Abstract
Over the last three decades, the California sea hare, Aplysia californica, has played an increasingly important role as a model organism in the neurosciences. Since 1995, the National Resource for Aplysia has supported a growing research community by providing a consistent supply of laboratory-reared individuals of known age, reproductive status, and environmental history. The purpose of the present study was to resolve the key biological factors necessary for successful culture of large numbers of high quality larval Aplysia. Data from a sequence of five experiments demonstrated that algal diet, food concentration, and veliger density significantly affected growth, attainment of metamorphic competency, and survival of Aplysia larvae. The highest growth and survival were achieved with a mixed algal diet of 1:1 Isochrysis sp (TISO) and Chaetoceros muelleri (CHGRA) at a total concentration of 250 x 103 cells/mL and a larval density of 0.5 – 1.0 per mL. Rapid growth was always correlated with faster attainment of developmental milestones and increased survival, indicating that the more rapidly growing larvae were healthier. Trials conducted with our improved protocol resulted in larval growth rates of >14 μm/d, which yielded metamorphically competent animals within 21 days with survival rates in excess of 90%. These data indicate the important effects of biotic factors on the critical larval growth period in the laboratory and show the advantages of developing optimized protocols for culture of such marine invertebrates.
Keywords: Aplysia californica, veliger larvae, diet, density, development, growth, survival
1. Introduction
The importance of the California sea hare (Aplysia californica, Cooper 1863) as a reductionist model for studies in molecular neurobiology, electrophysiology, learning, and memory (Kandel 1976, Moroz et al. 2006) has steadily increased over the past three decades. The large, identifiable neurons, simple nervous system and capacity for basic learned behaviors of this opisthobranch have made Aplysia the most widely studied molluscan model for neuroscience. This marine algavore inhabits intertidal and sublittoral zones along the Pacific coast of the United States and Mexico where it lays large benthic egg masses (Audersirk 1976, Kandel 1979, Carefoot 1987). Eggs hatch after 7–10 days (Kriegstein et al. 1974, Kandel and Capo 1979), releasing planktotrophic veliger larvae that have been reported to remain in the plankton for at least 35 days before attaining metamorphic competency (Kriegstein et al. 1974, Kriegstein 1977, Capo et al. 1979, Nadeau et al. 1989). During metamorphosis, veliger larvae settle and begin feeding on macroalgae such as Agardhiella sp. (Capo 1979, Nadeau et al. 1989). This begins the benthic phase, characteristic of juvenile and adult Aplysia (Kriegstein et al. 1974, Capo et al. 2002). Details of the natural history (Eales 1921, Audersirk 1976, Audersirk 1979), biology (Carefoot 1987) and basic neurobiology (Kandel 1976, Kriegstein 1977) of Aplysia are well documented in the literature.
Early neurobiologcal research on Aplysia was conducted on wild-caught animals, potentially introducing several undesirable sources of variation to experimental results, such as uncertainty associated with parental background, environmental history, nutritional status, reproductive state and age. Due to their annual life cycle, there are seasonal fluctuations in availability of entire size classes of wild animals (Audersirk 1979). Reliance on field-collected specimens provides little or no information on how environmental conditions (e.g. food availability, sea state, salinity, and temperature) influence growth, development, and survival rates (Audersirk 1979, Carefoot 1987, Stommes et al. 2005). Growth rate and age in wild Aplysia is typically estimated from body size (Kriegstein et al. 1974, Kriegstein 1977, Audersirk 1979) which is an unreliable indicator for soft-bodied organisms (Capo et al. 2002, Stommes et al. 2005, Fieber et al. 2005). Age can be more reliably determined by the diameter of the internal shell (Peretz and Adkins 1982), but this approach assumes a constant growth rate based on Kriegstein et al. (1974) and requires that the animals be sacrificed. Clearly there is a need for a reliable source of large numbers of cultured animals of known age and physiological condition. Current research on developmental genetics, bifurcated cell culture, and genomics require large numbers of postmetamorphic Aplysia reared under stringent environmental conditions.
Historically, researchers studying the early life history of biomedically important opisthobranch models focused on describing planktotrophic larval culture (Kriegstein et al. 1974, Strenth and Blankenship 1978, Paige 1986), morphological development (Kriegstein 1977, Kempf 1981), and metamorphosis (Kriegstein et al. 1974, Capo 1979, Bickell and Kempf 1983, Nadeau et al. 1989, Avila 1998). As a result, the early life history of A. californica (Kriegstein et al. 1974, Kriegstein 1977), as well as several other ecologically important opisthobranchs, is well described (Thompson 1958, Franz 1975, Switzer-Dunlap and Hadfield 1977, Perron and Turner 1977, Harrigan and Alkon 1978, Williams 1980, Kempf 1981, Plaut et al. 1995). However, experimentally-derived information resolving the interactions and effects of factors controlling the feeding larval phase of opisthobranchs are extremely limited (Switzer-Dunlap and Hadfield 1977, Hubbard 1988, Plaut et al. 1995, Avila et al. 1997). Previous larval Aplysia rearing trails have been characterized by limited survival rates and reduced growth of <10μm/d (Kriegstein et al 1974, Nadeau et al. 1989, Pawlik 1989). Several authors have discussed the effects of diet on the growth on other opisthobranch larvae and the effects of larval density on their growth and survival (Switzer-Dunlap and Hadfield 1977, Harrigan and Alkon 1978, Williams 1980, Hubbard 1988, Avila et al. 1997). The beneficial effects of a mixed diet on bivalve larvae has been well demonstrated (Davis and Guillard 1958, Loosanoff and Davis 1963, Bayne 1965, Chanely 1975, Gallager et al. 1986).
The National Resource for Aplysia (the Resource) was established in 1995 to support the growing demand for large numbers of all size-classes of Aplysia californica raised under uniform conditions and of known age. Since then, the techniques for rearing larvae and juveniles at the Resource have continually evolved, leading to incremental improvements in animal quality and production (Capo et al. 1999, Capo et al. 2002, Capo et al. 2003, Fieber et al. 2005, Stommes et al. 2005). The present study was derived from previously unpublished baseline information on the successful rearing techniques developed at the Resource. The experiments described here were designed to determine optimal biological parameters for the successful culture of larvae, a prerequisite to production of large numbers of robust animals. Previous research with Opisthobranchs has demonstrated that algal diet (monospecific and mixed), algal concentration and veliger density can each have significant effects on larval growth and development (Switzer-Dunlap and Hadfield 1977, Harrigan and Alkon 1978, Williams 1980, Hubbard 1988, Avila et al. 1997). Specifically, we conducted a sequence of five experimental trials on Aplysia larvae to quantify the effects of algal diet, food concentration, and veliger density on their growth, attainment of metamorphic competency and survival. We draw comparisons with earlier studies and discuss the possible biological implications of our results and their application to the culture of other opisthobranchs used in neurobiology.
