Abstract
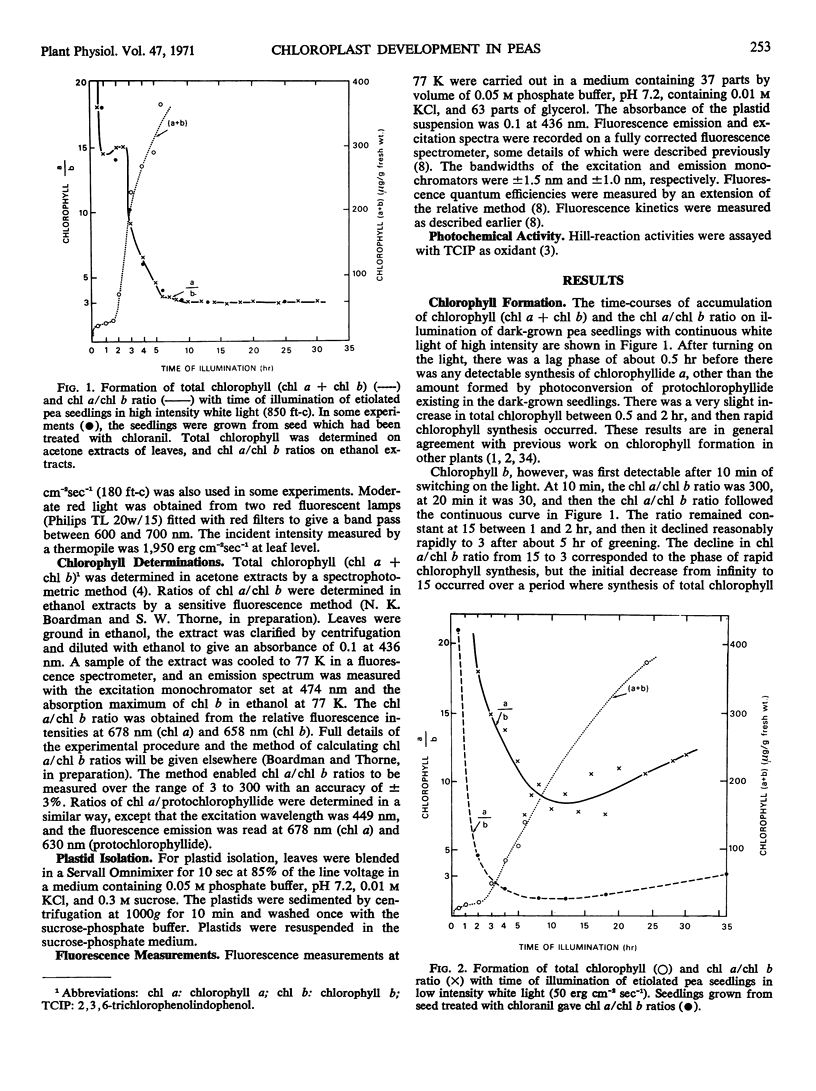
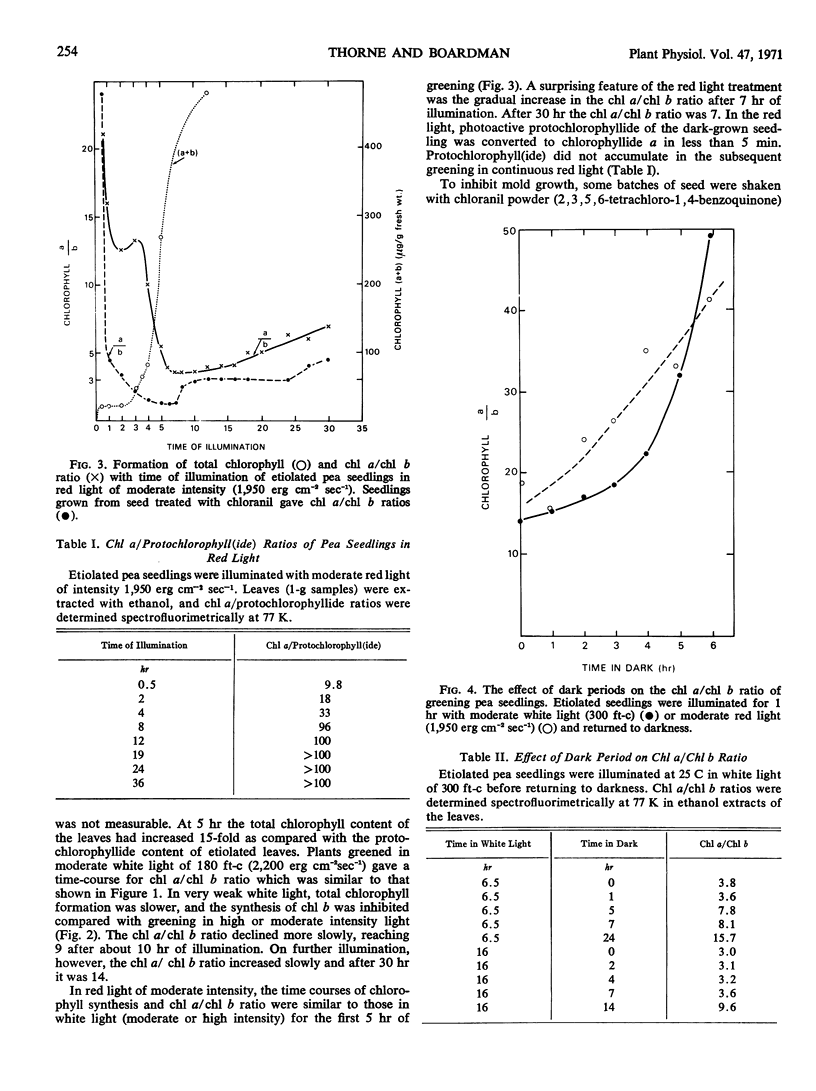
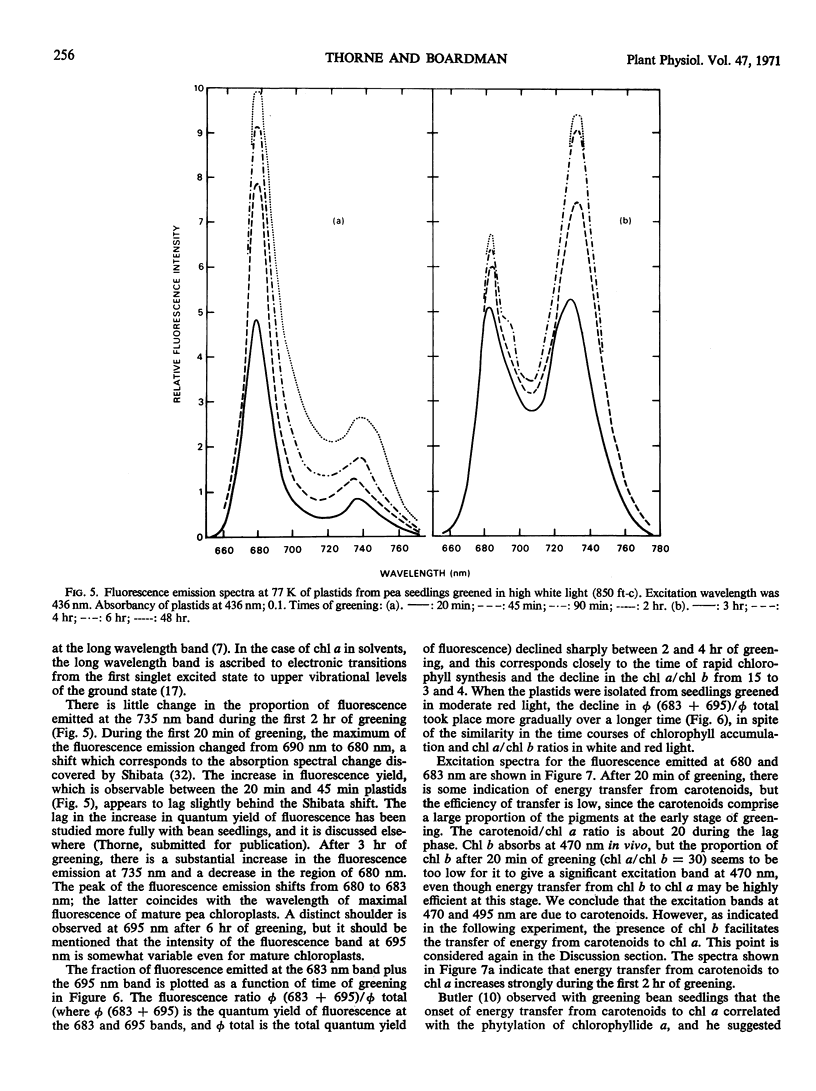
Chlorophyll b was first detectable after 10 minutes of illumination of etiolated pea seedlings (Pisum sativum L. var Greenfeast) with continuous white light. The chlorophyll a/b ratio decreased from 300 at 10 minutes to 15 after 1 hour. There was little change in