Abstract
The sympathetic nervous system plays an important role in some forms of human hypertension as well as the Dahl salt-sensitive rat model of hypertension; however, the sympathetic targets involved remain unclear. To address this, we examined the role of the renal and splanchnic sympathetic nerves in Dahl hypertension by performing either sham surgery (n = 10) or targeted sympathetic ablation of the renal nerves (renal denervation, n = 11), the splanchnic nerves (celiac ganglionectomy, n = 11) or both renal and splanchnic nerves (n = 11) in hypertensive Dahl rats. Mean arterial pressure increased from ~120 mmHg while on a 0.1% sodium chloride diet, to ~140 mmHg after being fed a 4.0% sodium chloride diet for three weeks. At that point rats underwent sham or targeted sympathetic ablation. Four weeks after treatment, mean arterial pressure was lower in renal denervated (150.4 ± 10.4) and celiac ganglionectomized (147.0 ± 6.1) rats compared to sham rats (165.0 ± 3.7), and even lower in rats that underwent both ablations (128.4 ± 6.6). There were no differences in heart rate or fluid balance between sham and renal denervated rats; however, rats that underwent either celiac ganglionectomy or both ablations exhibited marked tachycardia as well as sodium and water retention following treatment. These data suggest that targeted sympathetic ablation is an effective treatment for established hypertension in the Dahl rat and that the kidneys and the splanchnic vascular bed are both independently important targets of the sympathetic nervous system in this model.
Keywords: renal denervation, celiac ganglionectomy, Dahl S hypertension, sodium balance, water balance
INTRODUCTION
Hypertension is the leading risk factor for death worldwide1, yet the underlying causes are poorly understood as evidenced by the staggering number of uncontrolled cases of hypertension2-5. Recent studies have shown that catheter-based renal nerve ablation results in a sustained reduction of arterial pressure in drug resistant hypertensives, suggesting that targeted sympathetic ablation may be an effective treatment for hypertension that avoids the side effects of globally acting sympatholytic drugs6-8.
While the kidneys appear to be sympathetic targets in some forms of hypertension, other vascular beds, such as the splanchnic circulation, are also likely important as evidenced by the ability of splanchnic nerve stimulation to increase arterial pressure9 and surgical splanchnic sympathectomy to attenuate drug resistant hypertension in humans10, 11. Additionally, we have shown that the splanchnic, but not renal nerves contribute to the pathophysiology of angiotensin II-salt (AngII-salt) hypertension12. Together, these findings suggest that renal nerve ablation may be effective in some, but not all, forms of hypertension and that targeted sympathetic ablation of non-renal vascular beds may be an effective strategy for the treatment of hypertension in some individuals.
With that in mind, the present study compared the effects of renal and splanchnic nerve ablation on arterial pressure and body fluid balance in a well-accepted and widely studied genetic model of neurogenic hypertension - the Dahl salt-sensitive (Dahl S) rat. Similar to a significant fraction of humans with essential hypertension, the Dahl S rat becomes hypertensive when fed a high salt diet, has increased sympathetic activity13-18 and has increased renal and splanchnic vascular resistance19. Although renal nerve ablation has been consistently reported to have no effect on the development of Dahl S hypertension20-23, the ability of renal nerve ablation to reverse hypertension in this model not been reported. This is an important distinction with respect to the development of new therapies, which for the foreseeable future will focus on the treatment rather than prevention of hypertension. To our knowledge, the effect of splanchnic nerve ablation on either the developmental or maintenance phase of Dahl S hypertension has not been reported.
The present study was designed to address the following questions. First, once hypertension is initiated does renal nerve ablation decrease arterial pressure in the Dahl S rat? If so, is this response due to increased renal excretion of sodium and water? Second, does ablation of sympathetic nerves innervating the splanchnic vascular bed decrease arterial pressure in the Dahl S rat and, if so, how does that response compare to renal nerve ablation? Third, is combined regional sympathectomy more effective in lowering arterial pressure than denervation of a single target?