2. Materials and Methods
2.1. Broodstock and eggs
Adult animals (Aplysia californica) collected by Santa Barbara Marine Biologicals were housed in the flow-through seawater system at the Resource as described by Capo et al. (2002). The animals were fed a daily ration of one or more of the following laboratory–cultured seaweeds: Gracilaria ferox, Agardhiella subulata (strain A2), and Ulva sp. (Capo et al. 1999, 2002). The light cycle was maintained at 12 h light:12 h dark and the seawater temperature was 14±1°C. Mating pairs were monitored throughout the day for active egg-laying. During oviposition, a 10 cm portion of egg strand was collected, immediately rinsed with 0.45Em filtered seawater of the same temperature and salinity, and incubated in a 2 L flask containing filtered seawater to which Na2EDTA (0.25mg/L) was added to bind heavy metals in the natural seawater that may deleteriously affect development (Capo et al. 2002). The eggs and seawater were vigorously aerated until one day prior to hatching in a temperature-controlled incubator at 22°C. Hatching occurred 7–8 days after eggs were deposited. The egg strand (cordon) was inspected under a dissecting microscope at six days post-oviposition to confirm normal and synchronized development of embryos; strands not meeting these criteria were discarded.
Estimation of the number of larvae/mm of cordon was conducted by cutting three portions, determining their lengths with an ocular micrometer, and dissolving each segment in 2% sodium hypochlorite. The veliger shells were counted and the total number of larvae/mm of egg strand calculated. Additionally, the shell lengths (SL) of 25 empty shells from each portion of cordon were measured using an ocular micrometer at 50x magnification. These techniques were used to determine the day 0 SL in microns for each experiment. To establish initial larval density, the appropriate length of cordon was aseptically cut, rinsed with 2μm filtered seawater, and transferred directly to the larval culture vessel.
2.2. Larval rearing conditions
Seawater from Bear Cut, Virginia Key, Florida was prepared by prefiltration through a 15 μm glass media filter, adjustment of salinity to 32 ppt with deionized water, addition of chloramphenicol (2.5 mg/l), Na2EDTA (0.25 mg/L), and aeration for 18–24 h followed by 2 μm vacuum filtration (Millipore AP2504700) (Kriegstein et al. 1974, Nadeau et al. 1989). The desired concentration of microalgae and estimated length of egg mass were added to filtered seawater in 2 L roller bottles (Corning). The air-water interface was eliminated by sealing the vessel with Parafilm and plastic wrap (Paige 1986, Capo et al. 1987, Tamse et al. 1990). Cultures were incubated on a continuously rotating (1 rpm) roller bottle apparatus (Wheaton), with a 24 hr fluorescent light regime (~0.001 μE/cm2/s) at a constant temperature of 22°C (Kriegstein et al. 1974, Nadeau et al. 1989, Tamse et al. 1990). Roller apparatus positions were randomly assigned to each culture vessel at the start of the experiment and remained fixed throughout the experiment.
After hatching, larvae were measured and the culture media was changed every 7 days. Larvae were collected on a 74 μm mesh screen, rinsed with filtered seawater (FSW) and transferred to a sterile crystallizing dish. An iodine-based surfactant (Betadine Surgical Scrub) was added to resuspend any larvae entrapped by the air-water interface. Larvae were treated with 1.25 mL of a solution of 2.5 mg/mL poly(vinylpyrrolidone)–iodine complex (Sigma) and 2.0 mg/mL pH 8.3 fish-grade Trizma (Sigma) solution for 5 min to inhibit bacterial growth. This treatment also acted to suppress swimming of the larvae and provided a non-lethal method to facilitate shell length measurements. The SL of 25 larvae was measured and each was staged according to Kriegstein et al. (1974). At the end of the exposure period, the iodine concentration was reduced by incremental addition of a 0.4% sodium thiosulfate solution to the treatment bath until the characteristic iodine color disappeared. The larvae were rinsed in FSW and transferred to a clean, acid-washed roller bottle with FSW containing the appropriate amount of microalgae and sealed. The bottles were then returned to the previously assigned roller bottle apparatus and position.
2.3. Microalgae and media preparation
Primary microalgae inocula were purchased from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton (CCMP, Table 1). All references to scientific names, strain identification, and culture numbers conform to CCMP’s nomenclature. Working stocks were aseptically transferred to fresh f/2 media (Guillard 1975) weekly and prepared with autoclaved 32 ppt ambient seawater. Stocks were aerated and housed at 22°C under continuous light from a single cool white fluorescent bulb. Bacterial contamination of the primary phytoplankton cultures was monitored monthly by streaking on Bacto marine agar 2216 (DIFCO) and any contaminated cultures were discarded. Cell concentrations were determined by hemocytometer and cells were collected by low speed centrifugation prior to transfer to filtered seawater.
Table 1.
List of algal species, strain, and culture collection numbers used for feeding studies.
| Diatoms | Strain | CCMP number |
|---|---|---|
| Chaetoceros muelleri | CHGRA | CCMP1316 |
| Chaetoceros calcitrans | CHCAL | CCMP1315 |
| Skeletonema costatum | SKEL | CCMP1332 |
| Thalassiosira pseudonana | 3H | CCMP1015 |
| Flagellates | ||
| Isochrysis sp | TISO | CCMP1324 |
| Tetraselmis suecica | TETRA | CCMP904 |
| Pavlova lutheri | PAV | CCMP1325 |
| Micromonas pusilla | MICRO | CCMP487 |
| Dunaliella tertiolecta | DUN | CCMP1320 |
| Rhodomonas salina | 3C | CCMP1319 |
2.4. Experimental design
Five experiments were conducted to evaluate the biological factors controlling larval growth, development, and survival of A. californica in culture (Table 2). The first two experiments examined the effects of feeding larvae different combinations of nine algal species. Utilizing the most successful diet combination, the third experiment examined food concentration effects. Building on these results, the last two experiments examined the effects of varying larval density under either fixed or rationed food quantities. Details of each experiment follow.
Table 2.