the chlorophyll a/b ratio between 1 and 2 hours, and it declined to 3 between 2 and 5 hours of illumination. In red light, the time courses of total chlorophyll synthesis and chlorophyll a/b ratio were similar to those in white light for the first 5 hours of illumination. But with increasing time of illumination with red light, there was an increase in the chlorophyll a/b ratio to 7 after 30 hours. Illumination with white light of very low intensity also gave high chlorophyll a/b ratios. Seedlings which had been illuminated for varying periods and then returned to darkness always showed an increase in chlorophyll a/b ratio during the dark period. It is concluded that the synthesis of chlorophyll b is controlled by light.
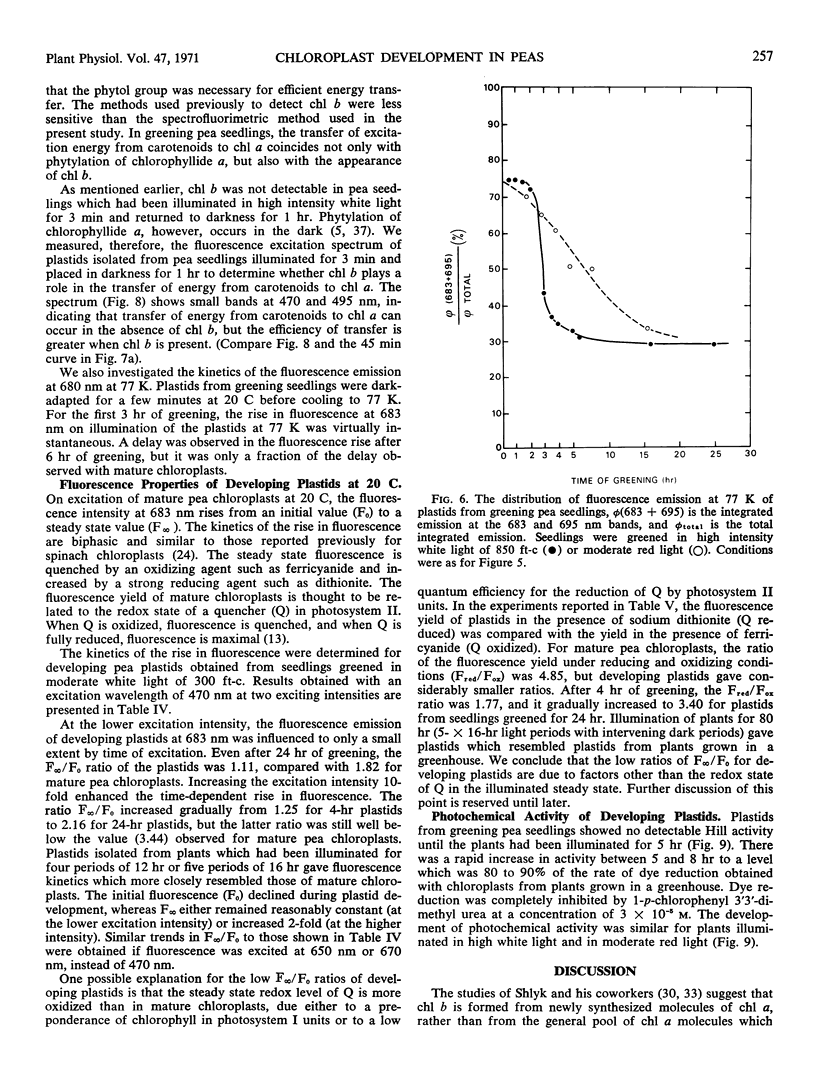