METHODS
Animals and General Procedures
Male Dahl S rats were purchased from Charles River Laboratories (Wilmington, MA) and housed in pairs in a temperature and light controlled room until the beginning of the study, at which time they were 64-70 days old and 250-340g. The rats were allowed access to standard rat chow and distilled water ad libitum during this pre-experimental period. All procedures were approved by the University of Minnesota Animal Care and Use Committee and were conducted in accordance with the institutional and National Institutes of Health guidelines. For all surgeries, rats were anesthetized with 2.0% isoflurane. Atropine sulfate (0.4 mg/kg, IP) and gentamicin sulfate (10 mg/kg, IM) were administered prior to surgery. For three days following surgery, buprenorphine (0.015 mg, SQ) was given twice per day and the drinking water was supplemented with amoxicillin (1 mg/ml).
Experimental Protocol
The timeline for the experimental protocol is shown in Figure 1. Rats were placed on a low salt diet (0.1% NaCl; Research Diets, New Brunswick, NJ) and instrumented with radio telemeters (model TA11PA-C40, DSI, Intl. St. Paul, MN) for monitoring of mean arterial pressure (MAP) and heart rate (HR) as previously described24. After a 7-day recovery period, rats were individually housed in metabolic cages (Techniplast 3701M001, Buguggiate, Italy) and allowed to acclimate for four days. Three days of baseline data were then collected (see below for details) and rats were placed on a high salt diet (4.0% NaCl; Research Diets) for the remainder of the protocol. After 21 days of high salt intake, rats were anesthetized with isoflurane and, via a midline approach, subjected to a sham (SHAM; n = 10), renal denervation (RDNX; n = 11), celiac ganglionectomy (CGX; n = 11) or combined renal denervation and celiac ganglionectomy (RDNX-CGX; n = 11) procedure. SHAM, RDNX and CGX procedures were performed as previously described25, 26 and the combined RDNX-CGX was achieved by performing the RDNX and CGX in single procedure. Rats were returned to their cages and monitored for an additional 4 weeks. Upon completion of the study, rats were anesthetized with isoflurane and the duodenum, liver, spleen and both kidneys of each rat were harvested, weighed, immediately frozen with liquid nitrogen and stored at −80 °C until they were assayed for tissue norepinephrine content as previously described26.
Figure 1.

Protocol timeline. CGX = celiac ganglionectomy, RDNX = renal denervation, RDNX-CGX = combined celiac ganglionectomy and renal denervation, and SHAM = sham surgery.
Daily Measurements
The transmitter signal was monitored by a receiver (Data Sciences, model RPC-1) mounted on the side of the metabolic cage and connected to a Data Exchange Matrix (Data Sciences, Int). The arterial pressure signal was sampled at 500 samples/second for 10 seconds every 4 minutes using commercially available software (Data Sciences, Int.). HR was determined from the arterial pressure profile using the same software. 24 hour (24h) averages of MAP and HR were determined and plotted for each day of the study.
24h food intake, water intake and urine output were measured gravimetrically. 24h sodium intake was calculated by multiplying food intake (grams) and sodium content of the diet (0.1% NaCl = 0.01711 mmol Na+/g food; 4.0% NaCl = 0.6844 mmol Na+/g food). 24h sodium excretion was calculated by multiplying 24h urine output (ml) and urinary sodium concentration (mmol Na+/ml), which was measured using an ion specific electrode (NOVA-5+ electrolyte analyzer, Nova Biomedical, Waltham, MA). 24h sodium and water balances were calculated as 24h intake minus 24h excretion. Cumulative sodium and water balances were determined from sequential summation of daily balances over the duration of the protocol.
Statistical Analysis
Data were analyzed by 2-way analysis of variance for repeated measures followed by the Holm-Sidak method for all post-hoc comparisons (SigmaPlot version 10.0). A p value less than 0.05 was considered to be statistically significant.
