Abstract
The suprachiasmatic nucleus (SCN) regulates a wide range of daily behaviors and has been described as the master circadian pacemaker. The role of daily rhythmicity in other tissues, however, is unknown. We hypothesized that circadian changes in olfactory discrimination depend on a genetic circadian oscillator outside the SCN. We developed an automated assay to monitor olfactory discrimination in individual mice throughout the day. We found olfactory sensitivity increased approximately 6-fold from a minimum during the day to a peak in the early night. This circadian rhythm was maintained in SCN-lesioned mice and mice deficient for the Npas2 gene but was lost in mice lacking Bmal1 or both Per1 and Per2 genes. We conclude that daily rhythms in olfactory sensitivity depend on the expression of canonical clock genes. Olfaction is, thus, the first circadian behavior that is not based on locomotor activity and does not require the SCN.
Keywords: olfaction, circadian rhythms, Bmal1 gene, oscillator, Period2 gene
The mammalian suprachiasmatic nucleus (SCN) controls circadian rhythms in physiological variables including wake-sleep, feeding-fasting, and their underlying and resultant daily surges in hormone release (Dibner et al., 2010). At the molecular level, circadian rhythms involve a transcription/ translation feedback loop in which transcription factors (CLOCK and BMAL1) activate transcription of the Period (Per) and Cryptochrome (Cry) genes. PER and CRY proteins accumulate in the cytoplasm, translocate into the nucleus, and after some delay, inhibit their own transcription. Turnover of the inhibitory PER and CRY leads to a new cycle of activation by CLOCK and BMAL1. NPAS2 is a functional homolog of CLOCK, binding with BMAL1 to drive transcription in the forebrain and SCN (Reick et al., 2001; Zhou et al., 1997). Locomotor activity in Npas2−/− or Clock−/− mice is circadian, while mice deficient for both genes are arrhythmic (Debruyne et al., 2007; Reick et al., 2001; Zheng et al., 2001). Similarly, mice lacking mPer1 or mPer2 show modest circadian phenotypes compared to the arrhythmic double knockouts (Bae et al., 2001; Zheng et al., 2001). Bmal1 is the single gene that, when knocked out, is known to abolish circadian locomotor patterns (Bunger et al., 2000).
Many mammalian cells show circadian rhythms in vitro (Abe et al., 2002; Abraham et al., 2005; Reyes et al., 2008; Stratmann and Schibler, 2006). Where examined, the genes involved in SCN rhythmicity also play a role in daily rhythms in other cell types (Bellet and Sassone-Corsi, 2010; Dibner et al., 2010; Maywood et al., 2007; Welsh et al., 2010; Yagita et al., 2001), although the function of these peripheral clocks in behavior is largely unknown (Bass and Takahashi, 2010; Le Martelot et al., 2009; Marcheva et al., 2010; Suter and Schibler, 2009). One exception is the food-entrainable oscillator that regulates food anticipatory behavior in the absence of the SCN (Stephan, 2002), although its location and genetic basis remain unresolved (Fuller et al., 2008; Mistlberger et al., 2008; Pendergast et al., 2009; Storch and Weitz, 2009).
As circadian oscillators are discovered in new organisms, tissues, and gene networks, we may find they share common roles in regulating responses to environmental stimuli (Barlow, 1983; Chatterjee et al., 2010; Dibner et al., 2010; Krishnan et al., 1999; Krishnan et al., 2008; McWatters et al., 2000; Merrow et al., 2001; Tosini and Menaker, 1996). For example, the main olfactory bulb (OB) in rodents is a circadian pacemaker, which regulates daily rhythms in firing rate and Period1 gene activity in the absence of time cues from the environment or from the SCN (Abraham et al., 2005; Granados-Fuentes et al., 2004a; Granados-Fuentes et al., 2004b; Granados-Fuentes et al., 2006). Here, we tracked olfactory discrimination across the day as a function of genotype and the SCN. We conclude that there is a SCN-independent circadian rhythm in olfactory discrimination that depends on the Period1 and Period2 and Bmal genes.
MATERIALS AND METHODS
Animals and Locomotor Activity Recording
Mice were maintained in the Danforth campus animal facility at Washington University. Wild-type (wt), Npas2-deficient (Npas2−/−) (founders generously provided by Dr. S. L. McKnight, University of Texas Southwestern, Dallas, TX), and Per1- and Per2-deficient (Per1mPer2m) (founders generously provided by Dr. S. M. Reppert, University of Massachusetts Medical School, Worcester, MA) mice were maintained by crossing homozygotes on a C57Bl/6 genetic background. Bmal1-deficient (Bmal1−/−) mice were generated by crossing heterozygous mutants maintained on a Balb/C background (founders were a generous gift of Dr. C. Bradfield, University of Wisconsin Medical School, Madison, WI).
We recorded running-wheel revolutions in 1-minute bins (Clocklab, Actimetrics, Evanston, IL) from mice housed individually in cages maintained in light-tight chambers, illuminated by fluorescent bulbs. Animals were in a 12-hour/12-hour light/ dark (LD) cycle (lights-on at 0700 h) and then switched to constant darkness (DD) 3 days before the discrimination test. All procedures were approved by the Animal Care and Use Committee at Washington University and conformed to National Institutes of Health guidelines.
