in the significant attenuation of NR2B mRNA expression, also occurs in gastric cancer cell lines 24, 30, which we demonstrated in this study to block NR2B protein expression and NMDA receptor-mediated Ca2+ permeation in MKN28 cells. We additionally demonstrate in RGM1 cells and in MKN28 cells with induced NR2B expression, that the effects of A/A are directly related to NMDA channels with NR2B subunit expression. Although it is not known what function NMDA channels provide normally in epithelial cells, studies by others in gastric cancer cells suggest that promoter methylation and down-regulation of the NR2B subunit, in particular, limits apoptosis and cell renewal to enhance cancer progression 24. The reduction in apoptosis that fosters bacterial persistence later in infection 37 may parallel the timing of NR2B promoter methylation in gastric cells. This interesting possibility should be examined further.
How A/A facilitates NMDA receptor activation is largely unknown, although is thought to occur by an A/A-induced depolarization of cell membrane potential, which also occurs with high K+ 23, 38. In cultured brain cells, 5 mM NH4Cl immediately decreased resting membrane potential from −96.2 to −89.1 mV, and 10 and 20 mM NH4Cl further reduce resting membrane potential to −66.3 and −50.4 mV, respectively 39. This A/A-induced depolarization of membrane potential is thought to release the Mg2+ block that normally inhibits channel activity so that Ca2+ is able to enter cells 23. This may explain why the sum of fluo-3 and the size of fluo-3 containing vacuoles with A/A exposure in our study were dependent on the A/A concentration. Additionally, A/A was recently shown to dose-dependently increase cAMP-PKA levels in cells 40 and Skeberdis et al 22 reported that cAMP-PKA is required to gate NMDA channels in brain. As such, the rate of NMDA-mediated Ca2+ permeation with A/A exposure may also depend on the level of intracellular cAMP-PKA that is generated. Our results in gastric cells are consistent with this idea as we showed that A/A-induced Ca2+ permeation is inhibited by cAMP inhibitors and that increasing cAMP with forskolin significantly increased Ca2+ permeation in the presence of A/A.
Cell signaling pathways involving A/A-induced cAMP-PKA may additionally function in regulating the membrane expression of receptors, thus influencing the rate of Ca2+ permeation. We show here that NR2B is localized to the cell membrane but in Western blots and in our immunostained images in vitro and in vivo it is clear that cytoplasmic localization of NR2B is also present. When the NR2A and NR2B subunits of NMDA receptors were transfected into HeLa cells either as heterotetramers or homotetramers, NR2B subunits were associated with Rab11-containing recycling endosomes that were cytoplasmic and played a dominant role in receptor trafficking to and from the membrane 41. Roma et al 42 recently showed that both Ca2+ and cAMP are required to move cytoplasmic recycling endosomes to the surface, suggesting that cAMP generated by A/A may play a role not only in channel gating but in regulating the expression of receptors at the apical surface.
In conclusion, gastric epithelial cells express NMDA channels that, in gastric RGM1 cells, regulate Ca2+-influx and cell viability in the presence of A/A. Ca2+ permeation also occurs in a urease-dependent manner in HP-infected MKN28 cancer cells that express active NMDA channels. NMDA channels may play an important pro-apoptotic function in gastric surface epithelial cells, and may prove to be important overall in HP-induced gastric cancer progression by regulating the rate of epithelial cell survival and death.
Supplementary Material
Acknowledgements
The authors thank Dr. Lay-Hong Ang for doing the NR2B immunostaining, Drs. Yi Zheng and Lay-Hong Ang for help with live-cell confocal instrumentation, Dr. Zeli Shen for growing Helicobacter pylori, and Dr. John Woodward for providing the NMDAR2B expression plasmid.
Grant Support: R01DK015681, P30DK037854, and S10RR017927 (S.J.H.), R01DK058587, R01CA77955, and P01116037 (R.M.P.), and R01CA067463 and R01AI/RR037750 (J.G.F.).
Abbreviations used in this paper
- A/A
ammonia/ammonium generated from ammonium chloride (NH4Cl)
- COX
cytochrome c oxidase 1
- DBA
Dolicho biflorus agglutinin
- ER
endoplasmic reticulum
- HP
Helicobacter pylori
- IF
intrinsic factor
- IP3
inositol-3-phosphate
- NH4Cl
ammonium chloride
- NMDA
N-methyl d-aspartate
- RGM1
Rat gastric mucosal 1cells
- NR2B
N-methyl d-aspartate receptor subunit 2B
- Rh
Rhodamine
- ureB
urease subunit B gene
- wkPI
weeks post infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors declare no conflict of interest.
Author contributions: SJH contributed the idea, discussed and reviewed results, analyzed tissues, and wrote the manuscript. JHS collected and analyzed data. RMP provided the MKN28 cells and HP. JGF grew HP and did the HP studies in mice.