RESULTS
Cardiovascular Responses to Targeted Sympathetic Ablation
As shown in Figure 2, MAP increased in all groups from ~120 mmHg on the final day of 0.1% NaCl diet (SHAM = 120.2 ± 2.2, RDNX =119.5 ± 2.0, CGX = 119.8 ± 1.3, RDNX-CGX = 118.3 ± 1.8 mmHg) to ~140 mmHg after three weeks of 4.0% NaCl (SHAM = 140.1 ± 3.1, RDNX = 140.9 ± 5.0, GCX = 139.4 ± 2.4, RDNX-CGX = 138.5 ± 3.4 mmHg). Following SHAM surgery, MAP fell transiently, most likely due to a transient decrease in food (and therefore sodium) intake (please see http://hyper.ahajournals.org Figures S1), but returned to the pre-surgery trajectory within 7 days, reaching 165.0 ± 3.7 mmHg on the final day of the protocol. In contrast, MAP also fell in the RNDX group but did not rebound to the same trajectory and, by the end of the protocol, MAP was ~15 mmHg lower (150.4 ± 10.4) than SHAM rats. The magnitude and time course of the MAP response to CGX was similar to that of RDNX rats with MAP reaching a final level of 147.0 ± 6.1 mmHg. Finally, RDNX-CGX resulted in a much greater decrease in MAP than RDNX or CGX alone with a final MAP of 128.4 ± 6.6, ~35 mmHg lower than SHAM rats and ~20 mmHg lower than RDNX and CGX rats on the final day of the protocol. Moreover, the maximum pressure response was greater in RDNX-CGX than all other groups (please see http://hyper.ahajournals.org Figures S2).
Figure 2.
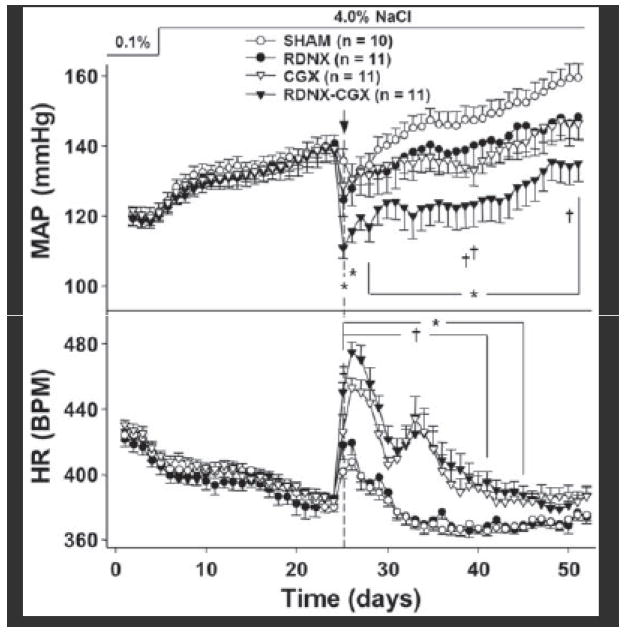
Effect of SHAM, RDNX, CGX and RDNX-CGX on MAP and HR. * = p < 0.05 for RDNX-CGX vs. SHAM. † = p < 0.05 for CGX vs. SHAM. ‡ = p < 0.05 for RDNX vs. SHAM.
The effects of SHAM and targeted sympathetic ablation on HR are also shown in Figure 2. HR on the final day of baseline was similar in all groups (SHAM = 422.5 ± 2.7, RDNX =416.9 ± 4.9, CGX = 425.1 ± 4.1, RDNX-CGX = 420.6 ± 4.8 bpm) and decreased to a similar level after three weeks of high salt intake (SHAM = 380.0 ± 3.1, RDNX = 385.2 ± 3.0, CGX = 383.6 ± 2.8, 384.3 ± 2.9 bpm). Following treatment surgery, HR transiently increased and then gradually fell in SHAM and RDNX rats such that HR was not statistically different between these groups throughout the protocol. In contrast both CGX and RDNX-CGX rats exhibited a marked biphasic increase in HR following treatment. During the first peak, HR increased to 425.87 ± 10.5 in CGX rats and 450.22 ± 6.2 in RNDX-CGX rats and began to fall but then increased to a second peak before falling to levels slightly higher than SHAM and RDNX rats by the end of the protocol.
Sodium and Water Balance Responses to Targeted Sympathetic Ablation
Daily sodium and water intake, excretion and balance measurements were similar between all groups during the baseline period, increased transiently when the diet was increased to 4.0% NaCl, and returned to baseline levels until the time of SHAM or targeted sympathetic ablation (please see http://hyper.ahajournals.org Figures S1 and S3). There were no differences in these parameters between SHAM and RDNX rats at any time during the 4 weeks following surgery with the exception of the day after surgery when urine output was higher in RDNX than SHAM rats. Sodium and water intake, excretion and balance decreased transiently after SHAM and RDNX but returned to pre-procedure levels within 10 days.