Experiments to test effects of biotic variables on growth, development and survival of A. californica larvae. Values in brackets indicate number of experimental conditions tested for that variable.
| Experiment | Diet type (1:1 ratio by cell number) |
Algal concentration (cells/mL) |
Larval density, (individuals/mL) |
Replicates per condition |
|---|---|---|---|---|
| 1 – Evaluation of Flagellates | CHGRA + Flagellate [6] | 250 x 103 | 0.75 | 4 |
| 2 – Evaluation of Diatoms | Diatom [4] + TISO | 250 x 103 | 0.75 | 3 |
| 3 – Algal concentration | CHGRA + TISO | 10 – 300 x 103 [8] | 0.75 | 4 |
| 4 – Larval density | CHGRA + TISO | 250 x 103 | 0.5 – 4.0 [5] | 3 |
| 5 – Larval density and ration | CHGRA + TISO | 333 x 103 | 1.0 – 4.0 [4] | 4 |
2.5. Experiment 1: Comparison of mixed larval diet composed of Chaetoceros muelleri(CHGRA) with a selected flagellate
This experiment compared diets composed of one of six flagellate taxa commonly used in molluscan culture (Table 1) in combination with the diatom, CHGRA. Treatments were replicated four times and larvae were fed one of the six flagellate species plus the diatom according to a standard feeding regime of 250 x 103 cells/mL in a 1:1 ratio based on cell/mL.
2.6. Experiment 2: Comparison of mixed larval diet composed of Isochrysis sp. (TISO) with a selected diatom
The second set of feeding trials paired one species of the flagellate, TISO, with one of four diatoms (Table 1) at a 1:1 ratio and a total concentration of 250 x 103 cells/mL. Trials were replicated 3 times.
2.7. Experiment 3: Effects of algal concentration
To evaluate the effects of algal concentration, eight algal feeding regimes with four replicates of each were compared. Based on experiments 1 and 2, a standard diet of CHGRA and TISO at a 1:1 ratio was used to provide starting total algal concentrations of 10, 20, 50, 100, 150, 200, 250, and 300 x 103 cells/mL.
2.8. Experiment 4: Evaluating larval density effects under fixed initial algal concentrations conditions
This experiment measured effects of larval density on survival, growth, and metamorphic competency under conditions of a fixed initial algal concentration of 250 x103 cells/mL using a CHGRA:TISO (1:1) mixture. The species of algae and concentrations used were based on the previous three experiments. Five veliger densities 0.5, 1.0, 2.0, 3.0, and 4.0 larvae/mL (l/mL) were evaluated.
2.9. Experiment 5: Evaluating larval density effects under rationed food conditions
This experiment was conducted to test the possibility that the slower observed growth and lower survival at higher larval densities observed in Experiment 4 resulted from food limitation as larval density increased. This experiment also used a CHGRA:TISO (1:1) mixture, while larval density was varied from 1 to 4 per mL. However, the initial algal concentration was increased proportionately with larval density, such that the initial algal density was set at 333 x103 cells/larva. Note that this was equivalent to an algal concentration of 250 x103 cells/mL for a larval density of 0.75 larvae/mL, as used in experiments 1–3. Thus, initial algal density was varied from 333 x103 cells/mL at a larval density of 1 larva/mL to 1,332 x 103 cells/mL at larval density of 4 larvae/mL. Weekly, dissolved oxygen levels were monitored with a YSI-55 multi-probe instrument.
2.10. Data analysis
Three metrics were used to assess larval performance under controlled diet type, food concentration and larval density treatments: growth rate, time to attainment of metamorphic competency, and survival. All three metrics were determined on a per roller bottle basis. Growth rates (μm/d) were calculated by regressing larval SL measurements against age (days post-hatch) for days 0 through 21. Metamorphic competency was defined as the time (in elapsed days) at which ≥ 80% of individuals possessed 4–6 red, lateral pigmentation spots (indicating that animals were developed sufficiently to undertake metamorphosis). Survival was expressed as the percentage of initial larvae that remained alive at the time when 80% competency was reached. Trials were terminated when any of the following conditions were met: (1) ≥ 80% of the larvae were competent to metamorphose; (2) < 25 individuals remained alive (considered a failed culture); or (3) once 50 days had elapsed. Confirmation of normal larval development was evaluated by observing metamorphosis following exposure of competent larvae to the metamorphosis inducing substrate Agardhiella subulata (Capo 1979, Nadeau et al. 1989, Capo et al. 2002). Effects of diet type on larval performance (i.e. Experiments 1 and 2) were assessed using analysis of variance (ANOVA), followed by t-tests. For the latter, experimentwise error rate was held at p< 0.10 using the Bonferroni method (Sokal and Rohlf 1987). Linear or non-linear regression was used in Experiments 3, 4 and 5, which addressed the effects of varying food concentrations and larval densities. Following Sokal and Rohlf (1987), growth and competency values were loge-transformed and survival values were arcsine-transformed prior to statistical analyses.
3. Results
3.1. Experiment 1: Comparison of mixed larval diet composed of CHGRA with a selected flagellate
Mean larval growth rates ranged 4.8–14.2 μm/d with the lowest and highest rates associated with CHGRA:TETRA and the CHGRA:TISO diets, respectively (Table 3). Larvae fed 4 of the 6 combinations reached metamorphic competency at a mean of 29–40 days. The larvae in the remaining 2 diet treatments died before attaining metamorphic competency. The CHGRA:TISO combination yielded significantly higher growth rates than three of the treatments. This combination was also associated with the shortest average time to metamorphic competency and the highest mean survival rate, although these differences were not statistically significant.
Table 3.
Effects of diet (Experiment 1) on A. californica larvae fed a diet of Chaetoceros muelleri (CHGRA) in combination with selected flagellates. Results represent the mean ± 1 standard deviation; a one-way ANOVA with a Tukey’s post hoc test was used to determine statistical significance (different letters indicate significant differences at p<0.05).
| CHGRA:Flagellate | Growth rate (μm/day) | Days to 80% competency | Percent larval survival at 80% competency |
|---|---|---|---|
| CHGRA:TISO | 14.2 ± 0.6a | 28.8 ± 3.5a | 75.3 ± 11.8a |
| CHGRA:DUN | 11.8 ± 1.1a,b | 37.5 ± 4.0a | 62.3 ± 10.9a |
| CHGRA:MONO | 9.3 ± 1.9b | 39.75 ± 7.6a | 60.1 ± 9.6a |
| CHGRA:MICRO | 11.5 ± 2.1a,b | 32.7 ± 7.6a | 50.1 ± 34a |
| CHGRA:3C | 5.5 ±0.5c | * | 0.0 ± 0.0b |
| CHGRA:TETRA | 4.8 ± 0.4c | * | 0.0 ± 0.0b |
indicates that 80% of larvae in treatment failed to reach competency within 50 days.