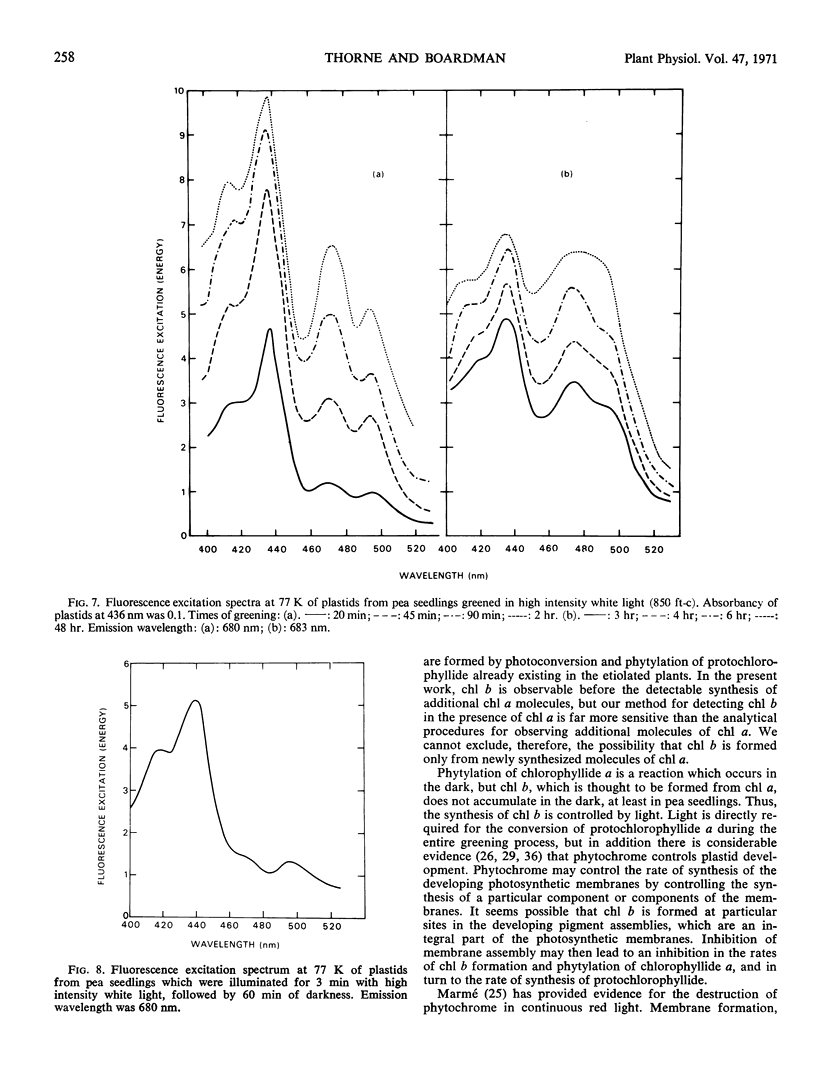
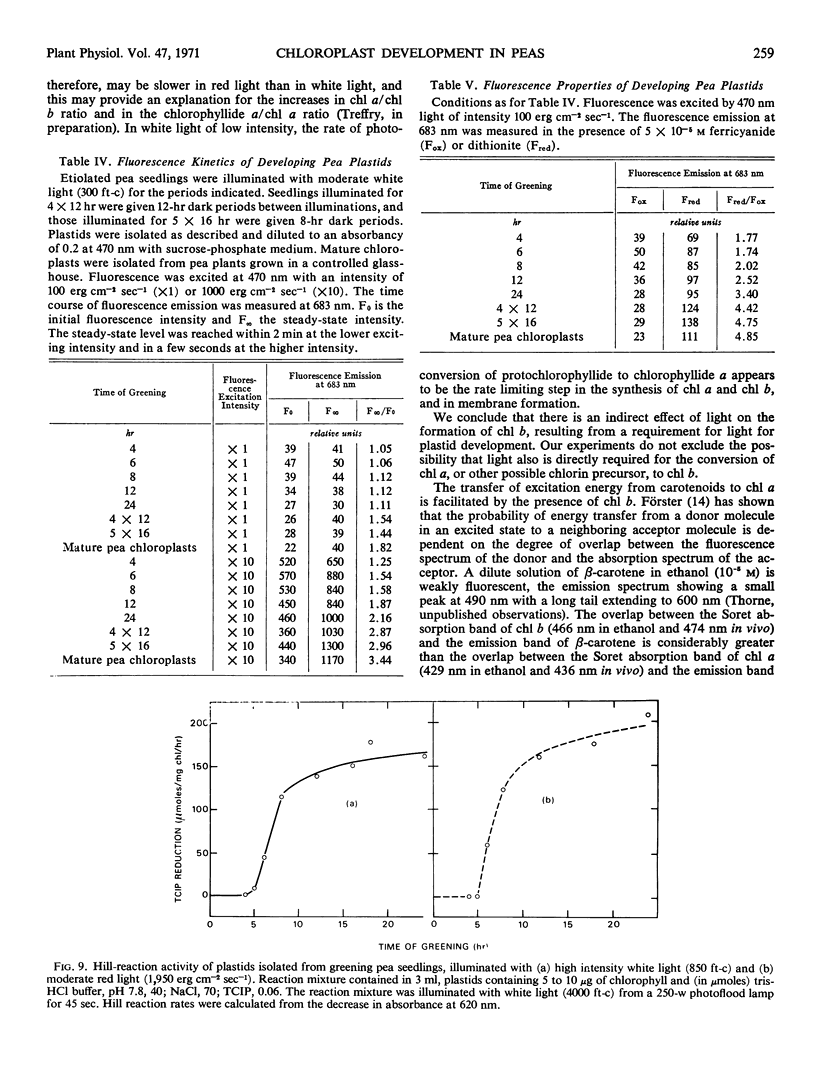
The onset of the transfer of excitation energy from carotenoids to chlorophyll a correlated with the appearance of chlorophyll b. The detection of chlorophyll b and the onset of energy transfer from carotenoids to chlorophyll a preceded by several hours the appearance of Hill activity in the isolated pea plastids. The detection of the large fluorescence emission band at 735 nm at 77 K also preceded the observation of Hill activity, but it correlated with the phase of rapid chlorophyll synthesis. There was a good correlation between Hill activity and the formation of grana. The fluorescence kinetics which are characteristic of mature chloroplasts appeared considerably later than grana or the observation of Hill activity.
