Abstract
ECOG 1696 was a Phase II multi-center trial testing vaccination with melanoma peptides, gp100, MART-1 and tyrosinase delivered alone, with GM-CSF, IFN-α2b or both cytokines to HLA-A2+ patients with metastatic melanoma. Here, the frequency of circulating CD8+tetramer+ (tet+) T cells and maturation stages of responding T cells were serially monitored and compared with baseline values in a subset of patients (n = 37) from this trial. Multiparameter flow cytometry was used to measure the frequency of CD8+ T cells specific for gp100, MART-1, tyrosinase and influenza (FLU) peptides. Expression of CD45RA/CCR7 on CD8+tet+ T cells and CD25, CD27, CD28 on all circulating T cells was determined. Vaccine-induced changes in the CD8+tet+ T cell frequency and phenotype were compared with results of IFN-γ ELISPOT assays and with clinical responses. The frequency of CD8+tet+ T cells in the circulation was increased for the melanoma peptides (p < 0.03–0.0001) but not for FLU (p < 0.9). Only gp100- and MART-1-specific T cells differentiated to CD45RA+CCR7− effector/memory T cells. In contrast to the IFN-γ ELISPOT frequency, previously correlated with overall survival (Kirkwood et al., Clin Cancer Res 2009;15:1443-51), neither the frequency nor differentiation stage of CD8+tet+ T cells correlated with clinical responses. Delivery of GM-CSF and/or IFN-α2b had no effects on the frequency or differentiation of CD8+tet+, CD8+ or CD4+ T cells. Phenotypic analyses of CD8+tet+ T cells did not correlate with clinical responses to the vaccine, indicating that functional assessments of peptide-specific T cells are preferable for monitoring of anti-tumor vaccines.
Keywords: CD8+ T cells, immune monitoring, melanoma, peptide-based vaccine, tetramer
Introduction
An improvement in overall survival (OS) for patients with inoperable metastatic melanoma has been an elusive goal. Two recent clinical advances that appear promising are trials with inhibitors of T lymphocyte-associated antigen 4 (CTLA-4) and oncogenic BRAF.1,2 Ipilimumab, which blocks CTLA-4 activity (1) and inhibitors of BRAF such as PLX4032 (2) were both reported to improve OS in patients with metastatic melanoma (1). Nevertheless, long-term therapeutic control of melanoma will require integration of specific active immune-therapy with these novel therapies. In the past, a combination of cytokines, peptides and DC-based vaccines has provided a suggestion of improved immunologic responses in association with clinically significant anti-tumour responses in patients with melanoma.3–5 Unfortunately, durable clinical benefits following vaccination therapies are uncommon. The multi-center phase II ECOG (E1696) trial completed in 2009, was the first to indicate that the ability of melanoma patients to mount an immune response to at least one vaccinating peptide is predictive of survival.6
To better understand the potential benefits of peptide-based vaccines in melanoma, well-powered, randomized studies are necessary, in which immunologic effects of the vaccine can be objectively evaluated and correlated with clinical endpoints. The hypothesis to be tested is that the immunodominant melanoma peptide epitopes delivered with immunostimulatory adjuvants induce a higher frequency of peptide-specific T cells and establish durable memory immune responses. Prior to the E1696 study, no cooperative group trial had evaluated the immunogenicity of vaccines comprised of multiple lineage melanoma antigens or compared vaccination performed in combination with immuno-modulatory cytokines to vaccination alone. The E1696 trial was designed to test immune responses induced among CD8+ T cells specific for HLA-A2-restricted peptides derived from the melanoma lineage antigens MART-1, gp100 and tyrosinase and administered with GM-CSF and/or IFNα2b serving as immunological adjuvants.6 This trial established that patients with immune response to this vaccine lived longer (p < 0.033) than those without response, suggesting that this triple peptide vaccine may have clinical benefits in patients with metastatic melanoma following failure of multiple prior therapies.6 This finding adds to evidence which demonstrated clinical benefits of one of the peptides (gp100) over the benefit of high-dose IL-2 in advanced melanoma.7
To further evaluate the frequency and the differentiation status of melanoma tumour antigen-specific CD8+ T cells in the HLA-A2+ subset of patients enrolled in ECOG 1696 trial, we performed additional analyses of immune cells in the peripheral circulation of these patients. MHC tetramers (tet) were used to measure CD8+ T-cell responses to the vaccine-delivered peptides, MART-127–35, gp100209–217 and tyrosinase368–376. As control, FLU M158–66 peptide-specific responses were also evaluated. In addition, the differentiation phenotype of tet+CD8+ T cells was established. The objective was to correlate the frequency of CD8+tet+ T cells and their differentiation state with clinical outcomes of the patients treated with vaccine and with immunologic responses to the same peptides measured in IFN-γ ELISPOT assays as previously reported.6 The results suggest that the functional status, as measured in IFN-γ ELISPOT assays, but not the frequency or phenotype of CD8+tet+ T cells, correlated with clinical responses to the peptide-based vaccine.
Material and Methods
Patients
All patients were enrolled in the E1696 clinical trial (4). Eligible patients all had histologically confirmed Stage IV melanoma and measurable disease. Other details are described in the trial report (6). Patients were HLA-A2 positive by serologic or genotypic analysis. All patients provided written informed consent, and the study was approved by the Institutional Review Board of each participating ECOG-affiliated institution. The patients whose specimens were available for immune monitoring were randomized to any one of the four arms of the trial (see in the following text).
Peptides
HLA-A2 restricted peptides used in the vaccine included: wt AAGIGILTV (MART-127–35),8 modified IMDQVPFSV (gp100209–217(2M))9 modified YMDGTMSQV (tyrosinase368–376(3D)).10 In addition, the influenza peptide GILGFVFTL (FLU M158–66) was used as a control for immune monitoring. The vaccine peptides were synthesized and provided by the Cancer Therapy Evaluation Program (CTEP) as Investigational New Drug #6123. They were placed in vials containing 1 mL of a sterile 1 mg/mL solution for injection.
Immunization protocol
Briefly, patients were randomized into one of the four treatment arms:
Arm A, multi-epitope peptide vaccine alone. Patients were given each multi-epitope peptide vaccine (six injections, three locations) subcutaneously (SC) on Days 0 and 15 for a maximum of 13 cycles (1 year). Each peptide was emulsified with Montanide ISA-51 (Seppic, France) and administered SC in two aliquots (1 mL each). The vaccine was injected in the same way in all treatment arms.
Arm B, GM-CSF +multiepitope peptide vaccine.Patients self-administered GM-CSF (Immunex, Seattle, WA) at a fixed dose of 250 µg SC daily for 14 days followed by 14 days off every 28 days for 1 year (13 cycles) or until disease progression.