3.2. Experiment 2: Comparison of mixed larval diet composed of TISO with a selected diatom
Mean larval growth rates ranged from 10.6–13.2 μm/d and were not statistically different between treatments with the exception of 3H:TISO, which produced the slowest growth (Table 4). The TISO in combination with CHCAL and CHGRA produced the highest growth rates of 13.1 and 13.2μm/d, respectively. These larvae also reached metamorphic competency significantly sooner than those fed SKEL:TISO or 3H:TISO, which required 7–9 additional days to reach competency. Survival rates were consistently high (> 77 %) for all groups, except for larvae fed 3H:TISO, which showed only 39% survival at competency.
Table 4.
Effects of diet (Experiment 2) on A. californica larvae fed Isochrysis galbana (TISO) fed in combination with a selected diatom. Results represent the mean ± 1 standard deviation; a one-way ANOVA with a Tukey’s post hoc test was used to determine statistical significance (different letters indicate significant differences at p<0.05).
| Diatom:TISO | Growth rate (μm/day) | Days to 80% competency | Percent larval survival at 80% competency |
|---|---|---|---|
| CHGRA:TISO | 13.1 ± 0.4a | 34.1 ± 0.2a | 81.6 ± 13.6a,b |
| CHCAL:TISO | 13.2 ± 0.6a | 34.0 ± 0.0a | 77.7 ± 21.8a,b |
| SKEL:TISO | 11.9 ± 0.5a,b | 41.0 ± 0.0b | 86.1 ± 10.4a |
| 3H:TISO | 10.6 ± 0.7b | 43.3 ± 4.0b | 39.0 ± 17.8b |
3.3. Experiment 3: Effects of algal concentration
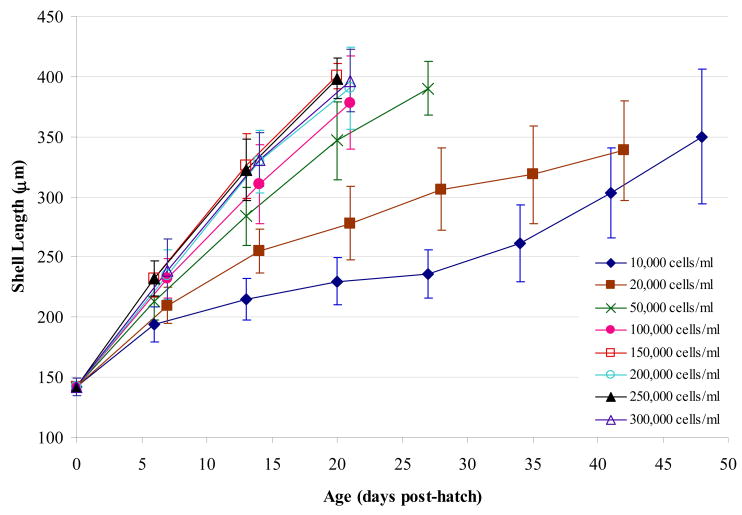
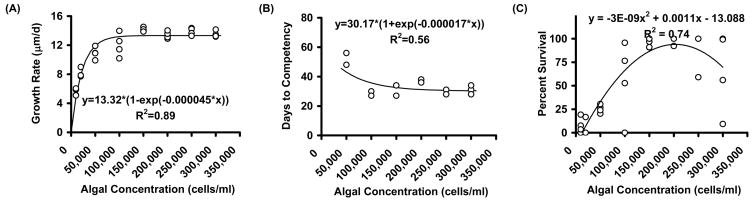
Mean larval growth trajectories were sharply reduced at the two lowest concentrations (Figure 1). The relationship between larval growth rates and algal concentration was asymptotic (R2 = 0.89; p < 0.001), with rates varying from 5.5 to 14.1 μm/d (Figure 2A). Increasing food concentrations from 10 x 103 up to 150 x 103 cells/mL yielded large increases in growth rate. Beyond this food level, little change in growth rates emerged. An asymptotic relationship (R2 = 0.56; p < 0.02) was observed in terms of days to competency (Figure 2B). Larvae fed the two lowest concentrations failed to reach 80% metamorphic competency during the 50 day maximum experimental time period, while levels ≥ 100 x 103 cells/mL produced very similar, rapid rates of progression to competency. The relationship between survival rate and algal concentration (Figure 2C) was parabolic (R2 = 0.82; p < 0.001) with peak levels at 200 x 103 cell/mL. Variation in survival rates was consistently high in all concentrations beyond 200 x 103 cell/mL, with a general tendency of decline thereafter. The results of this experiment suggested that the best algal concentration ranged from 150 – 250 x 103 cells/mL.
Figure 1.
Effect of algal concentration (Experiment 3) on veliger shell length trajectories. Shell length was measured weekly from day of hatch until 80% competency (or termination of experiment). Diet was 1:1 ratio of CHGRA:TISO; larval density was 0.75 individuals per mL.
Figure 2.
Effect of algal concentration (Experiment 3) on growth rate (A), days to competency (B) and percent survival (C). Diet was 1:1 ratio of CHGRA:TISO; larval density was 0.75 individuals per mL.
3.4. Experiment 4: Evaluating larval density effects under fixed initial algal concentrations
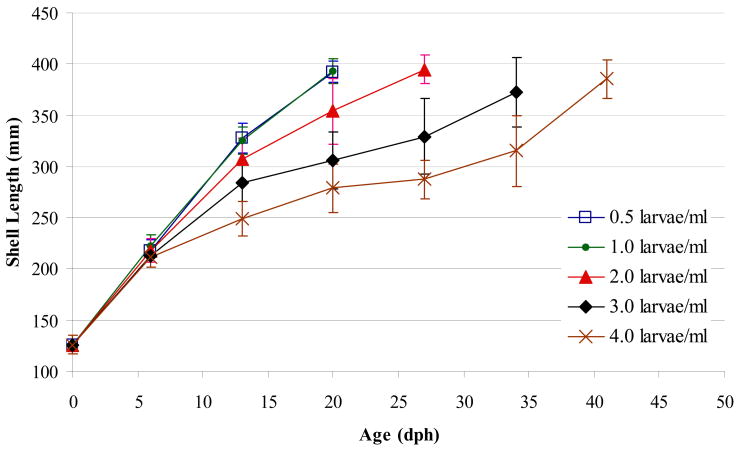
Growth trajectories indicated that growth rates slowed as larval densities increased beyond 1 larvae/mL (Figure 3). The negative linear relationship between larval growth rates and larval density ranged from 15.5-9.3 μm/d (R2 = 0.94; p<0.01, Figure 4A). A positive linear relationship described the trend between larval density and days to competency (R2 = 0.923; p<0.01, Figure 4B), whereas a negative linear relationship emerged for survival rates (R2 = 0.899; p<0.01, Figure 4C). The results from this experiment suggest that with regard to survival, growth rate, and time to metamorphosis the best stocking density of larvae was ≤1 larva/mL when the initial algal concentration provided was 250 x 103 cells/mL.