Cumulative sodium and water balances over the duration of the protocol were calculated from the daily balance measurements in all 4 groups (Figure 3). There were no differences for cumulative sodium or water balance between RDNX and SHAM rats over the entire protocol. On the other hand, CGX rats retained sodium and water following nerve ablation such that cumulative balances were significantly higher by the end of the protocol as compared to SHAM rats. This response was significantly attenuated by combining CGX with RDNX.
Figure 3.

Effect of SHAM, RDNX, CGX and RDNX-CGX on cumulative sodium and water balance. * = p < 0.05 for RDNX-CGX vs. SHAM. † = p < 0.05 for CGX vs. SHAM. ‡ = p < 0.05 for CGX vs. RDNX-CGX.
Tissue Norepinephrine Content
Tissues were collected at the end of the study to determine the extent of denervation 4 weeks post-procedure. Results of the norepinephrine assay clearly show that RDNX selectively denervated the kidneys, CGX selectively denervated the gut and RDNX-CGX denervated both (Figure 4).
Figure 4.

Effect of SHAM, RDNX, CGX and RDNX-CGX on norepinephrine (NE) content in the duodenum, liver, spleen, left kidney and right kidney. * = p < 0.05 vs. SHAM. † = p < 0.05 vs. RDNX. ‡ = p < 0.05 vs. CGX.
DISCUSSION
The sympathetic nervous system plays an important role in some forms of human hypertension; however, the degree to which sympathetic activity to the kidneys, relative to non-renal vascular beds, contributes to the pathogenesis and maintenance of essential hypertension remains unclear27-29. The recent success of catheter based renal nerve ablation to treat drug resistant hypertension supports the concept that the kidneys are a key target of the sympathetic nervous system in hypertension, but it is still not clear whether other vascular beds are also important.
The present study was designed to address this question by measuring the cardiovascular and fluid balance responses to targeted ablation of sympathetic nerves to the kidneys and the splanchnic vascular bed, separately and in combination, in Dahl S rats. The main findings of this study were: 1) RDNX decreased arterial pressure independent of changes in sodium and water balance; 2) CGX decreased arterial pressure to a similar magnitude as RDNX despite compensatory increases in HR and sodium and water balance; 3) combined RDNX and CGX induced the greatest fall in arterial pressure suggesting that the responses to RDNX and CGX are mediated by separate mechanisms. Overall, these results suggest that targeted sympathetic ablation is an effective treatment for established hypertension in the Dahl S rat and that both the renal and splanchnic vascular beds are important sympathetic targets in this model.
Renal denervation partially reverses salt-induced hypertension in Dahl S rats
RDNX has no effect on the developmental phase of Dahl S hypertension20-22. However, the ability of RDNX to reverse this model of hypertension has not been reported. Compared to SHAM rats, arterial pressure was ~15 mmHg lower in RDNX rats throughout the 4 week post-procedure period. The mechanism(s) by which RDNX attenuate(s) any form of hypertension is a subject of great debate. One explanation is that RDNX increases renal sodium and water excretion and causes a subsequent contraction of blood volume30 by suppressing sympathetically mediated renin secretion and/or sodium reabsorption. However, we found no differences in daily or cumulative sodium and water balance between SHAM and RDNX rats. This is consistent with our previous reports that RDNX decreases arterial pressure in normotensive Sprague Dawley rats independent of sodium balance or renin release31, 32. It is important to note that RDNX did not affect the salt sensitivity of arterial pressure in Sprague Dawley rats31 or Dahl S rats.
Another possibility is that RDNX results in renal vasodilation. Although this hypothesis remains to be tested in the Dahl S rat, it was recently reported that renal nerve ablation in patients with drug resistant hypertension decreases renal vascular resistance with no change in glomerular filtration rate33.
A third possibility is that RDNX attenuates hypertension by ablation of centrally projecting afferent renal nerves reducing sympathetic activity to non-renal vascular beds. As Dahl S rats become increasingly hypertensive, renal injury worsens34-36, and evidence suggests that kidney disease can drive afferent renal nerve-dependent sympathetically mediated hypertension37, 38. The progressive nature of this proposed kidney disease dependent sympathoexcitation may explain the difference in effectiveness of RDNX to reverse, rather than prevent Dahl S hypertension. However, it is important to note that the arterial pressure response to RDNX may not require augmented afferent signaling since it occurs in normotensive Sprague-Dawley rats25, 39.