Olfactometer
We built two olfactometers for these experiments. The olfactometers were designed to be placed over a standard, size “B” mouse cage (Nalgene, Rochester, NY). The control and data acquisition module of the olfactometer was accessible with a local laptop computer or over the Internet so that programming, running, and data downloading were remote from the mice. Mice were trained to sniff in a central nose cone and then poke their nose in a cone to the left or right to indicate whether they detected an odor or not. Mice worked for a water reward delivered to the lateral cones following correct responses. The three cones each had an infrared beam and detector to mark the times when nose pokes began and ended. From these times, we determined the proportion of correct and false alarm responses, decision latency, and sniff duration.
Serial dilutions of vanilla (pure vanilla extract, Durkee, Memphis, TN) in Milli-Q water (Millipore, Billerica, MA) were prepared every 4 hours. Each dilution was pipetted onto sterile cotton in a clean 10-mL chamber. Clean air was pumped continuously at a rate of 1 L/min through a charcoal filter, a humidified chamber, and a flow meter before exiting through the central nose cone of the olfactometer. Vanilla vapor was added by opening a pinch valve (225P031-21, NResearch, West Caldwell, NJ) to split the air flow into a selected 10-mL chamber saturated with odor. The reward for correct responses (30 uL of tap water) was delivered by a pinch valve (1615NC, Takasago Electric, Nagoya, Japan) to the lateral cones. Olfactometers were cleaned with 70% ethanol and dried at 36 °C overnight.
Olfactometer Calibration
Using a portable photoionization gas detector (PID, ppbRAE3000, RAE Systems, San Jose, CA) connected directly to the output port of each olfactometer, we averaged odor concentration over 30 seconds for five concentrations from undiluted to 1:3 × 1012 vanilla: water. The detection limit of the PID was 3 × 1012–fold diluted vanilla. To establish any changes in odor delivery between the first and last trials, we measured the vanilla concentration immediately after preparation, 3 hours after preparation, and 3 hours after preparation following 3 minutes of constant air flow. We found the odor concentration varied by less than 1% at the nose cone between these conditions and between repeated trials. Both olfactometers produced a linear odor concentration over the range of undiluted to 105-fold diluted. Delivered odor concentration was reported as fold diluted (the ratio of PID counts at the given odor dose to counts for undiluted vanilla). For testing, we used only vanilla concentrations that were directly calibrated.
Training
Mice were deprived of water for 18 hours prior to training. Training consisted of two stages in which mice learned to 1) poke their noses into a lateral cone for a water reward and 2) sniff in a center cone and then, depending upon whether they detected vanilla (1:400 dilution), poke into either the left or right cone to receive a water reward. Some groups were trained to indicate odor detection by poking to the left and others to the right. Training took approximately 15 days for wt mice performing 30-minute sessions twice per day until the mice reached 85% correct. Mice below 85% correct were not tested.
Testing
We tested olfactory discrimination of 1:400 vanilla:water as a function of circadian time. We pipetted 500 µL of water or diluted vanilla onto fresh cotton prior to each testing session. Mice initiated each trial by poking into the center cone. Odor was delivered until the mouse removed its nose from the center cone (141.3 ± 8.8 milliseconds [mean ± SEM]). Trials were separated by 3 seconds to prevent desensitization. Mice were tested on bedding-free cages. We used the first 125 trials from each mouse at each circadian time. Beyond 125 trials, the intertrial sniff interval tended to increase, indicative of a decrease in motivation. Mice that failed to run 125 trials at any time were excluded (n = 6 of 65 tested). Mice were tested under constant dim red light. Time of testing was defined relative to the daily onset of locomotor activity (circadian time, CT12) or, for arrhythmic SCN-lesioned mice, as projected CT12, which was extrapolated from the activity onsets of the last 5 days prior to the lesion. For Per1mPer2m and Bmal1−/− mice, we defined projected CT12 as the time of lights-off (1900 h) on the days prior to testing. Data were collected at 8-hour intervals to ensure that mice were thirsty; it took 48 hours to collect data for 6 times per day. We recorded locomotor activity continuously except during the 30-minute tests. At the end of each test, mice returned to their home cages. Groups of mice were started at random times to avoid effects due to the time of testing.
Surgical Procedures
Some mice were SCN-lesioned (SCNx). Mice were anesthetized with isofluorane (Butler Animal Health, Dublin, OH) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Bilateral lesions were made by passing an anodal current (40 seconds of 1.25 mA DC) through a tungsten electrode (563410, A–M Systems, Carlsborg, WA) placed 0.6 mm posterior to, ±0.1 mm lateral to, and 5.7 mm below the bregma (Paxinos, 2003). SCNx mice were tested 8 days after the surgery. SCN lesions were defined as complete if locomotor activity showed no significant circadian periodicity by χ2 periodogram analysis, and the lesion was subsequently confirmed by Nissl staining. Mice that remained rhythmic in wheel-running activity and later corroborated by histology to be SCN intact were used as lesion controls (n = 5 of 10 mice).