Figure 3.
Effect of larval density (Experiment 4) on veliger shell length trajectories. Shell length was measured weekly from day of hatch until 80% competency. Diet was a was 1:1 ratio of CHGRA:TISO at a fixed initial algal concentration of 250 x 103 cells/mL.
Figure 4.
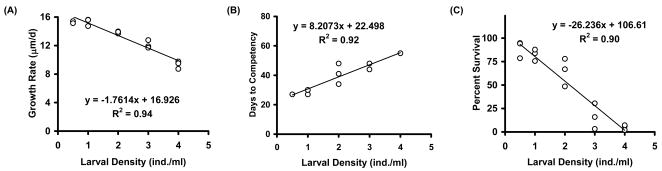
Effects of larval density (Experiment 4) on growth rate (A), days to competency (B), and percent survival (C). Diet was a was 1:1 ratio of CHGRA:TISO at a fixed initial algal concentration of 250 x 103 cells/mL.
3.5. Experiment 5: Evaluating larval density under rationed food conditions
A negative linear relationship (R2 = 0.74; p<0.01) emerged between larval growth rates and larval densities when a food ration equivalent to 333 x103 cells/larva was provided to all treatments (Figure 5A). As was seen in Experiment 4, along the larval density gradient, positive and negative linear relationships emerged for days to competency (R2 = 0.69; p<0.01, Figure 5B) and survival rates (R2 = 0.63; p<0.01, Figure 5C), respectively. These results suggest that food limitation was not a significant driver of the relationships found in Experiment 4. Dissolved oxygen levels increased in culture bottles by an average of 2 mg/L between weekly water changes (presumably due to photosynthesis by the algae), indicating that oxygen levels were not limiting in these experiments.
Figure 5.
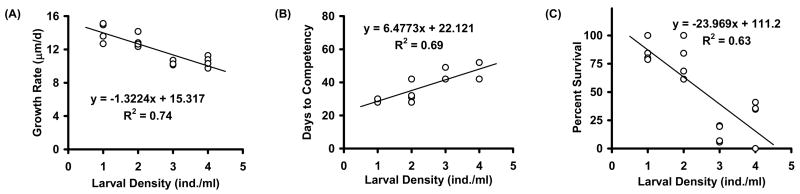
Effect of larval density with fixed algal food ration of 333 x 103 cells/larva (Experiment 5). Shown are growth rate (A), days to competency (B) and percent survival (C) as a function of larval density. Diet was 1:1 ratio of CHGRA:TISO.
3.6. Comparison of morphological development schedules
Growth of larvae under conditions of a mixed algal diet of 1:1 CHGRA:TISO at a total concentration of 250 x 103 cells/mL and a larval density of 0.5 – 1.0/mL also yielded rapid progression of larvae through the 7 described stages of larval development. These milestones were reached more rapidly than in previously reported studies (Kriegstein 1977) as shown in Table 5.
Table 5.
Comparison of morphological development schedules of A. californica larvae as reported by Kriegstein (1977) compared to the present study. Values are the number of days post-hatch until the specified developmental stage was observed.
| Morphological characteristic | Stage | Kriegstein 1977 | Present study |
|---|---|---|---|
| Newly hatched | 1 | 1 | 1 |
| Larval eyes | 2 | 14 | 7 |
| Larval heart | 3 | 21 | 14 |
| Maximum shell size | 4 | 28 | 17 |
| Propodium | 5 | 30 | 19 |
| Competency | 6 | 32 | 21 |
| Metamorphosis | 7 | 34 | 22 |
4. Discussion
A limited understanding of environmental interactions and the effects of biological factors controlling veliger growth, development, and survival have hampered our ability to meet the increasing demand for >30,000 post-metamorphic animals per year for the Aplysia research community. The year-round availability of large numbers of Aplysia of specific ages and sizes grown under controlled laboratory conditions is essential for the future advancements for this community. Our aim was to examine the variables involved in the nutrition and stocking density of these larvae and determine the optimal parameters which would yield rapid development, attainment of competency, and low mortality in larval cultures of A. californica.
4.1. Diet Type
Several authors have discussed the effects of food on the growth of opisthobranch larvae and the effects of larval density on their growth and survival (Switzer-Dunlap and Hadfield 1977, Harrigan and Alkon 1978, Williams 1980, Hubbard 1988, Avila et al. 1997). Certain algal diets have been shown to improve rearing success of larvae of some commercially valuable bivalves (Walne 1963) and gastropods (Paulson and Scheltema 1968, Harrigan and Alkon 1978, Hubbard 1988, Avila et al. 1997). The nutritional benefits of a mixed algal diet, including both flagellates and diatoms, on bivalve larval growth and survival have been well demonstrated (Davis and Guillard 1958, Loosanoff and Davis 1963, Bayne 1965, Chanely 1975, Gallager et al. 1986) and several researchers conducted mixed diet and feeding concentration trials with the planktotrophic larvae of congeners of A. californica and some nudibranchs (Switzer-Dunlap and Hadfield 1977, Harrigan and Alkon 1978, Hubbard 1988, Plaut 1995, Avila 1997). Hubbard (1988) reported success with a mixed feeding regime of a 1:1 ratio of a Tahitian strain of Isochrysis and Ochromonassp. at 0.5–1 x 105 cells/mL when feeding the larval nudibranch Hypselodoris infucata. Larvae exposed to the higher concentration of the mixed diet grew faster than on lower algal concentrations or on a single algal species (Hubbard 1988). There are no previous studies on the effects of feeding a mixed diet specific to A. californica. Our results confirmed that the species composition of the algal diet can have profound effects on larval growth, development, and survival in A. californica.