Arm C, interferon α-2b + multi-epitope peptide vaccine. IFN (IFNα-2b, Schering-Plough, Kenilworth, NJ) was administered at 10 MU/m2 SC three times a week (Monday, Wednesday, Friday) for 52 weeks, or until disease progression.
Arm D, IFNα-2b + GM-CSF + multi-epitope peptide vaccine. Both IFNα-2b and GM-CSF were administered in the exact same schedule as in Arms B and C, respectively.
The duration of therapy for all arms was 13 cycles (52 weeks). Patients who did not progress during treatment were followed every 3 months to 24 months after study enrolment and every 6 months to 60 months following study enrolment. Follow-up included physical exam and whole body CT scans.
Patient cells
Peripheral blood in green top tubes and containing heparin anticoagulant was delivered by overnight shipments (from sites other than Pittsburgh, PA) to the ECOG Central Immunologic Laboratory located at the University of Pittsburgh Cancer Institute for immediate lymphocyte isolation on Ficoll-Hypaque gradients and cryopreservation performed as described elsewhere.11 Cells were stored under cGLP conditions in liquid nitrogen in monitored freezers. For each patient, batched frozen PBMC were available for at least two (Day 0 and Day 43 or 85) time points. In many patients, three serial samples (Day 0, Days 43 and 85) were available. Samples obtained from each patient were tested simultaneously.
Tetramers, antibodies and staining
The PE-labeled HLA-A*0201 tetramers used in this study were all purchased from Beckmann Coulter (Fullerton, CA). The tetramers were folded to include modified peptides: MART-1/Melan A26–35(2L), Tyrosinase368–376(3D), gp100209–217(2M) and FLU matrix58–66. Titrations of tetramers and specificity assays were as follows: (i) all tetramers were pre-titered on melanoma peptide-specific or wt p53-specific T cell lines maintained in our laboratory to establish optimal reagent concentrations and distinguish positive from negative signals; (ii) a negative control used to define a cut-off for tet+ vs. tetneg T cells, a nonsense tetramer also purchased from Beckmann Coulter was used. The values for the nonsense tetramer never exceeded 0.01% of CD8+ T cells; (iii) a cut-off for tetramer binding to HLA-A2 negative PBMC of normal donors (n = 10) was established as previously described.12The LLD was defined as the reciprocal frequency of 10,000 or 0.01%. This value was used as a cut-off for all tetramer results described here.
The staining for tetramers was performed as recommended by the manufacturer. Briefly, PBMC were thawed and resuspended at a concentration 10 × 106 cells/mL. Each tetramer (10 µL) was added to a 100 µL aliquot of cells (1 × 106) and incubated for 30 min, 4°C in the dark. Next, 5 µL of each surface mAb was added directly to the cell suspension, followed by 30-min incubation at 4°C in the dark. Finally the cells were washed with 3 mL of PBS, centrifuged at 400 g for 5 min and then resuspended in 500 µL of PBS/ 0.5% (wt/vol) paraformaldehyde. For intracellular staining of IFN-γ in tet+ cells, PBMC were first pre-stained with PE-labeled tetramers for 30 min to visualize peptide-specific T cells, then incubated with a relevant peptide (10 µM) for 4 h at 37°C with Brefeldin A (Sigma) added for the last 3 hr of incubation. Subsequently, the cells were stained with PE-labeled tetramers and anti-CD8 Abs. Finally, the cells were permeabilized with 1% saponin in PBS at 4°C and stained with anti-IFN-γ PE-Cy7 (BD Pharmingen). For flow cytometry of tetramer-stained samples, 4 × 105 CD8+ T cells were acquired/sample.
All mAbs used for the surface staining of lymphocytes (anti-CD8-FITC; anti-CD4-FITC; anti-CD45RA-ECD, anti-CD27-PE; anti-CD28-PE), including the respective isotype controls, were purchased from Beckmann Coulter (Miami, FL), except PerCP-labeled mAbs against CCR7 (R&D, Minneapolis, MN). Optimal working dilutions of mAbs used for surface staining were determined by titrations with normal PBMC. For surface staining without tetramers, 5 µL of each surface antibody was added to 100 µL of the cell suspension and incubated for 30 min at 4°C in the dark, followed by two washes with PBS/0.1% BSA/0.1% NaN3 and fixation with 500 µL 0.5% of PBS/0.5% paraformaldehyde. Flow cytometry was performed within 1 hr after fixation using a Beckmann Coulter Epics XL cytometer. Expo 32 ADC software (Beckmann Coulter) was used for subsequent analysis of the acquired data. The cytometer performance was checked daily using quality control beads system.
ELISPOT assays
ELISPOT data were generated prior to tetramer analyses and are presented in the paper by Kirkwood et al.6 The direct ELISPOT assay for IFN-γ was performed ex vivo, using the same peptides as those in the vaccine: wt MART27–35, gp100209–217(2M) and tyrosinase368–376(3D). In addition, FLU M158–66 was used as a control peptide. Cryopreserved and thawed PBMC were used to isolate responder CD8+ T cells as fully described in the clinical trial report.6 Briefly, wells plated in triplicate (50,000 cells/well) were read by an automatic ELISPOT reader. A permutation test was used to select positive triplicates. Controls included responder or T2 cells alone, responders + irrelevant ova peptide and responders + OKT3. Background spots were subtracted from those in experimental wells.
Immune score
Total “immune score” was determined by considering any ELISPOT positivity (one, two, or all three vaccine peptides) and any tetramer positivity (one, two, or three vaccine peptides) for a possible 6/6 total immune score. For patients without data for any of the six assay responses, the percent of the total possible positive responses (e.g., ×/4 or ×/5 possible was used).
Statistical analysis
The descriptive statistics were provided using the median/ range and box plots. To assess the association between the two continuous variables, the Spearman correlation was used. To assess the association between categorical and continuous variables, Wilcoxon rank sum test (for two groups) or Kruscal Wallis test (for multiple groups) was applied. To assess the changes over time, the generalized estimating equations were used. In this longitudinal model, the correlation structure among repeated measures over time was assumed to be unstructured. Given the exploratory nature of this article, Type I error rates have not been adjusted for multiple testing. All the reported p-values are based on two-sided tests.