Data Analysis
We calculated the percentage of correct responses as the number of trials in which the mouse correctly identified the presentation of odor (hits) or air (correct rejections) divided by the total number of trials as in previous publications (Slotnick and Restrepo, 2005). We also recorded sniff duration (the interval from when a mouse nose poked into and out of the central cone) and decision latency (the time from when the mouse removed its nose from the central cone until it poked into either lateral cone). We used a 2-way ANOVA for repeated measures and a Tukey post hoc test for differences across time and genotype. For other comparisons, we used a Student t test. Significance was set at p < 0.05 (Origin 8.0, OriginLab, Northampton, MA).
RESULTS
Daily Rhythms in Olfactory Discrimination Persisted in Constant Conditions
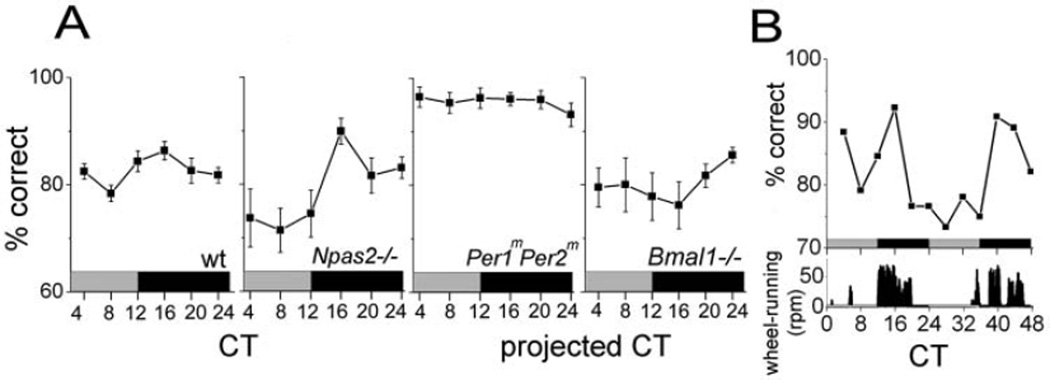
We tested olfactory discrimination of mice (n = 18) at six circadian times. In constant dim red light, mice showed a daily increase in the mean percentage of correct responses around the early subjective night (2-way ANOVA, F5,17 = 3.9 for the time effect, p = 0.003) (Fig. 1A). The correct response rate dropped from 86.4% ± 1.7% (mean ± SEM) at CT16 to 78.4% ± 1.5% at CT8. Two of these mice were tested at 8-hour intervals for an additional 48 hours. Both showed repeated nightly peaks in olfactory discrimination (Fig. 1B). Thus, mice detected 400-fold diluted vanilla at all times but with a higher fidelity at night.
Figure 1.
Circadian rhythms in olfactory discrimination. (A) Wild-type (wt) mice (n = 18) trained to detect vanilla diluted 400-fold showed a higher proportion of correct responses (mean ± SEM) during the early subjective night. Npas2−/− (n = 6) were also circadian, whereas Per1mPer2m (n = 5) and Bmal1−/− (n = 5) mice were arrhythmic. (B) Representative olfactory discrimination rhythms from one mouse (top) showed peak olfactory discrimination shortly after the daily onset of locomotor activity (bottom) over multiple days in constant darkness. The gray and black bars indicate subjective day and night, respectively.
Odor Sensitivity Increased 6-Fold at Night
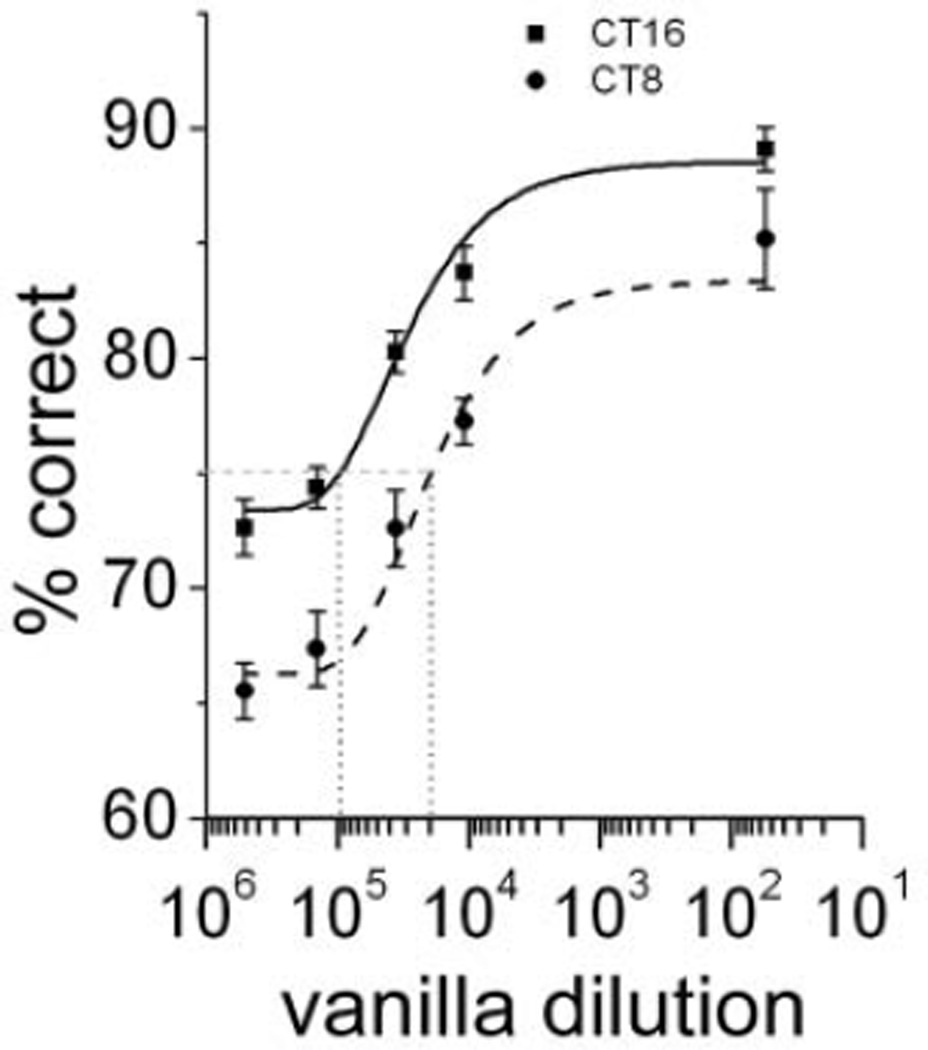