The benefits of supplementing the diet of molluscan larvae specifically with diatoms in culture have been well demonstrated (Gabbot and Holland 1972, Webb and Chu 1981). Providing a diet that includes both flagellates and diatoms may more closely replicate the natural diet of molluscs. Diatoms are a better source of some nutritional components, such as lipids, than flagellates. Lipids have been shown to be especially critical for successful growth and metamorphosis in bivalves (Gabbott and Holland 1972). Interestingly, the species in the diets that we found to produce the highest growth rates in A. californica were not necessarily the same ones that worked best for other species of molluscs (Gabbott and Holland 1972, Harrigan and Alkon 1978, Thompson et al. 1993). This suggests that the species of algae that result in the best growth and survival rates may depend on diets similar to those the opisthobranch evolved to consume. However, the low or variable growth rates and poor survival in previous diet and feeding studies on other opisthobranch molluscs precludes us from drawing any hard conclusions as to what species of algae may be best for other species. Although it was known that adding diatoms to molluscan diets improved results in rearing trials due to their lack of motility, water movement is needed to keep the diatoms in suspension so that they remain available to the larvae. As a result, attempts to incorporate diatoms into the diet of Aplysia with static larval rearing techniques proved unreliable (Kriegstein et al. 1974, Capo et al. 1987, Nadeau et al., 1989). In the method used in the present study, the rolling of the culture bottle provides the necessary water movement to keep the diatoms in suspension, thereby providing a reliable mechanism for larval access to this important food item.
4.2. Diet Concentration
Numerous studies have demonstrated the importance of algal concentration on molluscan larval growth, development, and survival. Our trial started at the most commonly reported food concentration in the literature for rearing of opisthobranch larvae, 10 x 103 cells/mL (Kriegstein et al. 1974, Switzer-Dunlap and Hadfield 1977, Kempf 1981, Bickell and Kempf 1983, Paige 1986, Avila et al. 1997). Pilot larval rearing trials that we conducted demonstrated faster growth and higher survival at higher algal concentrations. In order to bracket the entire feeding range we chose 300 x 103 cells/mL as our upper limit. This value is similar to that successfully used by Hubbard (1988) who fed a 1:1 of a Tahitian strain of Isochrysis and Ochromonassp. at a concentration of 50 – 100 x 103 cells/mL to the tropical sponge-eating nudibranch, Hypselodoris infucata. Our findings suggest concentration of 150 – 250 x 103 cells/mL should be supplied to achieve maximum growth rates and metamorphic competency of the larvae. This range of concentrations provided the greatest success in larval production with the least amount of mortality. In a short term feeding study, Gallager and Mann (1980) found larval Aplysia grazing rates increased with algal cell concentration, but began to decline at about 300 x 103 cells/mL. In their study and in ours, it was noted that when algal concentrations exceed this level it is probable that the uneaten algal cells result in lower growth rates, either due to fouling of the water by decaying algae or impeding of the feeding mechanisms of the veliger larvae. Inspection of dissolved oxygen levels suggests this was not a contributing factor. We found an increase in oxygen levels, probably as a result of photosynthesis of the algae in the culture bottles producing levels that exceeded larval consumption rates.
4.3. Veliger density
While there are many documented studies evaluating growth, metamorphic competency and survival of molluscan larvae as a function of initial veliger density (Bayne 1965, Switzer-Dunlap and Hadfield 1977, Pechenik et al. 1990, Avila et al. 1997), none have examined these relationships for A. californica. We found that increasing larval densities from 0.5 to 4 larvae/mL significantly reduced all three larval performance metrics. Growth rates declined from approximately 16μm/d at the lowest stocking densities to about 9μm/d at the highest.
Interestingly, the lowest growth rates correspond to the average rate obtained by Kriegstein et al. (1974; 9.8 μm/d). Likewise, time to competency increased by about two-fold and survival rates declined from about 85% to 5% or less over the veliger density range tested. The general trend of poor survival at increasing larval culture densities has been demonstrated for other planktotrophic larval opisthobranchs (Hubbard 1988, Avila et al. 1997), but it is unclear as to why this occurred in our experiments. Based on the results of Experiment 5, we do not consider insufficient food quantities to be the direct underlying cause of the observed larval performance metric declines. Rather, the declines may have been driven by collisions among individuals leading to a range of effects from inhibition of feeding to injury. Greater transmission of disease at high densities is another possibility, although we observed no evidence of bacterial, fungal, protozoan or other such contamination. Dissolved oxygen levels increased in the culture bottles, indicating that oxygen levels were not limited at these high larval densities. Similarly, high larval densities might be expected to result in elevated levels of toxic wastes. We plan to monitor NH3 and NH4 levels in future trials, in conjunction with more frequent water changes, to explore how this parameter affects larval performance.
4.4. Protocol advancements
Three innovations that contributed to the success of the culture of invertebrate larvae were developed by our laboratory and are worth mentioning here. The first is the use of roller bottles. Our laboratory first adapted the use of roller bottles from tissue culture techniques in 1988. Numerous researchers have discussed the importance of keeping the larvae, as well as phytoplanktonic food, in constant and homogeneous suspension (Loosanoff and Davis 1963, Strathmann and Leise 1979, Nadeau et al. 1989,). The roller bottles maintain the non-motile diatom and flagellate diet in constant suspension while providing a homogenous non-turbulent environment for rapid larval growth. Second, it has been a long held belief that larval Aplysia can not survive contact with the air-water interface for even a short time period during the water changing process (Kriegstein 1974). Researchers have reared larvae using a variety of elaborate techniques to avoid contact of the veliger with the air-water interface. Our consistent rapid growth and repeated high survival confirms that limited exposure to the air water-interface during water changes does not detrimentally affect the larvae. Third, in past studies the seawater was changed frequently under the belief that this was the only way could water quality be properly maintained.
We changed our culture media weekly, as compared to more frequently reported changing intervals, and consistently obtained larval survival in excess of 75%. To date, our techniques have been used successfully with several additional species of planktotrophic larvae, including Bursatella leachii, A. brasiliana, Strombus gigas, Lytechinus variegatus, and Diadema antillarum. Thus, the techniques described in the present study have the potential to resolve the key factors that limit the rearing of other fastidious biomedically and ecologically important larval organisms.