Results
Frequency of CD8+tet+ T cells
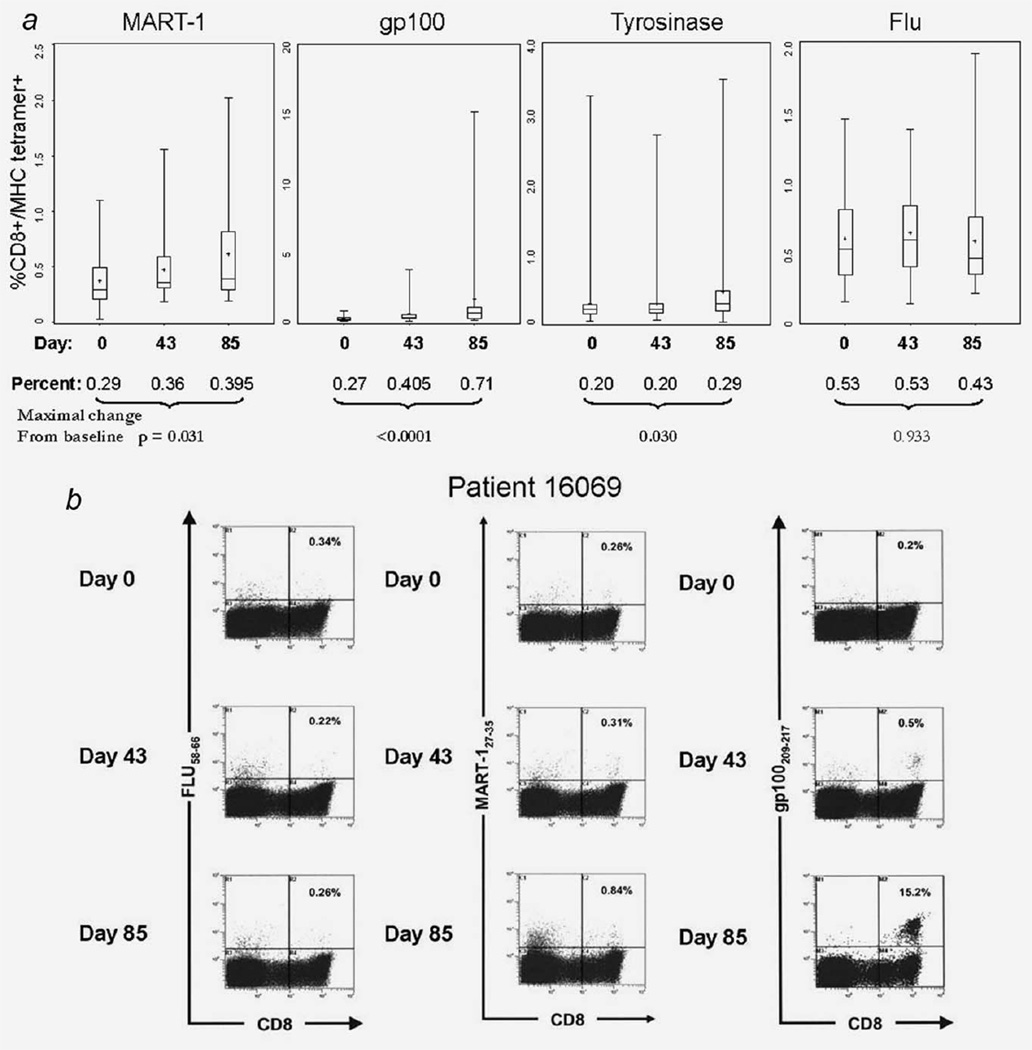
Cryopreserved, batched PBMC (Day 0, post vaccine Days 43 and 85) were thawed and tested by flow cytometry for the frequency of CD8+tet+ T cells, using HLA-A2 tetramer binding as a read out. Of 73 patients whose cells were available for immune monitoring, a subgroup of 37 was tested for tetramers and T-cell differentiation markers by flow cytometry based on the availability of PBMC after the ELISPOT assay was performed. Figure 1 shows the median frequency levels of circulating CD8+tet+ T cells as measured at the three time points. A broad range of values characterizes the responses to individual peptides, although it is clear that the frequency of each of the melanoma tumour antigen peptide-specific CD8+ T cells significantly increased after vaccination, relative to the baseline frequency (see Fig. 1 and Table 1). In contrast to the melanoma peptide-specific T cells, the frequency of FLU-specific T cells remained constant to Day 43 and then decreased slightly (Fig. 1).
Figure 1.
Vaccine-induced changes in the frequency of CD8+tet+ T cells. (a) Box plots show the frequency of CD8+tet+ T cells in the circulation of patients at baseline (Day 0) and the two post-vaccination time points (Days 43 and 85). The bars are median values, the box shows the interquartile range (25–75%), and the whiskers indicate the maximum and minimum values. The maximal change from baseline defines the percent increase (or decrease) from baseline on Days 43 or 85, whichever gives the highest value. (b) Examples of dot plots for patient 16,069 are shown for two of the vaccine peptides (MART-1 and gp100) and the Flu control. The calculated CD8+tet+ frequencies are shown in each upper-right quadrant.
Table 1.
Median frequency and ranges of melanoma peptide-specific and FLU-specific CD8+ T cells detected in the peripheral blood of patients with metastatic melanoma at baseline and on days 43 and 85 after vaccination1
| Time point | gp100 | n | MART-1 | n | Tyrosinase | n | FLU | n |
|---|---|---|---|---|---|---|---|---|
| % CD8+tet+ T cells | ||||||||
| Baseline | 0.27 (0.10–0.84) | 37 | 0.29 (0.03–1.10) | 37 | 0.20 (0.03–3.26) | 37 | 0.53 (0.15–1.46) | 37 |
| Day 43 | 0.40 (0.1–3.86) | 32 | 0.36 (0.18–.56) | 36 | 0.20 (0.05–2.7) | 36 | 0.53 (0.14–1.38) | 32 |
| Day 85 | 0.71 (0.17–5.2) | 20 | 0.39 (0.19–2.02) | 20 | 0.29 (0.02–3.49) | 21 | 0.43 (0.201–0.910) | 16 |
| Maximal change from baseline | p < 0.0001 | p = 0.031 | p = 0.030 | p = 0.933 |
As a reference, PBMC of 10 HLA-A2+ normal donors were used to determine the mean frequency of CD8+tet+ T cells for gp100 (0.01%), MART-1 (0.02%) tyrosinase (0.01%) and for FLU (0.04%).
The lower limit of detection (LLD) for melanoma peptide tetramers was established using cells obtained from 10 HLA-A2neg donors as 0.01%.
When the frequency of CD8+tet+ T cells was examined in individual patients, it appeared that nearly all were positive at baseline (Tables 1 and 2). The frequency values for all CD8+tet+ T cells were higher in melanoma patients at baseline than in randomly selected healthy HLA-A2+ donors (Table 1). An increase from baseline to Day 85 was observed for the majority of patients. Table 2 shows that up to 70% of the evaluated patients had detectable CD8+tet+ T cells on Days 43 and/or 85 after vaccination. Further, 63% of patients were shown to respond to 2/3 peptides and 48% responded to all three peptides after vaccination. An example of the tetramer frequency for MART-1, gp100 and FLU in patient 16069 is shown in Figure 2. This patient was negative for tyrosinase-specific CD8+ T cells.