To test for a change in olfactory threshold, we measured the detection rate of a new group of mice (n = 15) as a function of vanilla concentration. Mice were randomly assigned to start testing at either CT8 or CT16 to avoid effects due to the time of testing. Their accuracy increased with odor concentration, and their threshold (75% of correct) (Bodyak and Slotnick, 1999) shifted from 18,000-fold diluted vanilla at CT8 to 102,000-fold diluted at CT16 (Fig. 2). The nighttime increase in accuracy was significant at all odor concentrations (2-way ANOVA, F1,4 = 2.8, p < 0.01 for the concentration effect and p < 0.01 for the time effect; CT8 v. CT16). Thus, olfactory sensitivity increased by approximately 6-fold each night when mice would typically be active.
Figure 2.
Olfactory sensitivity peaks at night. Sensitivity increased by about 6-fold during the early night (CT16) in wild-type (wt) mice (n = 15). Mice were able to discriminate vanilla dilutions below 2:104 at CT16 but not at CT8. Dashed lines indicate the dose required to evoke a response above threshold (75% correct) and reflect the day-night shift in sensitivity.
Circadian Rhythms in Olfactory Discrimination Depended on Clock Genes
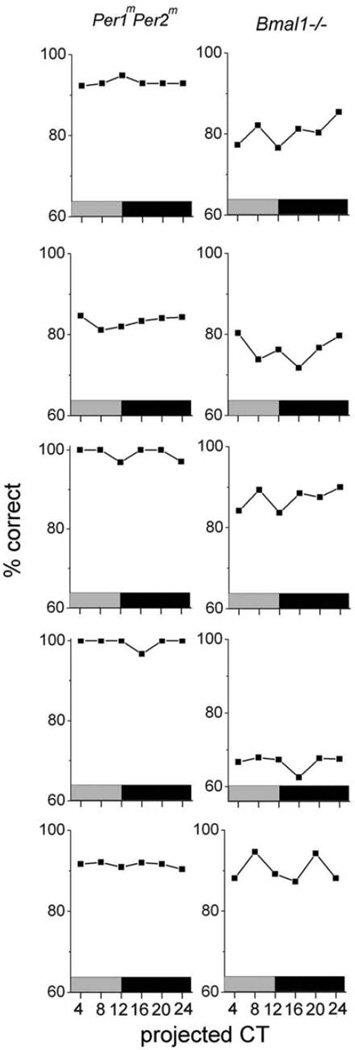
To assess if the molecular mechanisms that drive rhythms in olfaction are similar to those involved in circadian locomotor behavior, we tested olfactory discrimination in Npas2−/− (n = 6), Per1mPer2m (n = 5), and Bmal1−/− (n = 5) mice. Discrimination in mice deficient for Npas2−/− peaked at CT16 (2-way ANOVA, F1,5 = 6.4, p < 0.001 for the time effect), with a daily decrease from 89.9% ± 2.4% to 71.4% ± 4.1% correct (Fig. 1A). The apparent difference in amplitude between Npas2-deficient and wt mice was not significant (2-way ANOVA, F1,5 = 1.6, p = 0.06 for the genotype effect). In contrast, olfactory performance of Per1mPer2m and Bmal1−/− mice did not vary with time of day as a group (2-way ANOVA, F1,4 = 1.1, p = 0.3 for the time effect in Per1mPer2m; and F1,4 = 0.4, p = 0.7 for the time effect in Bmal1−/−) (Fig. 1A) or as individuals (Fig. 3). Interestingly, Per1mPer2m mice showed enhanced performance compared to wt mice at all times, while Bmal1−/− mice performed similar to the mean of wt mice (2-way ANOVA, F1,5 = 78.3, p < 0.001 for percentage correct in Per1mPer2m v. wt mice; and F1,5 = 2.1, p = 0.1 for percentage correct in Bmal1−/− v. wt mice). Thus, clock gene mutations produced similar changes in olfactory and locomotor rhythms.
