Abstract
Background:
Surrogate biomarkers for metastatic colorectal cancer (mCRC) are urgently needed to achieve the best outcomes for targeted therapy.
Methods:
A clinical association analysis was performed to examine the three single-nucleotide polymorphisms (SNPs) that were previously proposed as markers of chemosensitivity to the cetuximab (124 patients) and bevacizumab regimens (100 patients) in mCRC patients. In addition, biological correlations were examined for the candidate SNPs in terms of their regulatory pathway.
Results:
For cetuximab regimens, patients homozygous for the wild-type alleles (GG) of LIFR rs3729740 exhibited a 1.9 times greater overall response rate (ORR) and 1.4 months longer progression-free survival (PFS) than those homozygous or heterozygous for the mutant allele (GA and AA; P=0.022 and 0.027, respectively). For bevacizumab regimens, patients homozygous for the minor alleles (TT) of ANXA11 rs1049550 exhibited an ORR twice as high as those homozygous or heterozygous for the ancestral allele (CC and CT; P=0.031). Overall response rate gain was achieved up to 10% in patients with wild-type LIFR rs3729740 patients either with wild-type KRAS or skin toxicity (P=0.001) respectively. Specifically in clones treated with cetuximab and bevacizumab regimens, active p-ERK and MMP-9 expressions were significantly reduced in clones expressing wild-type LIFR rs3729740 (P=0.044) and in those expressing minor-type ANXA11 rs1049550 (P=0.007), respectively.
Conclusion:
LIFR rs3729740 and possibly ANXA11 rs1049550 may be useful as biomarkers for predicting whether mCRC patients are sensitive to relevant target regimens, although further validation in large cohorts is needed.
Keywords: colorectal cancer, targeted chemotherapy, marker, LIFR rs3729740, ANXA11 rs1049550
Surrogate biomarkers are urgently needed to achieve the best outcomes without unnecessarily costly expenditure from targeted therapy. Many molecular biomarkers have been investigated, but only a few have proved clinically useful for predicting responses accurately and timely.
Analysis of pooled results of randomised controlled trials (RCTs) has provided firm evidence across all efficacy end points for metastatic colorectal cancer (mCRC) patients with wild-type KRAS treated with anti-EGFR mAbs (Bokemeyer et al, 2009; Peeters and Price, 2012; Custidio and Feliu, 2013). Based on the results of these clinical trials, the consensus guidelines recommend that all candidates for anti-EGFR therapy should have their tumours tested for KRAS mutations in an accredited laboratory and that subsequent treatment should be in line with the results of these tests (Allegra et al, 2009). In addition to KRAS mutations, alterations of EGFR effector pathways and ligands, that is, BRAF and PIK3CA mutations, and EREG mRNA expression, may help predict anti-EGFR unresponsiveness in as many as 51%–70% of mCRC patients (De Roock et al, 2010; Kuramochi et al, 2012).
In contrast to biomarkers of responsiveness to cetuximab, there are few informative tools that predict the benefit of bevacizumab therapy in mCRC patients. A variety of clinicopathologic parameters and biomarkers have been widely investigated: hypertension, microvessel density, circulating endothelial or circulating endothelial progenitor cells, VEGF or VEGFR expression, single-nucleotide polymorphisms (SNPs) of relevant genes, and BRAF and BRAS mutations. However, no predictive factors have been identified (Gerger et al, 2011).
The aim of this study was to examine the feasibility as surrogate predictive markers of three novel SNPs described in our previous study (Kim et al, 2011), two of which may reflect chemosensitivity to cetuximab and one to bevacizumab regimens. Two SNPs, namely LIFR rs3729740 to cetuximab and putatively ANXA11 rs1049550 to bevacizumab, were suggested to be candidate biomarkers on the basis of correlations with clinical responses and/or in vitro assays of biological effectiveness.