Table 2.
Numbers of patients who showed an increased or decreased frequency of CD8+tet+ melanoma peptide-specific T cells after vaccination1
| HLA-A2+ tetramer | Positive at baseline2 |
Increase (pre to post)3 |
Decrease (pre to post)4 |
Increase > 50% (pre to post) |
|---|---|---|---|---|
| MART-1 | 36 (98) | 29 (70) | 8 (21) | 17 (46) |
| gp100 | 35 (95) | 29 (70) | 3 (8) | 25 (67) |
| tyrosinase | 33 (89) | 26 (63) | 7 (19) | 17 (46) |
| FLU (control) | 37 (100) | 7 (17) | 1 (2) | 7 (17) |
37 patients were tested for changes in the frequency of CD8+tet+ T cells after vaccination either on day 43 or day 85. 20 patients were tested on day 43; 15 on days 43 and 85; and 5 on day 85 only.
Baseline (pre-vaccination) greater than 0.05% (MART-1 and Tyrosinase) or 0.10% (gp100 and Flu) is considered as positive. All values in parentheses are %.
Any increase (pre to post) in frequency ≥ 0.03% tet+ (or 3 × background frequency of 0.01%, as indicated in the legend to Table 1) was considered to be positive.
Any decrease (pre to post) in frequency ≥ 0.03% tet+.
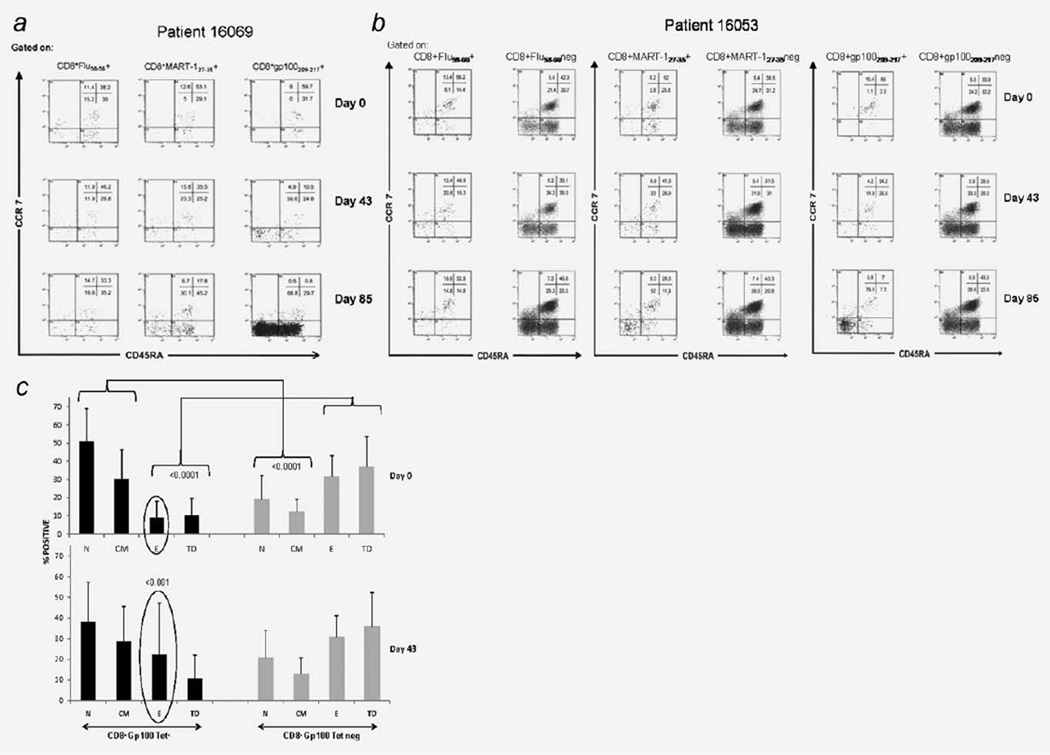
Figure 2.
Vaccine-induced changes in the differentiation phenotypes. (a) The dot plots for the expression of CCR7 and CD45RA on CD8+tet+ T cells in patient 16,069 (see Fig. 2). The gate is set on CD8+tet+ T cells for FLU, MART-1 and gp100 tetramers. The tetramer frequency calculated for each quadrant is shown in the upper-right of each dot plot. For this analysis, 4 × 105 cells/ sample were acquired. Note that almost all gp100+ cells are in the EM and TD compartments. The gp100+ T cells represent 15% of CD8+ T cells in this patient on Day 85 (see Fig. 2b). (b) The dot plots for expression of CCR7 and CD45RA on CD8+tet+ versus CD8+tetneg (“global”) T cells in patient 16,053. Note that vaccine-induced changes in the differentiation phenotype are evident only for CD8+MART-1 and CD8+gp100+ tetramers but not for “global” CD8+ T cells. The patient’s CD8+tyr+ T cells were at 0.2%, and no changes were seen in the differentiation phenotype after vaccination (data not shown). (c) Differentiation profiles of CD8+gp100tet+ T cells, and CD8+tetneg T cells at baseline (n= 37) and on Day 43 after vaccination (n= 35) for all tested patients. The Day 83 profile was analogous to that on Day 43 and is not shown. Phenotype of tet+ tumour-specific T cells is significantly different than that of the tetneg CD8+ T cell population p < 0.0001. EM gp100+ T cells increased significantly after vaccination (p= 0.003). The bars indicate mean% ± SD.
Influenza (FLU) peptide-specific CD8+ T cell responses were also determined simultaneously with the melanoma antigen-specific peptide responses. As shown in Table 2, only seven patients showed an increased frequency of CD8+FLU+ T cells after vaccination with melanoma peptides. The frequency of CD8+FLU+ T cells remained unchanged from pre to post vaccination in all other patients, except that it decreased in one patient. Table 2 also shows that in all cases, a considerably higher percentage of the patients showed increases rather than decreases in the frequency of CD8+tet+ T cells following vaccination.
It was also of interest to determine whether the frequency of CD8+tet+ T cells at baseline correlated with the magnitude of the increase in frequency after vaccination. Interestingly, all three melanoma T-cell frequencies showed a significant negative correlation with responses to the vaccine, i.e., cases with higher baseline values had smaller frequency changes on Days 43 and 85 than cases with no or low baseline frequency values (Supporting Information Table 1). The same trend was seen for the correlation between baseline and maximum changes achieved over 85 days.