Figure 3.
IndividualPer1mPer2m (left column; n= 5) and Bmal1−/− (right column; n = 5) mice showed no daily rhythms in olfactory discrimination.
Rhythms in Olfactory Discrimination Persisted in SCNx Mice
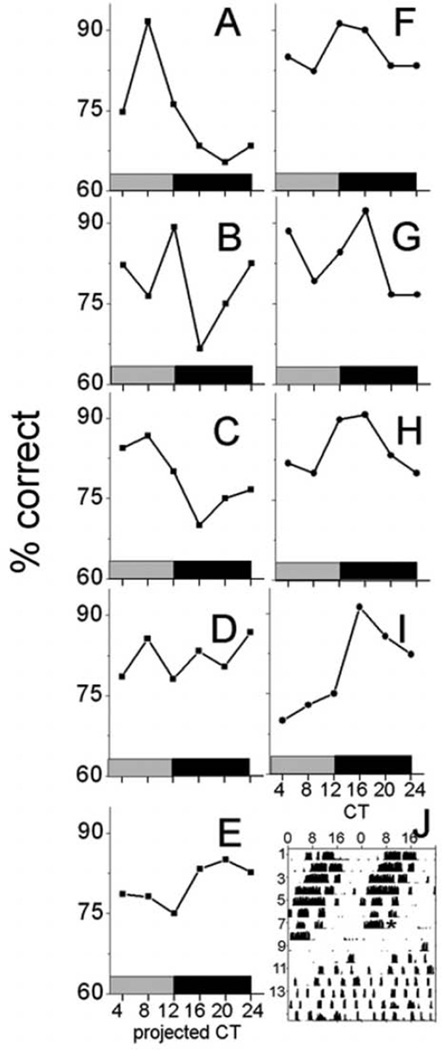
Rhythms in olfactory discriminability could result from activation of pathways that indirectly affect olfaction. We tested whether the rhythms in olfaction depended on the SCN, the master circadian regulator of many physiological and behavioral processes including sleep-wake, vigilance, and motivation (Saper et al., 2005; Van Dongen and Dinges, 2005). Four of five mice that showed arrhythmicity in wheel-running activity after SCN ablation showed clear circadian rhythms in olfactory discrimination (Fig. 4A–C and 4E); the remaining mouse showed arrhythmic wheel-running activity and olfactory discrimination (Fig. 4D). The peak-to-trough amplitude of olfactory discrimination rhythms in SCNx mice (16.3% ± 3.9%, n = 4) did not differ from that of lesion control mice (14.5% ± 1.8%, n = 5; Student t test, p = 0.6). Notably, the time of peak performance differed between SCN-lesioned mice by as much as 12 hours when plotted either according to the projected CT or time of day. These results suggest that rhythms in olfactory discrimination free ran in the absence of the SCN. Future experiments should test if light-dark cycles entrain olfactory rhythms in SCNx mice.
Figure 4.
Circadian cycling of olfactory discrimination in SCN-lesioned mice. Olfactory performance in SCNx (A–E) and control mice (F–I) was scored as circadian in all mice except mouse D. In contrast to control animals, mice tested 8 to 10 days after SCN ablation varied in the time of their daily peak in olfactory sensitivity. (J) Running-wheel activity from SCN-lesioned mouse E showing representative arrhythmic locomotor activity. * denotes time of surgery.
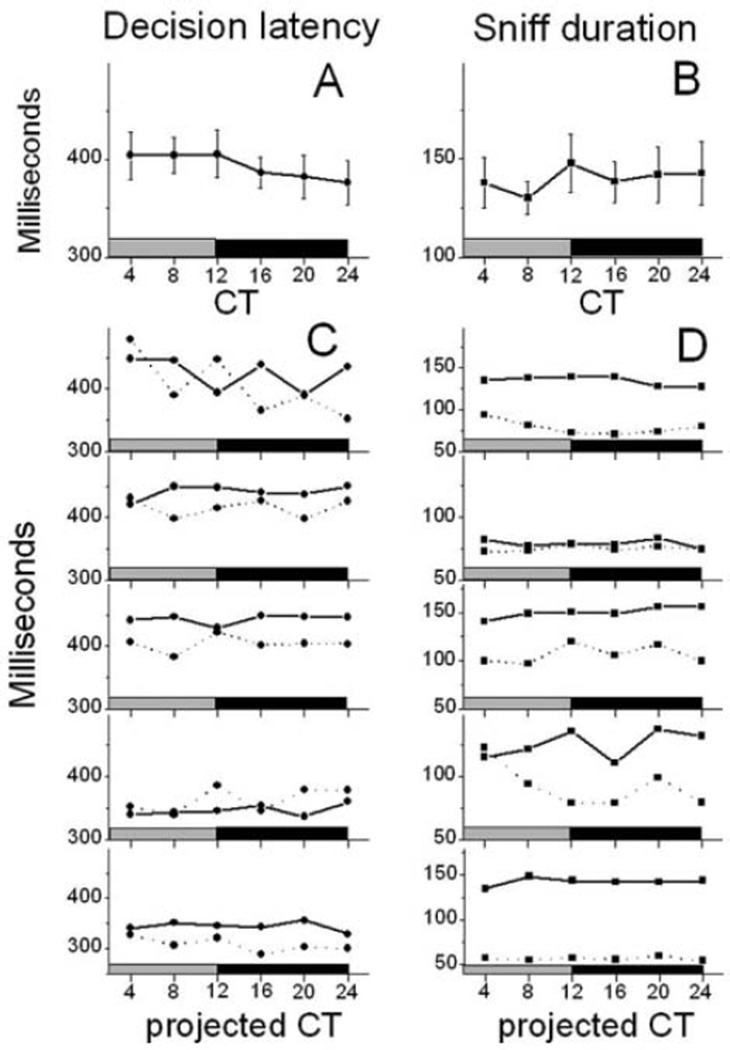
To further examine the possibility that olfactory performance depended on changes in motivation, alertness, or task recall, we measured the decision latency and sniff duration of SCN-intact and SCNx mice. Neither decision latency (1-way ANOVA, F5,89 = 0.7, p = 0.6) nor sniff duration (1-way ANOVA, F5,89 = 1.0, p = 0.4) (Fig. 5A and 5B) varied with time of day in wt or SCNx mice (Fig. 5C and 5D). Thus, olfactory discrimination rhythms did not correlate with how quickly mice performed.
