Abstract
Pathways of electron transport have been studied in mitochondria isolated from hypocotyls of etiolated mung bean seedlings and skunk cabbage spadices that show cyanide-resistant respiratory activity. The residual flux through cytochrome c oxidase is shown to be small in comparison with the flux through an unidentified alternative oxidase that is known to have a high affinity for oxygen. This alternative oxidase is not a cytochrome. Skunk cabbage and mung bean mitochondria contain cytochromes a and a3 that have absorption peaks differing slightly from those of animal preparations. A slow oxidation-reduction of cytochrome a3-CN has been demonstrated. Cytochromes b undergo oxidation and reduction in the presence of cyanide but play no essential role in the cyanide-resistant pathway. Antimycin inhibits to an extent similar to that of cyanide; the respiratory chain bifurcates on the substrate side of the antimycin-sensitive site. Evidence is presented for the selective inhibition by thiocyanate, α, α′-dipyridyl, and 8-hydroxyquinoline of the alternative oxidase pathway, which may therefore contain a non-heme iron protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDALL D. S. Cytochromes and some respiratory enzymes in mitochondria from the spadix of Arum maculatum. Biochem J. 1958 Nov;70(3):381–390. doi: 10.1042/bj0700381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. D., Bendall D. S. Reversed electron transport in mitochondria from the spadix of Arum maculatum. Biochem J. 1968 Sep;109(3):47P–47P. doi: 10.1042/bj1090047pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
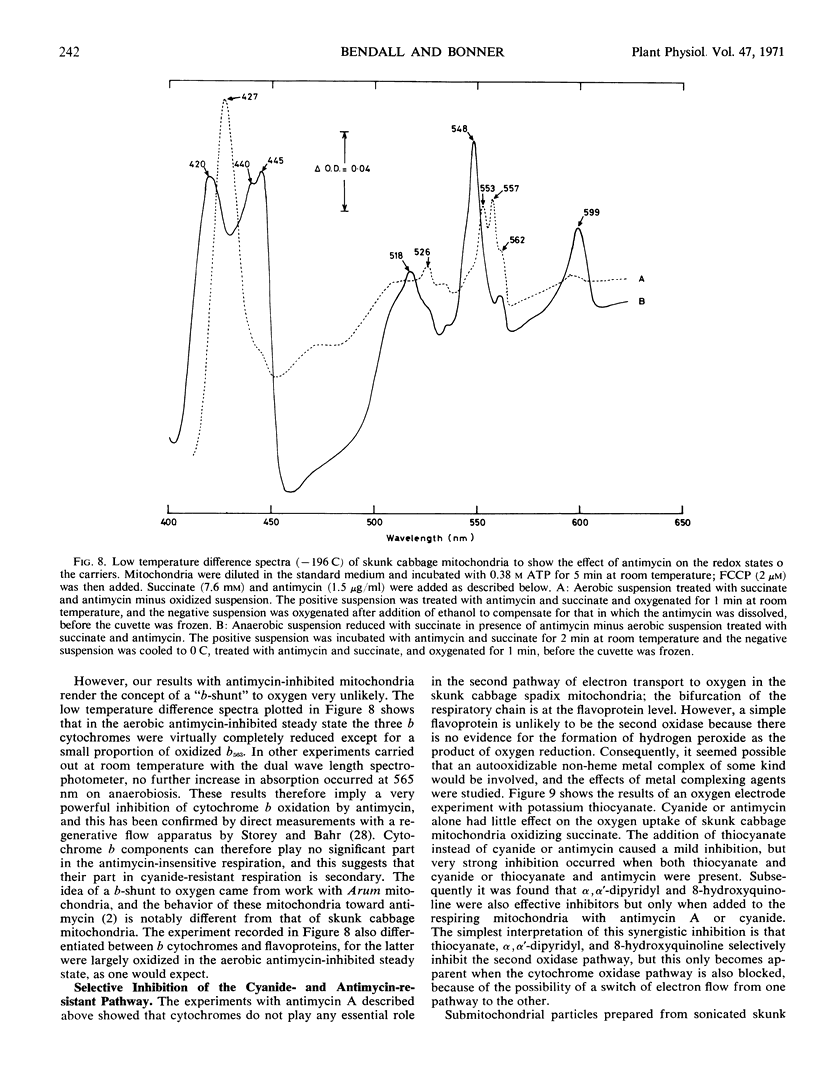
- Chance B., Hackett D. P. The Electron Transfer System of Skunk Cabbage Mitochondria. Plant Physiol. 1959 Jan;34(1):33–49. doi: 10.1104/pp.34.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
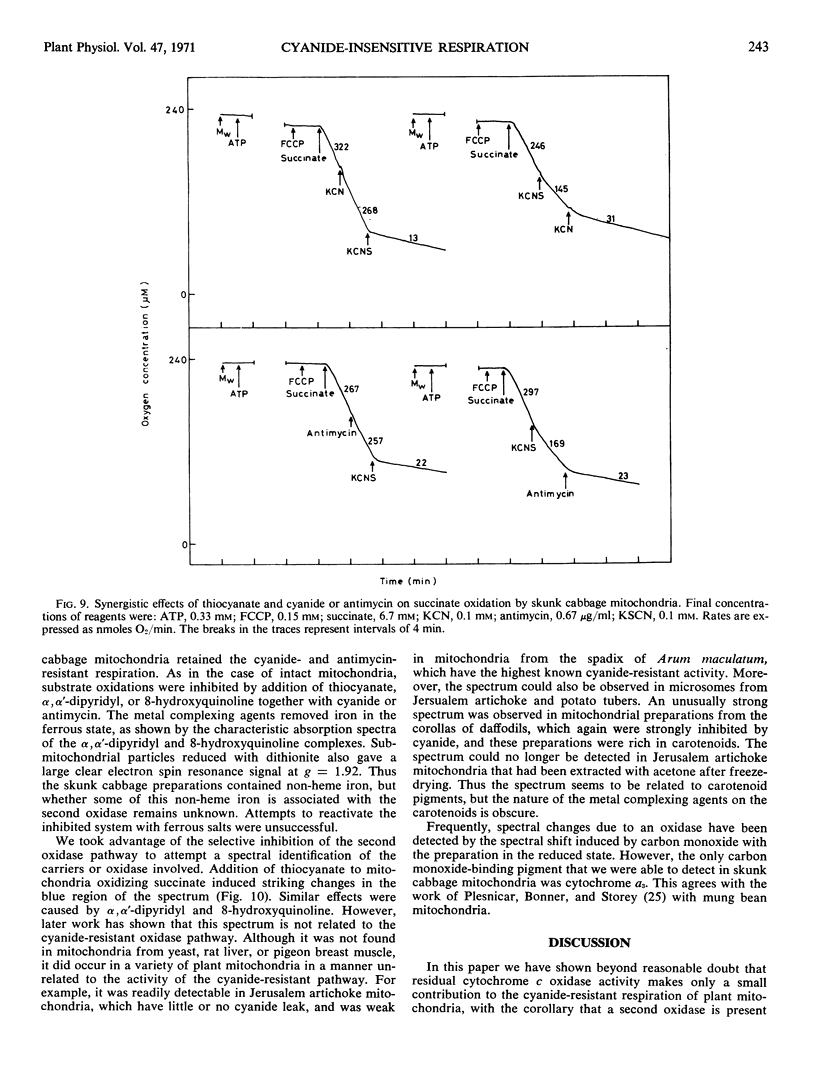
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverall B. J., Daly J. M. Metabolism of Indoleacetic Acid in Rust Diseases. II. Metabolites of Carboxyl-labeled Indoleacetic Acid in Tissues. Plant Physiol. 1964 Jan;39(1):1–9. doi: 10.1104/pp.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour M. V., Wilson D. F., Lemberg R. The low-temperature spectral properties of mammalian cytochromes oxidase. II. The enzyme isolated from beef-heart mitochondria. Biochim Biophys Acta. 1967;143(3):487–499. doi: 10.1016/0005-2728(67)90054-0. [DOI] [PubMed] [Google Scholar]