Celiac Ganglionectomy Partially Reverses Hypertension in the Dahl S Rat: Possible Role of Blood Volume Redistribution
A novel finding of this study is that CGX decreased arterial pressure in Dahl S rats to a similar magnitude as RDNX. To our knowledge this the first study, in any experimental model, demonstrating the ability of CGX to reverse hypertension. Although the mechanisms by which CGX reversed Dahl S hypertension were not explored, it is likely that, similar to our studies in the AngII-salt model12, CGX decreased splanchnic vascular resistance and increased total vascular conductance, both of which would promote a redistribution of blood from the arterial to the highly compliant venous compartment. This hypothesis is in line with our recently published mathematical model of salt-sensitive hypertension in which the distribution of blood volume between a high compliant (i.e. splanchnic) and low compliant (i.e. kidney) vascular bed is determined by neural input to each vascular bed40. Additional experiments will be needed to test this hypothesis.
Sympathetic nerve activity was not measured in this study and therefore it remains to be tested whether the responses to CGX were due to decreased sympathetic nerve activity per se. Alternative explanations include the possibility that ablation of sensory nerves in the gut may affect arterial pressure in non-sympathetically mediated ways (i.e. changes in immune system function or circulating levels of vasopressin). However, we have reported in other models that CGX decreases non-hepatic splanchnic norepinephrine spillover41, and increases total vascular capacitance12. These findings combined with measurements of tissue NE in the present study are most consistent with idea that CGX reduces sympathetic input to splanchnic vasculature resulting in decreased arterial pressure. This hypothesis remains to be tested by studies of the splanchnic hemodynamic responses to CGX in the Dahl S rat.
The magnitude and time course of the arterial pressure response to CGX was nearly identical to RDNX suggesting these procedures may act via a common pathway. However, the responses of the other variables suggest that RDNX and CGX reduced arterial pressure by separate mechanisms. Specifically, CGX resulted in marked tachycardia and sodium and water retention compared to SHAM and RDNX rats. These responses are consistent with activation of compensatory mechanisms to maintain arterial pressure following loss of sympathetic input to the splanchnic vascular bed which may reduce effective blood volume and therefore cardiac output (via increased venous capacitance and reduced atrial filling) and total peripheral resistance42. These results are consistent with compensatory increases in both cardiac and renal sympathetic activity following CGX. The greater tachycardic response to RDNX-CGX compared to CGX suggests a baroreflex mediated increase in cardiac sympathetic activity since the antihypertensive effect of RDNX-CGX was much greater than that of CGX. The blunted sodium and water retention following RDNX-CGX compared to CGX is consistent with the activation of renal sympathetic nerves following CGX. In addition, reduced atrial filling would be expected to inhibit the release of atrial natriuretic peptide (ANP) promoting sodium retention. The extent to which these mechanisms buffer the arterial pressure response to CGX in Dahl S rats remains to be determined.
Another possibility is that the compensatory HR and fluid balance responses to CGX were secondary to volume depletion due to decreased sodium and water absorption from the small intestine, since some rats exhibited diarrhea following CGX similar to previous studies using CGX43, 44. While we cannot discount this entirely, we do not feel it was a major contributor to the responses to CGX for several reasons. First, in past studies we have shown that CGX increases total vascular capacitance but has no effect on absolute blood volume12. Second, although some rats exhibited transient diarrhea (1-2 weeks), sodium retention persisted during the 2-3 weeks following the cessation of diarrhea. Finally, there was no correlation between final body weight (an index of nutrient absorption) and final arterial pressure in any group (please see http://hyper.ahajournals.org Figure S4). We conclude that the compensatory responses of HR and fluid balance to CGX are secondary to a decrease in effective blood volume rather than a reduction in absolute volume. This hypothesis will be tested in future studies in which we measure the effect of CGX on mean circulatory filling pressure in Dahl S rats.