Figure 5.
Neither decision latency (A) nor sniff duration (B) was circadian in wild-type (wt) mice (n = 15). The time spent in the odor port (sniff duration, C) and required to poke in a side port (decision latency, D) did not vary with time of day in individual SCNx mice (n = 5; dashed lines) or controls (n = 5 representative mice; continuous lines), indicating that daily rhythms in olfactory performance did not arise from changes in motivation or alertness.
SCNx mice showed similar decision latencies compared to controls (359.8 ± 14.7 milliseconds for SCNx v. 401.2 ± 14.5 milliseconds for lesion controls; Student t test, p = 0.14) (Fig. 5C) and lower sniff durations (81.7 ± 9.7 milliseconds for SCNx v. 141.3 ± 8.8 milliseconds for lesion controls; Student t test, p = 0.002) (Fig. 5D). Thus, SCN ablation changed an indicator of motivation or alertness without eliminating olfactory discrimination rhythms.
DISCUSSION
Circadian Regulation of Olfactory Discrimination
The nightly increase in olfactory discrimination found here is consistent with previous reports of circadian regulation of olfactory responsivity measured by odor-evoked c-Fos expression in the olfactory bulbs of mice and rats (Amir et al., 1999; Funk and Amir, 2000; Granados-Fuentes et al., 2006). In Drosophila, circadian oscillations in chemosensory cells of the antennae underlie daily changes in olfactory response (Krishnan et al., 1999) and their attraction to and repulsion from odorants (Zhou et al., 2005). Cockroaches and moths also modulate olfactory sensitivity in a circadian manner (Page and Koelling, 2003; Saifullah and Page, 2009; Decker et al., 2007; Merlin et al., 2007), and in humans, odorevoked potentials are largest at approximately 1600 h (Nordin et al., 2003). Interestingly, because discriminability of odors strongly depends on stimulus intensity (Daly et al., 2008), changes in olfactory sensitivity may also impact discrimination acuity.
The location of the circadian pacemaker underlying daily changes in mammalian olfaction is unknown. Interestingly, the circadian system underlying olfactory performance appears to parallel the system underlying daily rhythms including sleep-wake, body temperature, hormone secretion, and food consumption. Whereas lesions of the SCN abolish each of these rhythms (Klein et al., 1991), the daily cycling of olfactory performance persists in the absence of the SCN. Similarly, a food-entrainable oscillator and a methamphetamine-sensitive oscillator can drive circadian rhythms in locomotor activity in the absence of the SCN. Definitive localization of these oscillators, however, has proven difficult in part because they do not appear to free run for more than a few days (Carneiro and Araujo, 2009; Mistlberger, 2009; Pezuk et al., 2010). Evidence provided here indicates the olfactory system can sustain circadian rhythms in behavior for at least 8 days in constant conditions.
Olfactory Sensitivity Changes Do Not Require Changes in Motivation or Alertness
The SCN regulates circadian changes in motivation, alertness, fatigue, and performance, which could impact sensory performance. Indeed, functional connectivity between the olfactory system (e.g., OB, piriform cortex, etc.) and other forebrain areas is strongly influenced by behavioral state (Murakami et al., 2005; Wilson, 2010; Wilson and Yan, 2010). We found, however, that circadian rhythms in olfactory discrimination persisted in mice after SCN ablation, peaking at different times for different mice and independent of their locomotor activity, sniff duration, or decision latency, suggesting circadian changes in olfaction do not result from changes in alertness or motivation.