Maturation phenotype of CD8+tet+ cells
The CD8+tet+ T cells were co-stained for expression of CD45RA and CCR7 to categorize their differentiation/maturation status as follows: CD45RA+/CCR7+ (na¨ıve, N); CD45RA−/CCR7+ (central memory, CM); CD45RA−/CCR7− (effector memory, EM); and CD45RA+/CCR7− (terminally differentiating, TD). As shown in Table 3 changes in the differentiation status of CD8+tet+ T cells during the course of vaccination followed a different pattern. While the median % of N gp100+ and N MART-1+ cells decreased, that of EM CD8+tet+ cells increased, the other two subsets remaining unchanged. For tyrosinase, only TD CD8+ tet+ T cells decreased in frequency after vaccination. In contrast, all four FLU-specific and CD8+tet− subsets remained at the baseline levels, without any evidence for differentiation after vaccination (Table 3). There were large individual differences in the range of tet+ cells within each compartment, which are best illustrated in the patient 16,069. The CD8+tet+ cells were at 15% on Day 85 (Fig. 1b), and as shown in Figure 2a, nearly all these cells were gp100+ and localized to the TD compartment. This was also the case for MART-1+ cells but not for FLU+ cells. In this patient, nearly all CD8+tet+ cells induced by the vaccine were terminally differentiating on Day 85. The data in Table 3 and Figure 2b indicate, however, that this was not so evident in other patients.
Table 3.
Differentiation phenotypes of peptide-specific CD8+tet+ and CD8+tet− T cells determined at baseline and on Days 43 and 85 after vaccination1
| CD8+tet+ | N | EM | CM | TD |
|---|---|---|---|---|
| gp100 | ||||
| Baseline | 50 (13–86) | 7 (0–38) | 29 (4–61) | 8 (0–41) |
| Day 43 | 40 (0.6–75) | 12 (0–90) | 28 (1.6–75) | 8 (0–59) |
| Day 85 | 34 (0.8–56.5) | 22 (0–80) | 27 (0.5–54) | 8 (0–95) |
| p < 0.076 | p < 0.048 | NSD | NSD | |
| MART-1 | ||||
| Baseline | 44 (11–71) | 11 (1–59) | 31 (4–57) | 8 (0–45) |
| Day 43 | 41 (0–79) | 17 (0–63) | 24 (8–70) | 11 (0–57) |
| Day 85 | 30 (5–70) | 18 (0–82) | 27 (5–71) | 13 (0–45) |
| p < 0.006 | p < 0.048 | NSD | NSD | |
| Tyrosinase | ||||
| Baseline | 47 (4–88) | 6 (0–39) | 29 (4–73) | 7 (4–73) |
| Day 43 | 42 (7–93) | 7 (0–30) | 32 (0–82) | 8 (0–76) |
| Day 85 | 51 (7–87) | 6 (0–47) | 30 (4–75) | 5 (0–75) |
| p < 0.014 | ||||
| FLU | ||||
| Baseline | 34 (9–73) | 18 (2–55) | 28 (4–57) | 15 (0–53) |
| Day 43 | 30 (0–61) | 17 (4–73) | 28 (0–70) | 15 (0–52) |
| Day 85 | 30 (8–57) | 17 (3–65) | 29 (6–78) | 15 (0–57) |
| CD8+tet− | ||||
| Baseline | 18 (5–63) | 30 (14–61) | 9 (2–27) | 38 (13–65) |
| Day 43 | 23 (4–59) | 29 (14–51) | 11 (2–30) | 35 (18–63) |
| Day 85 | 18 (5–61) | 32 (9–51) | 13 (2–46) | 40 (9–61) |
The data are median percentages of positive cells among all CD8+tet+ T cells or all CD8+tetneg T cells with the ranges in parentheses. At baseline, the differentiation phenotypes were determined in 37, on Day 43 in 35, and on Day 85 in 21 patients. Naïve T cells (N) are defined as CD45RA+CCR7+; effector memory T cells (E) as CD45RA−CCR7−, central memory (CM) as CD45RA−CCR7+ and terminally differentiating (TD) as CD45RA+CCR7−.
In fact, the mean % of TD CD8+tet+ cells was lower (p < 0.0001) than that of TD CD8+tet− (“global” CD8+) after vaccination for all patients (Fig. 2c). Importantly, no vaccine-induced changes in the differentiation phenotype of CD8+tet− (“global”) T cells were seen (Table 3, Figs. 2b and 2c). The overall differentiation profile for CD8+tet+ after vaccination was characterized by variable contractions or expansions in the frequency of tet+ T cells in all compartments but with a consistently increased frequency of gp100+ EM cells (p < 0.001 in Fig. 2c).
Interestingly, Figure 2c indicates that the mean % of N and CM gp100+ T cells were increased in patients with melanoma (p < 0.0001) relative to CD8+tet− (“global”) T cells regardless of vaccination. This suggests that the maturation to EM of tumour-reactive T cells in melanoma patients is slower or delayed relative to that of “global” T cells.
Correlation of the tetramer frequency with IFN-γ ELISPOT results
The functional T-cell response (i.e., the ability of CD8+ T cells to produce IFN-γ) upon ex vivo stimulation with the vaccinating melanoma peptides was also measured in IFN-γ ELISPOT assays as previously described.6 It was, therefore, possible to determine whether the (pre vs. post) frequency of CD8+et+ cells correlated with the ELISPOT assay responses to the same melanoma peptides. Table 4 illustrates the significant positive correlation for the frequency of gp100+ and tyrosinase+ T cells on Day 85, indicating that CD8+tet+ T cells were more numerous and produced more IFN-γ at this time point. The negative correlations for tyrosinase on Days 0 and 43 imply that higher numbers of tyrosinase+CD8+ T cells produced less IFN-γ suggesting a disparity between the tetramer frequency and the ability of these tet+CD8+ T cells to produce IFN-γ.
Table 4.
Spearman correlations between the frequency of CD8+tet+ T cells vs. the frequency of IFN-γ producing CD8+ T cells at baseline and on Days 43 and 85 after vaccination1
| gp100 | MART-1 | Tyrosinase | FLU | |
|---|---|---|---|---|
| Pre vaccination | ns | −0.26 (0.073) | −0.67 (<0.001) | ns |
| Day 43 | ns | ns | −0.53 (0.005) | ns |
| Day 85 | 0.55 (0.006) | ns | 0.62 (<0.001) | ns |
The frequency of CD8+tet+ T cells was measured by flow cytometry, while that of IFN-γ producing CD8+ T cells was measured by a direct ELISPOT assay. Significant positive correlations are in bold, p values are in parentheses.