Additive Effects of Renal and Splanchnic Denervation in the Treatment of Hypertension
Further evidence that RDNX and CGX act via separate pathways is the fact that the combination of these treatments resulted in a greater response than either treatment alone. In addition, based on analysis of tissue norepinephrine content, RDNX had no effect on the duodenum, liver or spleen and CGX had no effect on the kidneys. Taken together we conclude that the renal nerves and splanchnic nerves contribute to the maintenance of Dahl S hypertension independently of one another and therefore RDNX and CGX reduce arterial pressure via two distinct mechanisms.
It is worth noting that the compensatory increases in HR and sodium and water retention seen in CGX rats were also observed in RDNX-CGX rats. The increase in HR during the first peak tended to be greater in RDNX-CGX rats than in CGX rats, which is consistent with a baroreflex mediated response as discussed above. Similarly, sodium retention in RDNX-CGX rats may have resulted from the greater fall in renal perfusion pressure, via the pressure-natriuresis relationship or suppression of plasma ANP as discussed above.
PERSPECTIVES
Renal nerve ablation has been shown to decrease arterial pressure in some human hypertensives; however, the lack of a method to quantitate the extent of denervation in humans makes it impossible to establish the efficacy of this treatment since failure to respond may be due to incomplete denervation or the fact that renal nerves do not contribute to all forms of human essential hypertension. The Dahl S rat is an excellent animal model for preclinical studies to address this issue. The response of arterial pressure to renal denervation in this model suggests that this approach can partially reverse hypertension but sympathetic nerves to other target organs are also important. Specifically, our study suggests that targeted splanchnic nerve ablation may have an additional therapeutic effect and should be pursued as a possible stand-alone or adjunct treatment for hypertension in the future. Further studies are needed to establish the mechanisms underlying the antihypertensive effects of these interventions.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
RDNX and CGX both decrease arterial pressure in hypertensive Dahl S rats.
CGX and RDNX-CGX, but not RDNX cause increases in heart rate as well as sodium and water retention.
RDNX-CGX has an additive antihypertensive effect.
The responses to RDNX and CGX are mediated by different mechanisms.
What Is Relevant?
RDNX decreased arterial pressure to a magnitude similar to that reported in humans.
CGX decreased arterial pressure as has been reported in human hypertensives.
Summary
The results of this study suggest that both the renal and splanchnic nerves contribute to the maintenance of Dahl S hypertension and that targeted ablation of the splanchnic sympathetic nerves should be considered as treatment option for hypertensive patients.
Acknowledgments
The authors would like to thank Dusty Van Helden and Marcos Kuroki for their help with metabolic data collection and analysis.
SOURCES OF FUNDING
This research was supported by NIH grant R01 HL076312
Footnotes
CONFLICT(S) OF INTEREST/DISCLOSURE(S) STATEMENT
J.W.O. is a paid consultant of Medtronic CardioVascular, Inc. Santa Rosa, CA
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Persell SD. Prevalence of resistant hypertension in the united states, 2003-2008. Hypertension. 2011;57:1076–1080. doi: 10.1161/HYPERTENSIONAHA.111.170308. [DOI] [PubMed] [Google Scholar]
- 3.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the united states, 1988 to 2008. Circulation. 2011;124:1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong KL, Cheung BMY, Man YB, Lau CP, Lam KSL. Prevalence, awareness, treatment, and control of hypertension among united states adults 1999-2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 5.Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, Papademetriou V, Probstfield J, Wright JT, Jr, Alderman MH, Weiss RJ, Piller L, Bettencourt J, Walsh SM Group ACR. Success and predictors of blood pressure control in diverse north american settings: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (allhat) Journal of clinical hypertension. 