References
- 1.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Sachs G, Weeks DL, Melchers K, et al. The gastric biology of Helicobacter pylori. Ann Rev Physiol. 2003;65:349–369. doi: 10.1146/annurev.physiol.65.092101.142156. [DOI] [PubMed] [Google Scholar]
- 4.Stark RM, Suleiman MS, Hassan IJ, et al. Amino acid utilisation and deamination of glutamine and asparagine by Helicobacter pylori. J Med Microbiol. 1997;46:793–800. doi: 10.1099/00222615-46-9-793. [DOI] [PubMed] [Google Scholar]
- 5.Mendz GL, Hazell SL. Amino acid utilization by Helicobacter pylori. Int J Biochem Cell Biol. 1995;27:1085–1093. doi: 10.1016/1357-2725(95)00069-2. [DOI] [PubMed] [Google Scholar]
- 6.Leduc D, Gallaud J, Stingl K, et al. Coupled amino acid deamidase-transport systems essential for Helicobacter pylori colonization. Infect Immun. 2010;78:2782–2792. doi: 10.1128/IAI.00149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Triebling AT, Korsten MA, Dlugosz JW, Paronetto F, Lieber CS. Severity of Helicobacter-induced gastric injury correlates with gastric juice ammonia. Dig Dis Sci. 1991;36:1089–1096. doi: 10.1007/BF01297452. [DOI] [PubMed] [Google Scholar]
- 8.Verdu EF, Armstrong D, Sabovcikova L, et al. High concentrations of ammonia, but not volatile amines, in gastric juice of subjects with Helicobacter pylori infection. Helicobacter. 1998;3:97–102. doi: 10.1046/j.1523-5378.1998.08068.x. [DOI] [PubMed] [Google Scholar]
- 9.Smoot DT, Mobley HLT, Chippendale GR, Lewison JF, Resau JH. Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect Immun. 1990;58:1992–1994. doi: 10.1128/iai.58.6.1992-1994.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lichtenberger LM, Dial EJ, Romero JJ, et al. Role of luminal ammonia in the development of gastropathy and hypergastrinemia in the rat. Gastroenterology. 1995;108:320–329. doi: 10.1016/0016-5085(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 11.Tsujii M, Kawano S, Tsuji S, Ito T, Nagano K, Sasaki Y, Hayashi N, Fusamoto H, Kamada T. Cell kinetics of mucosal atrophy in rat stomach induced by long-term administration of ammonia. Gastroenterology. 1993;104:796–801. doi: 10.1016/0016-5085(93)91015-a. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki H, Yanaka A, Shibahara T, et al. Ammonia-induced apoptosis is accelerated at higher pH in gastric surface cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G986–G995. doi: 10.1152/ajpgi.00482.2001. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura E, Hagen SJ. Role of glutamine and arginase in protection against ammonia-induced cell death in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:1264–1275. doi: 10.1152/ajpgi.00235.2002. [DOI] [PubMed] [Google Scholar]
- 14.Hagen SJ, Takahashi S, Jansons R. The role of vacuolation in the death of gastric epithelial cells. Am J Physiol Cell Physiol. 1997;272:C48–C58. doi: 10.1152/ajpcell.1997.272.1.C48. [DOI] [PubMed] [Google Scholar]
- 15.Athmann C, Zeng N, Kang T, et al. Local pH elevation mediated by the intrabacterial urease of Helicobacter pylori cocultured with gastric cells. J Clin Invest. 2000;106:339–347. doi: 10.1172/JCI9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boron WF, Waisbren SJ, Bodlin IM, Geibel JP. Unique permeability barrier of the apical surface of parietal and chief cells in isolated perfused gastric glands. J Exp Biol. 1994;196:347–360. doi: 10.1242/jeb.196.1.347. [DOI] [PubMed] [Google Scholar]
- 17.Kubota Y, Kato K, Dairaku N, et al. Contribution of glutamine synthetase to ammonia-induced apoptosis in gastric mucosal cells. Digestion. 2004;69:140–148. doi: 10.1159/000078152. [DOI] [PubMed] [Google Scholar]
- 18.Igarashi M, Kitada Y, Yoshiyama H, et al. Ammonia as an accelerator of tumor necrosis factor alpha-induced apoptosis of gastric epithelial cells in Helicobacter pylori infection. Infect Immun. 2001;69:816–821. doi: 10.1128/IAI.69.2.816-821.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wroblewski LE, Shen L, Ogden S, et al. Helicobacter pylori dysregulation of gastric epithelial tight junctions by urease-mediated myosin II activation. Gastroenterology. 2009;136:236–246. doi: 10.1053/j.gastro.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amagase K, Nakamura E, Endo T, et al. New frontiers in gut nutrient sensor research: prophylactic effect of glutamine against Helicobacter pylori-induced gastric disease in Mongolian gerbils. J Pharmacol Sci. 2010;112:25–32. doi: 10.1254/jphs.09r11fm. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes HB, Baimbridge KG, Church J, et al. Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA-induced apoptosis in YAC128 model of Huntington's disease. J Neurosci. 