In a subset of 10 patients who had sufficient numbers of post vaccination cells available for testing, expression of IFN-γ by CD8+tet+ T cells was monitored using gp100 tetramers and cytokine flow cytometry (CFC). The data (not shown) indicated that from 10 to 40% of CD8+gp100+ T cells expressed IFN-γ after vaccination. The assay as performed ensured that potential TCR-downregulation of tet+ cells was prevented. These data confirmed that peptide-specific CD8+ T cells indeed are the source of IFN-γ, albeit only a fraction of these T cells expressed this cytokine.
In addition, we asked whether the change from baseline in the frequency of CD8+tet+ T cells correlated with ELI-SPOT results on Days 43 or 85 (Supporting Information Table 2). The data showed a significant positive correlation for gp100 (p < 0.001) on Day 85 and for tyrosinase on Days 43 (p < 0.001). However, no significant correlations for MART-1 or FLU peptides were observed.
Correlation of CD8+tet+ T cell maturation stage with IFN-γ ELISPOT results
The CD8+tet+ differentiation phenotype was compared with the frequency of IFN-γ producing cells in ELISPOT at different time points as well as to the changes in their frequency over time. For the 12 possible comparisons for each peptide, the only positive correlations between the differentiation phenotype and IFN-γ production were obtained for gp100 (Supporting Information Table 2) and found to be largely due to differentiated EM T cells.
Correlation with clinical outcome
Given the observed increases in the frequency of melanoma peptide-specific CD8+ tet+ T cells after vaccination as well as evidence for changes in maturation of some of these T cells, we next evaluated a correlation between the tetramer frequency (pre to post) and the clinical response. To this end, patients were designated as clinical “responders” (CR+PR+SD; n = 13) and clinical “non-responders”: (PD; n = 24), and the two groups were compared for immunologic reactivity to the vaccine. No significant correlation between clinical response and increases in the post vaccination tetramer frequency was observed. Similarly, for most subsets, changes in the frequency of naïve and differentiated CD8+tet+ T lymphocytes observed during vaccination did not correlate with clinical responses. Only the frequency of terminally differentiating tyrosinase-speciic CD8+ T cells (pre-vaccine vs. Day 85) was higher in patients who had clinical responses than in patients with PD. The median frequency change in the “responder” group (n = 6) was 8.35, range (−5.4, 36.9), while that in the PD group (n = 15) was −1.3, range (−32.4, 40.9) and this correlation had a 2-sided p value = 0.071, with a trend toward significance.
Total immune score, tetramer and ELISPOT results
The “immune score” defines the breadth of immune responsiveness based on an expectation that a broad antigenic immune response may be more important for the patient clinical outcome than the total frequency of T cells specific for any single peptide.13–15 There were 32/37 patients who had the frequency data for all six tetramer and ELISPOT values (i.e., Days 0, 43 and 85) for both assays, their total immune score ranging from 1–5 (out of 0–6 possible). Five patients had fewer than six measurements. The total measured immune response fraction (positive immune score/no. measured) was compared in clinical non-progressors (PR, SD) vs. progressors (PD). The median (range in parentheses) of immune score for non-progressors (n = 17) is 0.5 (0.17, 1) and for progressors (n = 20), it is 0.4 (0.17, 0.83). Although progressors had a lower total immune score, this was not statistically significant (p = 0.609).
Discussion
The ECOG 1696 was the first large, randomized, multi-institutional trial of a multiepitope peptide vaccine for patients with metastatic melanoma who have failed prior therapy.6 The study addressed laboratory as well as traditional clinical endpoints that were considered critical to future vaccine development. Previous large randomized multi-center melanoma vaccine trials either could not evaluate therapeutic impact, because they were conducted in the adjuvant setting16–18 or have not employed serial immunological monitoring using defined peptides as immunogens.19 More recently, when peptide-specific assays were performed, they usually employed a single immune measure, i.e., either ELI-SPOT or tetramer assays.20,21 In E1696, the primary endpoint was immune response measured by the direct IFN-γ ELI-SPOT assay. The study demonstrated safety and significant clinical effects of this vaccine in relation to patient survival, linking peptide-specific IFN-γ production by CD8+ T cells with survival benefits as previously reported.6
After the ELISPOT assay was performed, sufficient cell numbers were available in a subset of patients for serial analyses of vaccine-induced peptide-specific immune responses in tetramer-based assays. We showed that the frequency of CD8+tet+ T cells increased for gp100 (p < 0.0001) as well as for MART-1 and tyrosinase peptides (p < 0.03) but not for the control FLU peptide (p < 0.9) after vaccination. Remarkably, in some patients, the frequency of CD8+tet+ T cells increased up to 15% of total CD8+ circulating T lymphocytes by Day 85, illustrating the broad range of peptide-specific responses to the vaccine in this genetically, biologically and clinically diverse patient population. In addition to the expected patient-related variability, the performance of tetramer-based flow cytometry, a rare-event analysis, is likely to affect results. We have previously reported on the lack of concordance between the three single-cell assays widely used for vaccine monitoring (ELISPOT, tetramer analysis and cytokine flow cytometry), and the fact, that in our hands, the tetramer frequency was consistently higher than that of the other two assays.11 Here, tetramer and ELISPOT assays were standardized to ensure reliability and included all the necessary controls. We observed that the frequency of CD8+tet+ T cells specific for gp100 and tyrosinase, but not MART-1, as measured by flow cytometry on Day 85 correlated (p < 0.006 and p < 0.001, respectively) with that for IFN-γ producing CD8+ T cells measured in ELISPOT. The lack of correlation for MART-1 could be due to the fact that the wt MART-1 peptide was used for vaccination and ELISPOT, while tetramer assays were done with the peptide modified for the monomer refolding. Nevertheless, vaccine-induced gp100+ and tyrosinase+ T cells were functional and able to produce IFN-γ upon ex vivo stimulation with the relevant peptide. Indeed, variable proportions of CD8+tet+ T cells were also shown to express intracytoplasmic IFN-γ by CFC in a limited number of tested patients. However, despite the observed significant association between the frequency of CD8+tet+ T cells and that of CD8+ T cells producing IFN-γ in ELISPOT in response to melanoma peptides, we saw no correlation between the frequency of CD8+tet+ T cells and OS. In contrast, a significant correlation was observed between T-cell reactivity in ELISPOT and OS.6 While this discrepancy might be due to the different patient numbers (73 patients tested in ELISPOT assays vs. 37 patients tested in tetramer assays), it does suggest that the frequency of CD8+tet+ T cells may not be as good a predictor of clinical outcome as the functional capability of CD8+tet+ T cells to produce IFN-γ. It has been reported that using CFC, only a subset of CD8+tet+ T cells stained for intracytoplasmic IFN-γ, as also observed here.22,23 This might reflect a difference in sensitivity of the two assays or the fact that IFN-γ expression and production are different cellular events. The finding that only functional activity of vaccine-induced CD8+ T cells as measured in ELISPOT assays, and not their phenotype, significantly correlated with patient OS is important. It implies that the selection of the immunologic assay for serial monitoring is a critical factor in the vaccine-based clinical trials.