4.5. Conclusions
By systematically testing a number of biotic variables in the rearing of larvae at the National Resource for Aplysia, we were able to define the components of the diet, concentration of food, and larval stocking densities that yielded the largest numbers of metamorphic stage larvae of Aplysia ever obtained in a laboratory study. Furthermore, we were able to increase growth rates considerably over other studies. This demonstrates that previously observed lower growth, high variation in developmental timing, and reduced survival were a result of a limited understanding of the biological criteria controlling the larval phase. While our study focused on A. californica, we believe that other opisthobranch models would benefit from similar studies. It is now possible for the Resource to supply >30,000 post-metamorphic animals year-round of known age, reproductive status, limited genetic background, and consistent environmental history. Controlled rearing of Aplysia has become essential in support of the increasing experimental criteria required for genomics, proteomics, and bifurcated neuron cell culture. Presumably, animals reared under our experimentally determined environmental regime will result in more homogeneously responding organisms in support of these increasingly stringent requirements of the research community.
Acknowledgments
This paper represents many years of collaborations and contributions from friends and colleagues, without whom this work could not have been completed. We are grateful to Eric Stenn of Algagen for preparing and maintaining all of our algal stocks. Susan Perritt Gagosian, David Leibowitz, Cay Tamse, John Paige, and Alan Kuzirian were all key in developing our understanding of Aplysia larval biology and these techniques. To Pat Walsh for initiating the NCRR Aplysia Resource Grant at the University of Miami we are thankful. Eric Kandel, Sam Schacher, and the late Jimmy Schwartz provided great insight into the importance of a dependable source of Aplysia to the neuroscience community. We are indebted to Eric Kandel for his commitment to the Aplysia Resource and for his early support with generous funding provided by HHMI- a vision realized. This work and the Resource have been supported by NIH NCCR (RR10294).
Footnotes
This paper is derived from a presentation given at the 4th Aquatic Animal Models of Human Disease Conference: hosted by Duke University’s Nicholas School of the Environment and Earth Sciences, and Duke’s Comprehensive Cancer Center, Durham, NC, USA, January 31 - February 3, 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Audersirk TE. The role of seasonal periodicity, chemical communication, and chemoreceptive organs in reproduction in Aplysia californica Cooper. Dissertation, University of Southern California; 1976. [Google Scholar]
- Audersirk TE. A field study of growth and reproduction in Aplysia californica. Biol Bull. 1979;157:407–421. doi: 10.2307/1541026. [DOI] [PubMed] [Google Scholar]
- Avila C. Competence and metamorphosis in the long-term planktotrophic larvae of the nudibranch mollusc Hermissenda crassicornis (Eschscholtz, 1831) J Exp Mar Biol Ecol. 1998;231:81–117. [Google Scholar]
- Avila C, Grenier S, Tamse CT, Kuzirian AM. Biological Factors affecting larval growth in the nudibranch mollusc Hermissenda crassicornis (Eschscholtz, 1831) J Exp Mar Biol Ecol. 1997;218:243–262. [Google Scholar]
- Bayne BL. Growth and the delay of metamorphosis of the larvae of Mytilus edulis (L) Ophelia. 1965;2:1–47. [Google Scholar]
- Bickell LR, Kempf SC. Larval and metamorphic morphogenesis in the nudibranch Melibe leonina (Mollusca: Opisthobranchia) Biol Bull. 1983;165:119–138. [Google Scholar]
- Capo TR, Fieber LA, Stommes DL, Walsh PJ. Reproductive output in the hatchery-reared California sea hare at different stocking densities. Contemp Topics. 2003;42:35–39. [PubMed] [Google Scholar]
- Capo TR, Fieber LA, Stommes DL, Walsh JW. The effect of stocking density on growth rate and maturation time in laboratory-reared California sea hares. Contemp Topics. 2002;41:18–23. [PubMed] [Google Scholar]
- Capo TR, Perritt SE, Berg CJ. New developments in the mariculture of Aplysia californica. Biol Bull. 1979;157:360. (Abstract) [Google Scholar]
- Capo TR, Perritt SE, Paige J. The mass culture of Aplysia californica. Fifty-third annual meeting of the American Malacological Union; 1987. p. 18. (Abstract) [Google Scholar]
- Capo TR, Jaramillo JC, Boyd AE, Lapointe BE, Serafy JE. Sustained high yields of Gracilaria (Rhodophyta) grown in intensive large-scale culture. J Appl Phycol. 1999;11:143–147. [Google Scholar]
- Carefoot TH. Aplysia: its biology and ecology, Oceanogr. Mar Biol Ann Rev. 1987;25:167–284. [Google Scholar]
- Chanley P. Laboratory cultivation of assorted bivalves. In: Smith WL, Chanley MH, editors. Culture of Marine Invertebrate Animals. Plenum Press; New York: 1975. pp. 297–318. [Google Scholar]
- Cooper JG. On rare mollusca inhabiting the Coast of California- No. II Proc Calif Acad Nat Sci. 1863;3:56–60. [Google Scholar]
- Davis HC, Guillard RR. Relative value of ten genera of microorganisms as foods for oyster and clam larvae. Fish Bull. 1958;58:293–304. [Google Scholar]
- Eales NB. Aplysia. Liverpool Marine Biology Committee. Proc Trans Liverpool Biol Com, LMBC Mem Vol 35. 1921;24:183–266. [Google Scholar]
- Fieber LA, Schmale MC, Jordi N, Orbesen E, Diaz GA, Capo TR. Von Bertalanffy growth models for hatchery-reared Aplysia californica Bull. Mar Sci. 2005;76:95–104. [Google Scholar]
- Franz DR. Opisthobranch culture. In: Smith WL, Chanley MH, editors. Culture of Marine Invertebrate Animals. Plenum Press; New York: 1975. pp. 245–256. [Google Scholar]
- Gabbott PA, Holland DL. Growth and metabolism of Ostrea edulis larvae. Nature. 1972;241:475–476. [Google Scholar]
- Gallager SM, Mann R, Sasaki GC. Lipid as an index of growth and viability in three species of bivalve larvae. Aquaculture. 1986;56:81–103. [Google Scholar]