2007;27:13614–13623. doi: 10.1523/JNEUROSCI.3455-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skeberdis VA, Chevaleyre V, Lau CG, et al. Protein kinase A regulates calcium permeability of NMDA receptors. Nature Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigo R, Cauli O, Boix J, et al. Role of NMDA receptors in acute liver failure and ammonia toxicity: therapeutical implications. Neurochem Int. 2009;55:113–118. doi: 10.1016/j.neuint.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Liu J-W, Kim MS, Nagpal J, et al. Quantitative hypermethylation of NMDAR2B in human gastric cancer. Int J Cancer. 2007;121:1994–2000. doi: 10.1002/ijc.22934. [DOI] [PubMed] [Google Scholar]
- 25.Hagen SJ, Yang DH, Tashima K, et al. Epithelial cell expression of BCL-2 family proteins predicts mechanisms that regulate Helicobacter pylori-induced pathology in the mouse stomach. Lab Invest. 2008;88:1227–1244. doi: 10.1038/labinvest.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagen SJ, Ohtani M, Zhou JR, et al. Inflammation and foveolar hyperplasia are reduced by supplemental dietary glutamine during Helicobacter pylori infection in mice. J Nutr. 2009;139:912–918. doi: 10.3945/jn.108.097790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javitt DC, Frusciante MJ, Zukin SR. Rat brain N-methyl-D-aspartate receptors requires multiple molecules of agonist for activation. Mol Pharmacol. 1990;37:603–607. [PubMed] [Google Scholar]
- 28.Javitt DC, Zukin SR. Biexponential kinetics of [3H]MK-801 binding: evidence for access to closed and open N-methyl-D-aspartate receptor channels. Mol Pharmacol. 1989;35:387–393. [PubMed] [Google Scholar]
- 29.Hermenegildo C, Marcaida G, Montoliu C, et al. NMDA receptor antagonists prevent acute ammonia toxicity in mice. Neurochem Res. 1996;21:1237–1244. doi: 10.1007/BF02532401. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe K, Kanno T, Oshima T, et al. The NMDA receptor NR2A subunit regulates proliferation of MKN45 human gastric cancer cells. Biochem Biophys Res Commun. 2008;367:487–490. doi: 10.1016/j.bbrc.2007.12.167. [DOI] [PubMed] [Google Scholar]
- 31.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 32.Hardingham GE. Coupling of the NMDA receptor to neruoprotective and neurodestructive events. Biochem Soc Trans. 2009;37:1147–1160. doi: 10.1042/BST0371147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27:6434–6451. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- 34.Goldenring JR, Nam KT, Wang TC, et al. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation ofmetaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosenko E, Llansola M, Montoliu C, et al. Glutamine synthetase activity and glutamine content in brain: modulation by NMDA receptors and nitric oxide. Neurochem Int. 2003;43:493–499. doi: 10.1016/s0197-0186(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 36.Nagata Y, Sato T, Enomoto N, et al. High concentrations of D-amino acids in human gastric juice. Amino Acids. 2007;32:137–140. doi: 10.1007/s00726-006-0262-9. [DOI] [PubMed] [Google Scholar]
- 37.Mimuro H, Suzuki T, Nagai S, et al. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptois, a bacterial strtegy to enhance colonization of the stomach. Cell Host Microbe. 2007;2:250–263. doi: 10.1016/j.chom.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Perez AM, Llansola M, Cauli O, et al. Modulation of NMDA receptors in the cerebellum: II. Signaling pathways and physiological modulators regulating NMDA receptor function. Cerebellum. 2005;4:162–170. doi: 10.1080/14734220510008003. [DOI] [PubMed] [Google Scholar]
- 39.Allert N, Köller H, Siebler M. Ammonia-induced depolarization of cultured rat cortical astrocytes. Brain Res. 1998;782:261–270. doi: 10.1016/s0006-8993(97)01288-2. [DOI] [PubMed] [Google Scholar]
- 40.Svoboda N, Zierler S, Kerschbaum HH. cAMP mediates ammonia-induced programmed cell death in the microglial cell line BV-2. Eur J Neurosci. 2007;25:2285–2295. doi: 10.1111/j.1460-9568.2007.05452.x. [DOI] [PubMed] [Google Scholar]
- 41.Tang TTT, Badger JD, Roche PA, et al. Novel approach to probe subunit-specific contributions to N-methyl-D-aspartate (NMDA) receptor trafficking reveals a dominant role for NR2B in receptor recycling. J Biol Chem. 2010;285:20975–20981. doi: 10.1074/jbc.M110.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roma JG, Crocenzi FA, Mottino AD. Dynamic localization of hepatocellular transporters in health and disease. World J Gastroenterol. 2008;14:6786–6801. doi: 10.3748/wjg.14.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.