Materials and methods
Study design, eligibility and treatments
We performed a clinical association analysis to investigate three SNPs that were previously identified as possible markers of chemosensitivity to cetuximab (LIFR rs3729740 and ISX rs361863) and bevacizumab regimens (ANXA11 rs1049550). A total of 170 mCRC patients (the test set) were enrolled between May 2006 and April 2012 to receive these two regimens. A separate cohort of 314 CRC patients (the reference set) curatively resected between February 2004 and November 2009 was used to identify correlations between these SNP markers and clinically prognostic parameters (Supplementary Table 1). The two cohorts were consecutively enrolled and treated in the Asan Medical Center (Seoul, Korea), agreeing with their tissue sample donation and examination. Genomic DNA was extracted from buffy coats preserved in liquid nitrogen, using a nucleic acid lysis buffer (Promega, Madison, WI, USA). The study protocol was approved by the Institutional Review Board for Human Genetic and Genomic Research (registration no. 2009-0091; valid until 9 August 2013), in accordance with the Declaration of Helsinki.
Eligibility criteria included histologically verified colorectal adenocarcinoma, Eastern Cooperative Oncology Group performance status of 0–3, and age ⩽75 years (Table 1). A total of 170 patients received metastatic chemotherapy using cetuximab (124 patients) and/or bevacizumab (100 patients) regimens, including 54 patients with crossover treatment (cetuximab to bevacizumab in 23 patients and bevacizumab to cetuximab in 31 patients). Chemotherapy regimens were administered in accordance with the NCCN Clinical Practice Guidelines in Oncology (www.nccn.org). Intravenous bevacizumab (2.5 mg kg–1 per week) or cetuximab (400 mg m–2 initial dose, then 250 mg m–2 per week) was administered every week or on day 1, combined with irinotecan or oxaliplatin in approximately 90% of patients. The percentage of prior chemotherapy lines was two or less in 71% and 81% of patients treated with cetuximab and bevacizumab, respectively.
Table 1. Demography, clinical features and tumour responses of patients treated with cetuximab and bevacizumab regimensa.
| |
Total no. of patients
(%), (n=170)b |
|
|
|---|---|---|---|
| Demographic and clinical features | Cetuximab regimens (n=124) | Bevacizumab regimens (n=100) | P-valuec |
| Gender, male/female |
78/46 (62.9/37.1) |
50/50 (50/50) |
0.058 |
| Age, mean (ranges) |
52 (25–75) |
52 (30–74) |
0.804 |
| ECOG performance status, 0/1/2 |
19/101/4 (15.3/81.5/3.2) |
13/86/1 (13/86/1) |
0.455 |
| Primary tumour site, colon/rectum |
75/49 (60.5/39.5) |
62/38 (62/38) |
0.891 |
| Curative tumour resection |
57 (46) |
49 (49) |
0.678 |
| No. of prior chemotherapy lines, 0/1/2/⩾3 |
16/19/53/36 (12.9/15.3/42.7/29) |
22/35/24/19 (22/35/24/19) |
<0.001 |
| No. of metastatic sites, 1/2/⩾3 |
33/34/57 (26.6/27.4/46) |
20/33/47 (20/33/47) |
0.447 |
| Tumour responsesd |
|
|
0.74 |
| CR | 3 (2.4) | 4 (4) | |
| PR | 40 (32.3) | 28 (28) | |
| SD | 46 (37.1) | 35 (35) | |
| PD |
35 (28.2) |
33 (33) |
|
|
Survival period, mean±s.e.m, months | |||
| PFS (ranges) | 6.2±0.4 (0.8–25) | 6.9±0.4 (1.1–14.3) | 0.123 |
| OS (ranges) | 10.2±0.6 (1–25) | 10.7±0.9 (0.8–37.8) | 0.86 |
Abbreviations: CR=complete response; ECOG=Eastern Cooperative Oncology Group; 5-FU=5-fluorouracil; OS=overall survival; PFS=progression-free survival; PD=progressive disease; PR=partial response; SD=stable disease.
Bold font, P<0.05.
Either irinotecan or oxaliplatin was combined to cetuximab and bevacizumab with 5-FU/leucovorin (FL) or capecitabine, namely, FOLFIRI and XELIRI or FOLFOX and XELOX.
Including 54 patients receiving crossover treatments.
Comparison between cetuximab and bevacizumab regimens by Pearson's χ2-test or unpaired t-test.
Assessment using RECIST criteria (Therasse et al, 2000).