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 6.Symplicity HTNI. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the symplicity htn-2 trial): A randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 7.Symplicity HTNI. Catheter-based renal sympathetic denervation for resistant hypertension: Durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 8.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 9.Kubicek WG, Kottke FJ, Laker DJ, Visscher MB. Renal function during arterial hypertension produced by chronic splanchnic nerve stimulation in the dog. Am J Physiol. 1953;174:397–400. doi: 10.1152/ajplegacy.1953.174.3.397. [DOI] [PubMed] [Google Scholar]
- 10.Smithwick RH, Thompson JE. Splanchnicectomy for essential hypertension; results in 1,266 cases. Journal of the American Medical Association. 1953;152:1501–1504. doi: 10.1001/jama.1953.03690160001001. [DOI] [PubMed] [Google Scholar]
- 11.Grimson KS, Orgain ES, Anderson B, D’Angelo GJ. Total thoracic and partial to total lumbar sympathectomy, splanchnicectomy and celiac ganglionectomy for hypertension. Annals of surgery. 1953;138:532–547. doi: 10.1097/00000658-195310000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin ii salt hypertension in rats. Hypertension. 2007;50:547–556. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- 13.Takeshita A, Mark AL, Brody MJ. Prevention of salt-induced hypertension in the dahl strain by 6-hydroxydopamine. The American journal of physiology. 1979;236:H48–52. doi: 10.1152/ajpheart.1979.236.1.H48. [DOI] [PubMed] [Google Scholar]
- 14.Friedman R, Tassinari LM, Heine M, Iwai J. Differential development of salt-induced and renal hypertension in dahl hypertension-sensitive rats after neonatal sympathectomy. Clinical and experimental hypertension. 1979;1:779–799. doi: 10.3109/10641967909068639. [DOI] [PubMed] [Google Scholar]
- 15.Goto A, Ikeda T, Tobian L, Iwai J, Johnson MA. Brain lesions in the paraventricular nuclei and catecholaminergic neurons minimize salt hypertension in dahl salt-sensitive rats. Clin Sci (Lond) 1981;61(Suppl 7):53s–55s. doi: 10.1042/cs061053s. [DOI] [PubMed] [Google Scholar]
- 16.Gordon FJ, Matsuguchi H, Mark AL. Abnormal baroreflex control of heart rate in prehypertensive and hypertensive dahl genetically salt-sensitive rats. Hypertension. 1981;3:I135–141. doi: 10.1161/01.hyp.3.3_pt_2.i135. [DOI] [PubMed] [Google Scholar]
- 17.Mark AL. Sympathetic neural contribution to salt-induced hypertension in dahl rats. Hypertension. 1991;17:I86–90. doi: 10.1161/01.hyp.17.1_suppl.i86. [DOI] [PubMed] [Google Scholar]
- 18.Bugenhagen SM, Cowley AW, Jr, Beard DA. Identifying physiological origins of baroreflex dysfunction in salt-sensitive hypertension in the dahl ss rat. Physiological genomics. 2010;42:23–41. doi: 10.1152/physiolgenomics.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boegehold MA, Huffman LJ, Hedge GA. Peripheral vascular resistance and regional blood flows in hypertensive dahl rats. The American journal of physiology. 1991;261:R934–938. doi: 10.1152/ajpregu.1991.261.4.R934. [DOI] [PubMed] [Google Scholar]
- 20.Osborn JL, Roman RJ, Ewens JD. Renal nerves and the development of dahl salt-sensitive hypertension. Hypertension. 1988;11:523–528. doi: 10.1161/01.hyp.11.6.523. [DOI] [PubMed] [Google Scholar]
- 21.Wyss JM, Sripairojthikoon W, Oparil S. Failure of renal denervation to attenuate hypertension in dahl nacl-sensitive rats. Canadian journal of physiology and pharmacology. 1987;65:2428–2432. doi: 10.1139/y87-385. [DOI] [PubMed] [Google Scholar]
- 22.Iwata T, Muneta S, Kitami Y, Okura T, Ii Y, Murakami E, Hiwada K. Effect of renal denervation on the development of hypertension in dahl-iwai salt-sensitive rats. Nihon Jinzo Gakkai shi. 1991;33:867–871. [PubMed] [Google Scholar]
- 23.Nagasu H, Satoh M, Kuwabara A, Yorimitsu D, Sakuta T, Tomita N, Kashihara N. Renal denervation reduces glomerular injury by suppressing nad(p)h oxidase activity in dahl salt-sensitive rats. Nephrol Dial Transpl. 2010;25:2889–2898. doi: 10.1093/ndt/gfq139. [DOI] [PubMed] [Google Scholar]