Circadian Rhythms in Olfactory Discrimination Share Genetic Mechanisms with the SCN
We found that genetic perturbations that abolish circadian rhythms in locomotor activity abolish rhythms in odor discrimination. Loss of Bmal1 or Per1 and Per2 abolished circadian olfactory behavior, whereas loss of Npas2 had no measurable effect. Importantly, this loss of rhythmicity at the behavioral level does not negate the possibility for daily rhythms at the level of cells or tissues, as has been found with the weak circadian cycling of SCN slices from Bmal−/− (Ko et al., 2010). Similarly, in flies, there is a common genetic basis for circadian rhythms in the antennae, which underlie rhythms in olfaction, and in lateral neurons of the brain, which underlie rhythms in locomotion (Zhou et al., 2005). Notably, Npas2 has been implicated in circadian regulation of the forebrain (Reick et al., 2001) but, according to our results, is not required for circadian modulation of olfactory discrimination. In contrast, Per1mPer2m mutant mice maintained high olfactory performance at all times, suggesting that Per1 and Per2 mediate decreases in olfactory sensitivity during the day. Present data suggest that an extra-SCN clock that controls rhythms in olfactory discrimination uses similar molecular mechanisms as the canonical clock in the SCN. It will be important to measure the olfactory thresholds of various clock and olfactory mutants to determine the mechanisms by which the circadian system increases and decreases olfactory sensitivity on a daily basis. This article provides the first circadian behavior in rodents that is not based on the presence of the master clock SCN.
ACKNOWLEDGMENTS
We thank Dr. Steven L. McKnight for the Npas2−/− mice, Dr. Steven M. Reppert for the Per1ldc Per2ldc mice, and Dr. Chris Bradfield for the Bmal1−/− mice. We also thank Lindsey McIntyre, Amaka Onwuzurike, and Tatiana Simon for expert assistance. This work was supported by National Institutes of Health (NIH) grant MH63104.
Footnotes
CONFLICT OF INTEREST STATEMENT
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham U, Prior JL, Granados-Fuentes D, Piwnica-Worms DR, Herzog ED. Independent circadian oscillations of Period1 in specific brain areas in vivo and in vitro. J Neurosci. 2005;25:8620–8626. doi: 10.1523/JNEUROSCI.2225-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir S, Cain S, Sullivan J, Robinson B, Stewart J. In rats, odor-induced Fos in the olfactory pathways depends on the phase of the circadian clock. Neurosci Lett. 1999;272:175–178. doi: 10.1016/s0304-3940(99)00609-6. [DOI] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential Functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Barlow RB. Circadian rhythms in the Limulus visual system. J Neurosci. 1983;3:856–870. doi: 10.1523/JNEUROSCI.03-04-00856.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet MM, Sassone-Corsi P. Mammalian circadian clock and metabolism: the epigenetic link. J Cell Sci. 2010;123:3837–3848. doi: 10.1242/jcs.051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodyak N, Slotnick B. Performance of mice in an automated olfactometer: odor detection, discrimination and odor memory. Chem Senses. 1999;24:637–645. doi: 10.1093/chemse/24.6.637. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Wilsbacher LD, Moran SM, Clendenin C, Radcliffe LA, Hogenesch JB, Simon MC, Takahashi JS, Bradfield CA. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro BTS, Araujo JF. The food-entrainable oscillator: a network of interconnected brain structures entrained by humoral signals. Chronobiol Int. 2009;26:1273–1289. doi: 10.3109/07420520903404480. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Tanoue S, Houl JH, Hardin PE. Regulation of gustatory physiology and appetitive behavior by the Drosophila circadian clock. Curr Biol. 2010;20:300–309. doi: 10.1016/j.cub.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly KC, Carrell LA, Mwilaria E. Characterizing psychophysical measures of discrimination thresholds and the effects of concentration on discrimination learning in the moth Manduca sexta. Chem Senses. 2008;33:95–106. doi: 10.1093/chemse/bjm068. [DOI] [PubMed] [Google Scholar]
- Debruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker S, McConnaughey S, Page TL. Circadian regulation of insect olfactory learning. Proc Natl Acad Sci U S A. 2007;104:15905–15910. doi: 10.1073/pnas.0702082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Amir S. Circadian modulation of fos responses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Res. 2000;866:262–267. doi: 10.1016/s0006-8993(00)02249-6. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J Neurosci. 2004a;24:615–619. doi: 10.1523/JNEUROSCI.4002-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Saxena MT, Prolo LM, Aton SJ, Herzog ED. Olfactory bulb neurons express functional, entrainable circadian rhythms. Eur J Neurosci. 2004b;19:898–906. doi: 10.1111/j.0953-816x.2004.03117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Tseng A, Herzog ED. A circadian clock in the olfactory bulb controls olfactory responsivity. J Neurosci. 2006;26:12219–12225. doi: 10.1523/JNEUROSCI.3445-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford University Press; 1991. [Google Scholar]
- Ko CH, Yamada YR, Welsh DK, Buhr ED, Liu AC, Zhang EE, Ralph MR, Kay SA, Forger DB, Takahashi JS. Emergence of noise-induced oscillations in the central circadian pacemaker. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000513. e1000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B, Dryer SE, Hardin PE. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature. 1999;400:375–378. doi: 10.1038/22566. [DOI] [PubMed] [Google Scholar]