The functional potential of T cells depends on their differentiation/maturation. T-cell surface markers associated with the N, CM, EM or TD phenotype (CD45RA/RO, CD27, CD28, CCR7) have been often used to measure the T-cell maturation state.24–27 The differentiation phenotype of melanoma peptide-specific cells has been previously investigated,22,23,28 and T-cell differentiation to activated EM cells after peptide-based vaccines has previously been observed in vaccine trials.29,30 Markers associated with trafficking to central (CD62L, CCR7) or peripheral sites (CCR5, CCR6), with the T-cell activation state (HLA-DR, CD25, CD69) and with early stages of T-cell apoptosis31–35 have all been used to evaluate responses to vaccines. More recent data suggest that T cells with the CM phenotype are the most desirable T-cell subset to be expanded after vaccination.36 In this study, the significant enlargement of gp100-specific and MART-1-specific T-cell populations within the EM compartment was observed following vaccination. The frequency of CD8+tet+ T cells in the CM compartment remained unchanged and that of TD CD8+tyrosinase+ T cells was decreased. Altogether, our data indicate that despite the use of the cytokine adjuvants, GM-CSF and IFN-α2b, this vaccine did not induce a significant expansion of melanoma peptide-specific CM T cells. It also did not alter the activation state of CD8+ or CD4+ T cells in the circulation (data not shown).
It has been suggested that the breadth of immune responses rather than any expanded response to a single peptide might correlate better with clinical responses to a vaccine.12–14 To test this hypothesis, and because the frequency of CD8+tet+ T cells significantly correlated with that of CD8+ T cells producing IFN-γ in ELISPOT assays, we determined an “immune score” for each patient by combining tetramer and ELISPOT results. This “immune score” was higher for patients who remained disease-free than those with progressive disease, although the difference did not reach statistical significance. Nevertheless, considering immunologic data acquired in this and other studies, it appears that patients with advanced metastatic melanoma who retained the capability to ex vivo mount peptide-specific immune responses had a better clinical outcome than those patients whose immune responsiveness to the vaccine was weak or negative. In this context, the observation that patients with a high baseline frequency of CD8+tet+ T cells generated small to moderate vaccine-induced immune responses, while those with no or low baseline frequency generated significantly higher responses suggests the presence of regulatory mechanisms. Thus, in melanoma patients, the magnitude of immune responses to the vaccine consisting of peptide self-epitopes appears to be carefully calibrated. The reasons for the observed negative correlation between the baseline and post-vaccine CD8+tet+ T cell frequency remain obscure, except for a possibility that peptide-specific T cell responses are controlled by regulatory T cells (Treg), which selectively prevent the expansion of peptide-specific effector T cells. The assessment of Treg subsets in future clinical trials, and particularly the recently completed adjuvant E4697 trial that tested the same three peptides studied in E1696, may elucidate their role in modulating vaccine-induced immune responses.
Supplementary Material
Acknowledgements
Grant sponsor: ECOG; Grant numbers: U10 CA 21115, NIH RO1 CA87904; Grant sponsor: NIH; Grant numbers: RO1 DE13918, PO1 CA109688; Grant sponsor: UPCI CCSG; Grant number: P30 CA047904
Supported in part by ECOG grant U10 CA 21115 and NIH RO1 CA87904 to JMK; NIH grants RO1 DE13918 and PO1 CA109688to TLW.
Abbreviations
- ELISPOT
enzyme-linked immune-spot
- ECOG
Eastern Cooperative Oncology Group
- FLU
influenza
- GM-CSF
granulocyte-macrophage colony stimulating factor
- HLA
human leukocyte antigen
- IFN
interferon
- PBMC
peripheral blood mononuclear cells
- tet
MHC-tetramer
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gozalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ. Improved survival with ipilmumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingstone E, Zimmer L, Piel S, Schadendorf D. PLAX4032: does it keep its promise for metastatic melanoma treatment? Exp Opin Invest Drugs. 2010;19:1439–1449. doi: 10.1517/13543784.2010.527945. [DOI] [PubMed] [Google Scholar]
- 3.Kirkwood JM, Manola J, Ibrahim J, Sondak V, Ernstoff MS, Rao U. A pooled analysis of eastern cooperative oncology group and intergroup trials of adjuvant high-dose interferon for melanoma. Clin Cancer Res. 2004;10:1670–1677. doi: 10.1158/1078-0432.ccr-1103-3. [DOI] [PubMed] [Google Scholar]
- 4.Astsaturov I, Petrella T, Bagriacik EU, de Benedette M, Uger R, Lumber G, Berinstein N, Elias I, Iscoe N, Hammond C, Hamilton P, Spaner DE. Amplification of virus-induced antimelanoma T-cell reactivity by high-dose interferon-alpha2b: implications for cancer vaccines. Clin Cancer Res. 2003;9:4347–4355. [PubMed] [Google Scholar]
- 5.Chianese-Bullock KA, Pressley J, Garbee C, Hibbitts S, Murphy C, Yamshchikov G, Petroni GR, Bissonette EA, Neese PY, Grosh WW, Merrill P, Fink R, et al. MAGE-A1-, MAGE-A10-, and gp100-derived peptides are immunogenic when combined with granulocyte-macrophage colony-stimulating factor and montanide ISA-51 adjuvant and administered as part of a multipeptide vaccine for melanoma. J Immunol. 2005;174:3080–3086. doi: 10.4049/jimmunol.174.5.3080. [DOI] [PubMed] [Google Scholar]
- 6.Kirkwood JM, Lee S, Moschos SJ, Albertini MR, Michalak JC, Sander C, Whiteside T, Butterfield LH, Weiner L. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/− granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, White DE, Steinberg SM, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14:5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, Appella E, Rosenberg SA. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180:347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfel T, Van Pel A, Brichard V, Schneider J, Seliger B, Meyer zum Buschenfelde KH, Boon T. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- 11.Whiteside TL, Zhao Y, Tsukishiro T, Elder EM, Gooding W, Baar J. Enzyme-linked immunospot, cytokine flow cytometry, and tetramers in the detection of T-cell responses to a dendritic cell-based multipeptide vaccine in patients with melanoma. Clin Cancer Res. 2003;9:641–649. [PubMed] [Google Scholar]