- 24.Veitenheimer B, Osborn JW. Role of spinal v1a receptors in regulation of arterial pressure during acute and chronic osmotic stress. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;300:R460–469. doi: 10.1152/ajpregu.00371.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob F, Ariza P, Osborn JW. Renal denervation chronically lowers arterial pressure independent of dietary sodium intake in normal rats. American journal of physiology Heart and circulatory physiology. 2003;284:H2302–2310. doi: 10.1152/ajpheart.01029.2002. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Autonomic neuroscience : basic & clinical. 2010;154:66–73. doi: 10.1016/j.autneu.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Joyner MJ, Charkoudian N, Wallin BG. A sympathetic view of the sympathetic nervous system and human blood pressure regulation. Exp Physiol. 2008;93:715–724. doi: 10.1113/expphysiol.2007.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- 29.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 30.DiBona GF, Esler M. Translational medicine: The antihypertensive effect of renal denervation. American journal of physiology. Regulatory, integrative and comparative physiology. 2010;298:R245–253. doi: 10.1152/ajpregu.00647.2009. [DOI] [PubMed] [Google Scholar]
- 31.Jacob F, Ariza P, Osborn JW. Renal denervation chronically lowers arterial pressure independent of dietary sodium intake in normal rats. Am J Physiol-Heart C. 2003;284:H2302–H2310. doi: 10.1152/ajpheart.01029.2002. [DOI] [PubMed] [Google Scholar]
- 32.Jacob F, LaBine BG, Ariza P, Katz SA, Osborn JW. Renal denervation causes chronic hypotension in rats: Role of beta(1)-adrenoceptor activity. Clin Exp Pharmacol P. 2005;32:255–262. doi: 10.1111/j.1440-1681.2005.04179.x. [DOI] [PubMed] [Google Scholar]
- 33.Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, Sobotka PA, Krum H, Scheller B, Schlaich M, Laufs U, Boehm M. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension. 2012;60:419–424. doi: 10.1161/HYPERTENSIONAHA.112.193870. [DOI] [PubMed] [Google Scholar]
- 34.Sterzel RB, Luft FC, Gao Y, Schnermann J, Briggs JP, Ganten D, Waldherr R, Schnabel E, Kriz W. Renal disease and the development of hypertension in salt-sensitive dahl rats. Kidney Int. 1988;33:1119–1129. doi: 10.1038/ki.1988.120. [DOI] [PubMed] [Google Scholar]
- 35.Tian N, Moore RS, Phillips WE, Lin L, Braddy S, Pryor JS, Stockstill RL, Hughson MD, Manning RD., Jr Nadph oxidase contributes to renal damage and dysfunction in dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1858–1865. doi: 10.1152/ajpregu.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD., Jr Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005;45:934–939. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 37.Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 38.Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, Dietl KH, Rahn KH. Sympathetic nerve activity in end-stage renal disease. Circulation. 2002;106:1974–1979. doi: 10.1161/01.cir.0000034043.16664.96. [DOI] [PubMed] [Google Scholar]
- 39.Jacob F, LaBine BG, Ariza P, Katz SA, Osborn JW. Renal denervation causes chronic hypotension in rats: Role of beta1-adrenoceptor activity. Clinical and experimental pharmacology & physiology. 2005;32:255–262. doi: 10.1111/j.1440-1681.2005.04179.x. [DOI] [PubMed] [Google Scholar]
- 40.Averina VA, Othmer HG, Fink GD, Osborn JW. A new conceptual paradigm for the haemodynamics of salt-sensitive hypertension: A mathematical modelling approach. The Journal of physiology. 2012;590:5975–5992. doi: 10.1113/jphysiol.2012.228619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kandlikar SS, Fink GD. Splanchnic sympathetic nerves in the development of mild doca-salt hypertension. Am J Physiol-Heart C. 2011;301:H1965–H1973. doi: 10.1152/ajpheart.00086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fink GD, O JW. The splanchnic circulation. In: Robertson D, editor. Primer on the autonomic nervous system. Amsterdam: Elsevier Academic Press; 2012. pp. 211–214. [Google Scholar]
- 43.Marlett JA, Code CF. Effects of celiac and superior mesenteric ganglionectomy on interdigestive myoelectric complex in dogs. Am J Physiol. 1979;237:E432–443. doi: 10.1152/ajpendo.1979.237.5.E432. [DOI] [PubMed] [Google Scholar]
- 44.Freedman MA, Hallenbeck GA, Code CF. The effect of vagotomy and of methantheline bromide on the diarrhea produced by celiac and superior mesenteric ganglionectomy. Surg Forum. 1953:481–486. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