- Gallager SM, Mann R. An apparatus for the measurement of grazing activity of filter feeders at constant food concentrations. Mar Biol Letts. 1980;1:341–349. [Google Scholar]
- Guillard RRL. Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH, editors. Culture of Marine Invertebrate Animals. Plenum Press; New York: 1975. pp. 29–60. [Google Scholar]
- Harrigan JF, Alkon DL. Larval rearing, metamorphosis, growth and reproduction of the aeolid nudibranch Hermissenda crassicornis (Eschscholtz, 1831) (Gastropoda, Opisthobranchia) Biol Bull. 1978;154:430–439. doi: 10.2307/1541069. [DOI] [PubMed] [Google Scholar]
- Hubbard EJA. Larval growth and the induction of metamorphosis of a tropical sponge-eating nudibranch. J Moll Stud. 1988;54:259–269. [Google Scholar]
- Kandel ER. Cellular Basis of Behavior. W.H. Freeman & Co.; San Francisco: 1976. p. 727. [Google Scholar]
- Kandel ER. Behavioral biology of Aplysia: a contribution to the comparative study of opisthobranch mollusks. W.H. Freeman and Co.; San Francisco, CA: 1979. p. 463. [Google Scholar]
- Kandel P, Capo TR. The packaging of ova in the egg cases of Aplysia californica. Veliger. 1979;22:194–198. [Google Scholar]
- Kempf SC. Long-lived larvae of the gastropod Aplysia juliana: Do they disperse and metamorphose or just slowly fade away? Mar Ecol Prog Ser. 1981;6:61–65. [Google Scholar]
- Kriegstein AR. Stages in the post-hatching development of Aplysia californica. J Exp Zool. 1977;199:275–288. doi: 10.1002/jez.1401990212. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Castellucci V, Kandel ER. Metamorphosis of Aplysia californica in laboratory culture. Proc Natl Acad Sci USA. 1974;71:3654–3658. doi: 10.1073/pnas.71.9.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosanoff VL, Davis HC. Rearing of bivalve molluscs. In: Russell FS, editor. Advances in Marine Biology. Vol. 1. Academic Press; London: 1963. pp. 1–136. [Google Scholar]
- Moroz LL, Edwards JR, Puthanveettil SV, Kohn AB, Ha T, Heyland A, Knudsen B, Sahni A, Yu F, Liu L, Jezzini S, Lovell P, Iannucculli W, Chen M, Nguyen T, Sheng H, Shaw R, Kalachikov S, Panchin Y, Farmerie W, Russo JJ, Ju J, Kandel ER. Neuronal transcriptome of aplysia: neuronal compartments and circuitry. Cell. 2006;127:1453–1456. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau L, Paige JA, Starczak V, Capo T, Lafler J, Bidwell JP. Metamorphic competence in Aplysia californica Cooper. J Exp Mar Biol Ecol. 1989;131:171–193. [Google Scholar]
- Paige J. The laboratory culture of two Aplysiids, Aplysia brasiliana Rang, 1828, and Bursatella leachii plei (Rang, 1828) (Gastropoda: Opisthobranchia) in artificial seawater. Veliger. 1986;29:64–69. [Google Scholar]
- Paulson TC, Scheltema RS. Selective feeding on algal cells by the veliger of Nassarius obsoletus (Gastropoda, Prosobranchia) Biol Bull. 1968;134:481–489. [Google Scholar]
- Pawlik JR. Larvae of the sea hare Aplasia californica settle and metamorphose on an assortment of macroalgal species. Mar Ecol Prog Ser. 1989;51:195–199. [Google Scholar]
- Pechenik JA, Eyster LS, Widdows J, Bayne BL. The influence of food concentration and temperature on growth and morphological differentiation of blue mussel Mytilus edulis L. larvae. J Exp Mar Biol Ecol. 1990;136:47–64. [Google Scholar]
- Perron FE, Turner RD. Development, metamorphosis, and natural history of the nudibranch Doridella obscura Verrill (Corambidae: Opisthobranchia) J Exp Mar Biol Ecol. 1977;27:171–185. [Google Scholar]
- Peretz B, Adkins L. An index of age when birthdate is unknown in Aplysia californica: shell size and growth in long-term maricultured animals. Biol Bull. 1982;162:333–344. [Google Scholar]
- Plaut I, Borut A, Spira ME. Growth and metamorphosis of Aplysia oculifera larvae in laboratory culture. Mar Biol. 1995;122:425–430. [Google Scholar]
- Sokal RR, Rohlf EJ. Introduction to biostatistics. W.H. Freeman; New York: 1987. p. 368. [Google Scholar]
- Stommes D, Fieber LA, Beno C, Gerdes R, Capo TR. Temperature Effects on Growth, Maturation, and Lifespan of the California Sea Hare (Aplysia californica) Contemp Topics. 2005;44:31–35. [PubMed] [Google Scholar]
- Strathmann RR, Leise E. On feeding mechanisms and clearance rates of molluscan veligers. Biol Bull. 1979;157:524–535. doi: 10.2307/1541035. [DOI] [PubMed] [Google Scholar]
- Strenth NE, Blankenship JE. Laboratory culture, metamorphosis and development of Aplysia brasiliana Rang, 1828. Veliger. 1978;21:99–103. [Google Scholar]
- Switzer-Dunlap M, Hadfield MG. Observations on development, larval growth and metamorphosis of four species of Aplysiidae (Gastropoda: Opisthobranchia) in laboratory culture. J Exp Mar Biol Ecol. 1977;1977:245–261. [Google Scholar]
- Tamse CT, Kuzirian AM, Capo TR. Roller culture system: The fitness machine for Hermissenda crassicornis larvae. Amer. Malacol. Union Abstr. 56th Ann. Mtg.; 1990. p. 61. [Google Scholar]
- Thompson PA, Guo M, Harrison PJ. The influence of irradiance on the biochemical composition of three phytoplankton species and their nutritional value for larvae of the Pacific Oyster (Crassostrea gigas) Mar Biol. 1993;117:259–268. [Google Scholar]
- Thompson TE. The natural history, embryology, larval biology and post-larval development of Adalaria proxima (Alder and Hancock)(Gastropoda Opisthobranchia) Phil Trans Roy Soc London B, Biol Sci. 1958;242:1–58. [Google Scholar]
- Walne PR. Observations on the food value of seven species of algae to the larvae of Ostrea edulis. J Mar Biol Assoc UK. 1963;43:767–784. [Google Scholar]
- Webb KL, Chu FE. In: Pruder GD, Langdon CJ, Concklin D, editors. Phytoplankton as a food source for bivalve larvae; Proc. 2nd Intern. Conference on Aquaculture Nutrition: Biochemical and Physiological Approaches to Shellfish Nutrition; New Orleans, USA. 1981. pp. 272–291. [Google Scholar]
- Williams LG. Development and feeding of larvae of the nudibranch gastropods Hermissenda crassicornis and Aeolidia papillosa. Malacologia. 1980;20:99–116. [Google Scholar]