- Krishnan P, Chatterjee A, Tanoue S, Hardin PE. Spike amplitude of single-unit responses in antennal sensillae is controlled by the Drosophila circadian clock. Curr Biol. 2008;18:803–807. doi: 10.1016/j.cub.2008.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Sasso GL, Moschetta A, Schibler U. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000181. e1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, O’Neill JS, Reddy AB, Chesham JE, Prosser HM, Kyriacou CP, Godinho SI, Nolan PM, Hastings MH. Genetic and molecular analysis of the central and peripheral circadian clockwork of mice. Cold Spring Harb Symp Quant Biol. 2007;72:85–94. doi: 10.1101/sqb.2007.72.005. [DOI] [PubMed] [Google Scholar]
- McWatters HG, Bastow RM, Hall A, Millar AJ. The ELF3 zeitnehmer regulates light signalling to the circadian clock. Nature. 2000;408:716–720. doi: 10.1038/35047079. [DOI] [PubMed] [Google Scholar]
- Merlin C, Lucas P, Rochat D, Francois MC, Maibeche-Coisne M, Jacquin-Joly E. An antennal circadian clock and circadian rhythms in peripheral pheromone reception in the moth Spodoptera littoralis. J Biol Rhythms. 2007;22:502–514. doi: 10.1177/0748730407307737. [DOI] [PubMed] [Google Scholar]
- Merrow M, Franchi L, Dragovic Z, Gorl M, Johnson J, Brunner M, Macino G, Roenneberg T. Circadian regulation of the light input pathway in Neurospora crassa. EMBO J. 2001;20:307–315. doi: 10.1093/emboj/20.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. Food-anticipatory circadian rhythms: concepts and methods. Eur J Neurosci. 2009;30:1718–1729. doi: 10.1111/j.1460-9568.2009.06965.x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Yamazaki S, Pendergast JS, Landry GJ, Takumi T, Nakamura W. Comment on “Differential rescue of light- and food-entrainable circadian rhythms”. Science. 2008;322:675. doi: 10.1126/science.1161284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Kashiwadani H, Kirino Y, Mori K. State-dependent sensory gating in olfactory cortex. Neuron. 2005;46:285–296. doi: 10.1016/j.neuron.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Nordin S, Lotsch J, Murphy C, Hummel T, Kobal G. Circadian rhythm and desensitization in chemosensory event-related potentials in response to odorous and painful stimuli. Psychophysiology. 2003;40:612–619. doi: 10.1111/1469-8986.00062. [DOI] [PubMed] [Google Scholar]
- Page TL, Koelling E. Circadian rhythm in olfactory response in the antennae controlled by the optic lobe in the cockroach. J Insect Physiol. 2003;49:697–707. doi: 10.1016/s0022-1910(03)00071-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G. The Mouse Brain in Stereotaxic Coordinates. New York: Academic Press; 2003. [Google Scholar]
- Pendergast JS, Nakamura W, Friday RC, Hatanaka F, Takumi T, Yamazaki S. Robust food anticipatory activity in BMAL1-deficient mice. PLoS ONE. 2009;4:e4860. doi: 10.1371/journal.pone.0004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M. Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms. 2010;25:432–441. doi: 10.1177/0748730410385204. [DOI] [PubMed] [Google Scholar]
- Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293:506–509. doi: 10.1126/science.1060699. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Pendergast JS, Yamazaki S. Mammalian peripheral circadian oscillators are temperature compensated. J Biol Rhythms. 2008;23:95–98. doi: 10.1177/0748730407311855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifullah AS, Page TL. Circadian regulation of olfactory receptor neurons in the cockroach antenna. J Biol Rhythms. 2009;24:144–152. doi: 10.1177/0748730408331166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Slotnick B, Restrepo D. Olfactometry with mice. Curr Protoc Neurosci Chapter Unit 8.20. 2005;8 doi: 10.1002/0471142301.ns0820s33. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: food as a zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci U S A. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann M, Schibler U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms. 2006;21:494–506. doi: 10.1177/0748730406293889. [DOI] [PubMed] [Google Scholar]
- Suter DM, Schibler U. Physiology: feeding the clock. Science. 2009;326:378–379. doi: 10.1126/science.1181278. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Dinges DF. Sleep, circadian rhythms, and psychomotor vigilance. Clin Sports Med. 2005;24:237–249. doi: 10.1016/j.csm.2004.12.007. vii-viii. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Single-unit activity in piriform cortex during slow-wave state is shaped by recent odor experience. J Neurosci. 2010;30:1760–1765. doi: 10.1523/JNEUROSCI.5636-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Yan X. Sleep-like states modulate functional connectivity in the rat olfactory system. J Neurophysiol. 2010;104:3231–3239. doi: 10.1152/jn.00711.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita K, Tamanini F, Der Horst GT, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science. 2001;292:278–281. doi: 10.1126/science.1059542. [DOI] [PubMed] [Google Scholar]
- Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC. Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/s0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- Zhou X, Yuan C, Guo A. Drosophila olfactory response rhythms require clock genes but not pigment dispersing factor or lateral neurons. J Biol Rhythms. 2005;20:237–244. doi: 10.1177/0748730405274451. [DOI] [PubMed] [Google Scholar]
- Zhou YD, Barnard M, Tian H, Li X, Ring HZ, Francke U, Shelton J, Richardson J, Russell DW, McKnight SL. Molecular characterization of two mammalian bHLH-PAS domain proteins selectively expressed in the central nervous system. Proc Natl Acad Sci U S A. 1997;94:713–718. doi: 10.1073/pnas.94.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]