- Hackett D. P., Haas D. W. Oxidative Phosphorylation and Functional Cytochromes in Skunk Cabbage Mitochondria. Plant Physiol. 1958 Jan;33(1):27–32. doi: 10.1104/pp.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. III. Effects of Respiratory Inhibitors. Plant Physiol. 1967 Nov;42(11):1535–1544. doi: 10.1104/pp.42.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance C., Bonner W. D. The respiratory chain components of higher plant mitochondria. Plant Physiol. 1968 May;43(5):756–766. doi: 10.1104/pp.43.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P., Kimelberg H. K. Cytochromes a and a3. Catalytic activity and spectral shifts in situ and in solution. Biochim Biophys Acta. 1968 Jul 16;162(1):11–21. doi: 10.1016/0005-2728(68)90209-0. [DOI] [PubMed] [Google Scholar]
- POTTER V. R., REIF A. E. Inhibition of an electron transport component by antimycin A. J Biol Chem. 1952 Jan;194(1):287–297. [PubMed] [Google Scholar]
- Plesnicar M., Bonner W. D., Jr, Storey B. T. Peroxidase associated with higher plant mitochondria. Plant Physiol. 1967 Mar;42(3):366–370. doi: 10.1104/pp.42.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F. Azide inhibition of mitochondrial electron transport. II. Spectral changes induced by azide. Biochim Biophys Acta. 1967 May 9;131(3):431–440. doi: 10.1016/0005-2728(67)90003-5. [DOI] [PubMed] [Google Scholar]
- Wiskich J. T., Bonner W. D. Preparation and Properties of Sweet Potato Mitochondria. Plant Physiol. 1963 Sep;38(5):594–604. doi: 10.1104/pp.38.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YLER D. D., ESTABROOK R. W., SANADI D. R. ELECTRON AND ENERGY REQUIREMENTS FOR CYTOCHROME B REDUCTION DURING THE OXIDATION OF TETRAMETHYL-P-PHENYLENE DIAMINE. Biochem Biophys Res Commun. 1965 Jan 18;18:264–269. doi: 10.1016/0006-291x(65)90751-5. [DOI] [PubMed] [Google Scholar]
- Yocum C. S., Hackett D. P. Participation of Cytochromes in the Respiration of the Aroid Spadix. Plant Physiol. 1957 May;32(3):186–191. doi: 10.1104/pp.32.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]