- 12.Hoffmann TK, Donnenberg AD, Finkelstein SD, Donnenberg VS, Friebe-Hoffmann F, Myers EN, Appella E, DeLeo AB, Whiteside TL. Frequencies of tetramer+ T cells specific for the wild-type sequence p53264-272 peptide in the circulations of patients with head and neck cancer. Cancer Res. 2002;62:3521–3529. [PubMed] [Google Scholar]
- 13.Butterfield LH, Ribas A, Dissette VB, Amarnani SN, Vu HT, Oseguera D, Wang HJ, Elashoff RM, McBride WH, Mukherji B, Cochran AJ, Glaspy JA, et al. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res. 2003;9:998–1008. [PubMed] [Google Scholar]
- 14.Ribas A, Glaspy JA, Lee Y, Dissette VB, Seja E, Vu HT, Tchekmedyian NS, Oseguera D, CominAnduix B, Wargo JA, Amarnani SN, McBride WH, et al. Role of dendritic cell phenotype, determinant spreading, and negative costimulatory blockade in dendritic cell-based melanoma immunotherapy. J Immunother. 2004;27:354–367. doi: 10.1097/00002371-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, et al. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 16.Sondak VK, Liu PY, Tuthill RJ, Kempf RA, Unger JM, Sosman JA, Thompson JA, Weiss GR, Redman BG, Jakowatz JG, Noyes RD, Flaherty LE. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: overall results of a randomized trial of the Southwest Oncology Group. J Clin Oncol. 2002;20:2058–2066. doi: 10.1200/JCO.2002.08.071. [DOI] [PubMed] [Google Scholar]
- 17.Hersey P, Coates AS, McCarthy WH, Thompson JF, Sillar RW, McLeod R, Gill PG, Coventry BJ, McMullen A, Dillon H, Simes RJ. Adjuvant immunotherapy of patients with high-risk melanoma using vaccinia viral lysates of melanoma: results of a randomized trial. J Clin Oncol. 2002;20:4181–4190. doi: 10.1200/JCO.2002.12.094. [DOI] [PubMed] [Google Scholar]
- 18.Hsueh EC, Morton DL. Antigen-based immunotherapy of melanoma: Canvaxin therapeutic polyvalent cancer vaccine. Semin Cancer Biol. 2003;13:401–407. doi: 10.1016/j.semcancer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood JM, Ibrahim JG, Sosman JA, Sondak VK, Agarwala SS, Ernstoff MS, Rao U. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 20.Slingluff CL, Jr., Petroni GR, Chianese-Bullock KA, Smolkin ME, Hibbitts S, Murphy C, Johansen N, Grosh WW, Yamshchikov GV, Neese PY, Patterson JW, Fink R, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 21.Faries MB, Hsueh EC, Ye X, Hoban M, Morton DL. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine. Clin Cancer Res. 2009;15:7029–7035. doi: 10.1158/1078-0432.CCR-09-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 23.Dunbar PR, Smith CL, Chao D, Salio M, Shepherd D, Mirza F, Lipp M, Lanzavecchia A, Sallusto F, Evans A, Russell-Jones R, Harris AL, et al. A shift in the phenotype of melan-A-specific CTL identifies melanoma patients with an active tumor-specific immune response. J Immunol. 2000;165:6644–6652. doi: 10.4049/jimmunol.165.11.6644. [DOI] [PubMed] [Google Scholar]
- 24.Tomiyama H, Matsuda T, Takiguchi M. Differentiation of human CD8(+) T cells from a memory to memory/effector phenotype. J Immunol. 2001;168:5538–5550. doi: 10.4049/jimmunol.168.11.5538. [DOI] [PubMed] [Google Scholar]
- 25.Valmori D, Scheibenbogen C, Dutoit V, Nagorsen D, Asemissen AM, Rubio-Godoy V, Rimoldi D, Guillaume P, Romero P, Schadendorf D, Lipp M, Dietrich PY, et al. Circulating Tumor-reactive CD8(+) T cells in melanoma patients contain a CD45RA(+)CCR7(−) effector subset exerting ex vivo tumor-specific cytolytic activity. Cancer Res. 2002;62:1743–1750. [PubMed] [Google Scholar]
- 26.Tomiyama H, Takata H, Matsuda T, Takiguchi M. Phenotypic classification of human CD8+ T cells reflecting their function: inverse correlation between quantitative expression of CD27 and cytotoxic effector function. Eur J Immunol. 2004;34:999–1010. doi: 10.1002/eji.200324478. [DOI] [PubMed] [Google Scholar]
- 27.Reiner SL, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T cell fate. Science. 2007;317:622–625. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- 28.Pittet MJ, Valmori D, Dunbar PR, Speiser DE, Lienard D, Lejeune F, Fleischhauer K, Cerundolo V, Cerottini JC, Romero P. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med. 1999;190:705–715. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speiser DE, Lienard D, Pittet MJ, Batard P, Rimoldi D, Guillaume P, Cerottini JC, Romero P. In vivo activation of melanoma-specific CD8(+) T cells by endogenous tumor antigen and peptide vaccines. A comparison to virus-specific T cells. Eur J Immunol. 2002;32:731–741. doi: 10.1002/1521-4141(200203)32:3<731::AID-IMMU731>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 30.Pittet MJ, Speiser DE, Lienard D, Valmori D, Guillaume P, Dutoit V, Rimoldi D, Lejeune F, Cerottini JC, Romero P. Expansion and functional maturation of human tumor antigen-specific CD8+ T cells after vaccination with antigenic peptide. Clin Cancer Res. 2001;7:796s–803s. [PubMed] [Google Scholar]
- 31.Pilla L, Rivoltini L, Patuzzo R, Marrari A, Valdagni R, Parmiani G. Multipeptide vaccination in cancer patients. Expert Opin Biol Ther. 2009;9:1043–1055. doi: 10.1517/14712590903085109. [DOI] [PubMed] [Google Scholar]
- 32.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, Corthesy P, Devevre E, Speiser DE, Rufer N. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 33.Schwendemann J, Choi C, Schirrmacher V, Beckhove P. Dynamic differentiation of activated human peripheral blood CD8+ and CD4+ effector memory T cells. J Immunol. 2005;175:1433–1439. doi: 10.4049/jimmunol.175.3.1433. [DOI] [PubMed] [Google Scholar]
- 34.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 35.Kondo T, Takata H, Takiguchi M. Functional expression of chemokine receptor CCR6 on human effector memory CD8+ T cells. Eur J Immunol. 2007;37:54–65. doi: 10.1002/eji.200636251. [DOI] [PubMed] [Google Scholar]
- 36.Turtle CJ, Swanson HM, Fujii N, Estey EH, Riddell SR. A distinct subset of self-renewing human memory CD8+ T cells survives cytotoxic chemotherapy. Immunity. 2009;31:834–844. doi: 10.1016/j.immuni.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.