It is concluded that the formation of photosynthetic membranes and the assembly of the photosystems is not a single step process, at least in the initial stages of chloroplast development from the etioplast.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Boardman N. K. Fractionation of the photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Bibl Laeger. 1966 Mar 14;112(3):403–421. doi: 10.1016/0926-6585(66)90244-5. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER W. L. A far-red absorbing form of chlorophyll. in vivo. Arch Biochem Biophys. 1961 May;93:413–422. doi: 10.1016/0003-9861(61)90287-9. [DOI] [PubMed] [Google Scholar]
- BUTLER W. L. Chloroplast development: energy transfer and structure. Arch Biochem Biophys. 1961 Feb;92:287–295. doi: 10.1016/0003-9861(61)90351-4. [DOI] [PubMed] [Google Scholar]
- Boardman N. K., Highkin H. R. Studies on a barley mutant lacking chlorophyll b. I. Photochemical activity of isolated chloroplasts. Biochim Biophys Acta. 1966 Oct 10;126(2):189–199. doi: 10.1016/0926-6585(66)90054-9. [DOI] [PubMed] [Google Scholar]
- Boardman N. K., Thorne S. W., Anderson J. M. Fluorescence properties of particles obtained by digitonin fragmentation of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Aug;56(2):586–593. doi: 10.1073/pnas.56.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman N. K., Thorne S. W. Studies on a barley mutant lacking chlorophyll b. II. Fluorescence properties of isolated chloroplasts. Biochim Biophys Acta. 1968 Feb 12;153(2):448–458. doi: 10.1016/0005-2728(68)90086-8. [DOI] [PubMed] [Google Scholar]
- Butler W. L. Development of photosynthetic system 1 and 2 in a greening leaf. Biochim Biophys Acta. 1965 May 25;102(1):1–8. doi: 10.1016/0926-6585(65)90198-6. [DOI] [PubMed] [Google Scholar]
- De Petrocellis B., Siekevitz P., Palade G. E. Changes in chemical composition of thylakoid membranes during greening of the y-1 mutant of Chlamydomonas reinhardi. J Cell Biol. 1970 Mar;44(3):618–634. doi: 10.1083/jcb.44.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEDHEER J. C. FLUORESCENCE BANDS AND CHLOROPHYLL A FORMS. Biochim Biophys Acta. 1964 Sep 25;88:304–317. doi: 10.1016/0926-6577(64)90186-x. [DOI] [PubMed] [Google Scholar]
- Goodchild D. J., Highkin H. R., Boardman N. K. The fine structure of chloroplasts in a barley mutant lacking chlorophyll B. Exp Cell Res. 1966 Oct;43(3):684–688. doi: 10.1016/0014-4827(66)90045-0. [DOI] [PubMed] [Google Scholar]
- Homann P. H., Schmid G. H. Photosynthetic reactions of chloroplasts with unusual structures. Plant Physiol. 1967 Nov;42(11):1619–1632. doi: 10.1104/pp.42.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoober J. K., Siekevitz P., Palade G. E. Formation of chloroplast membranes in Chlamydomonas reinhardi y-1. Effects of inhibitors of protein synthesis. J Biol Chem. 1969 May 25;244(10):2621–2631. [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. II. Plastid differentiation during greening of a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):553–584. doi: 10.1083/jcb.35.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Ohad I. Biogenesis of chloroplast membranes. 3. Light-dependent induction of proton pump activity in whole cells and its correlation to cytochrome f photooxidation during greening of a Chlamydomonas reinhardti mutant (y-I). Biochim Biophys Acta. 1969 May;180(1):165–177. doi: 10.1016/0005-2728(69)90203-5. [DOI] [PubMed] [Google Scholar]
- WOLFF J. B., PRICE L. Terminal steps of chlorophyll A biosynthesis in higher plants. Arch Biochem Biophys. 1957 Dec;72(2):293–301. doi: 10.1016/0003-9861(57)90205-9. [DOI] [PubMed] [Google Scholar]
- Woo K. C., Anderson J. M., Boardman N. K., Downton W. J., Osmond C. B., Thorne S. W. Deficient Photosystem II in Agranal Bundle Sheath Chloroplasts of C(4) Plants. Proc Natl Acad Sci U S A. 1970 Sep;67(1):18–25. doi: 10.1073/pnas.67.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]