Evaluation of clinical responses
Tumour responses were assessed every 6–8 weeks with imaging tools such as computed tomography, magnetic resonance and positron emission tomography/computed tomography. Treatment responses were determined according to the intent-to-treat analysis using RECIST criteria (Therasse et al, 2000). Overall response (OR) included complete response and partial response, and disease-control response included OR and stable disease. Progression-free survival (PFS) was calculated from the start of the targeted regimen to the date of disease progression, whereas overall survival (OS) was calculated from the start of the targeted regimen to the date of death or last follow-up. The best response time to this event was measured as the primary end point in patients receiving crossover treatment. During the study period, all patients with bevacizumab regimens received complete follow-up evaluation, whereas four patients with cetuximab regimens were lost and censored without event.
Genotyping of the four candidate SNPs, and KRAS mutation analysis
Single-nucleotide polymorphism genotypes were assayed by pyrosequencing using previously designed sequencing primers (Supplementary Table 2). PCR optimised samples were prepared and analysed on a Vacuum Prep Workstation (Biotage AB, Uppsala, Sweden) according to standard protocols. PCR amplification and direct sequencing of KRAS exon 2 were performed using tumour DNAs of the test set, as previously reported (Di Fiore et al, 2007).
Biological correlations
The two candidate SNPs by clinical association analysis were assessed using a biological utility assay that included mutagenesis and transfection, and correlations with their regulatory pathway. LIFR and ANXA11 cDNA (KRIBB, Daejeon, Korea) was amplified by PCR and sub-cloned into HA-tagged pcDNA3 vector and Myc/His-tagged pcDNA3 vector, respectively. The mutant or minor allele of each clone was generated using a site-directed mutagenesis kit (Intron Biotechnology, Seongnam, Korea), confirmed by DNA sequencing analysis. RKO CRC cells without KRAS mutation (ATCC, Manassas, VA, USA) was chosen for their short doubling time and sensitivities to the targeted regimens. Transient transfection was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Stably expressing cells were generated by G418 selection for 10 days, selecting at least two clones each bearing the wild-type and mutant alleles. Two RKO clones each expressing the same allelotype of LIFR rs3729740 and ANXA11 rs1049550, were treated with cetuximab and bevacizumab regimens in duplicate, respectively. The final concentrations of combined regimens were determined on the basis of IC50 values according to the previous study (Roh et al, 2012): FXC (50 μg ml–1 of 5-FU, 10 μg ml–1 of leucovorin, 40 μg ml–1 of oxaliplatin and 20 μg ml–1 cetuximab) and FRB (50 μg ml–1 of 5-FU, 10 μg ml–1 of leucovorin, 20 μg ml–1 of irinotecan and 20 μg ml–1 bevacizumab).
Correlations of the regulatory protein expression between clones with different gene SNPs were identified by western blotting, as previously described (Cho et al, 2010). Briefly, all tissues and cells were extracted in Laemmli sample buffer (Bio-Rad, Hercules, CA, USA), separated by SDS–polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes. Membranes were incubated with specific primary antibodies (Supplementary Table 3), followed by horseradish peroxidase-conjugated secondary antibody (Pierce, Rockford, IL, USA). Immunoblotting was identified using enhanced chemiluminescence system (Thermo Scientific, Rockford, IL, USA). The intensity of respective protein expression was measured using a calibrated densitometer (GS800, Bio-Rad).
Statistical analysis
The lowest sample number needed in the test group for efficient allele discrimination was determined using Altman's nomogram. Drug responses and clinicopathological variables were related to the corresponding genotype in case–control associations by cross-table analysis using Fisher's exact test with two-sided verification. Potential variables were verified by multivariate analysis using binary logistic regression. Progression-free survival and OS rates were compared and verified using the Kaplan–Meier method by a log-rank test and Cox's regression model. The biological correlations were compared using the paired Student's t-test. Statistical significance was defined as P<0.05. All calculations were performed using SPSS software (ver.19, SPSS Inc., Chicago, IL, USA).
Results
Patients' characteristics, tumour responses and adverse events
The two treatment groups had similar demographic findings and tumour sites (Table 1). Responsiveness was similar in the cetuximab and bevacizumab groups, with overall response rates (ORR) of 35.7% and 32%, and disease-control rates (DCRs) of 71.8% and 67%, respectively. Similarly, PFS and OS were comparable in the two groups. Adverse events of skin toxicity mostly occurred in those receiving cetuximab (76.6% of patients).
Association of LIFR rs3729740 and ISX rs361863 with cetuximab responses
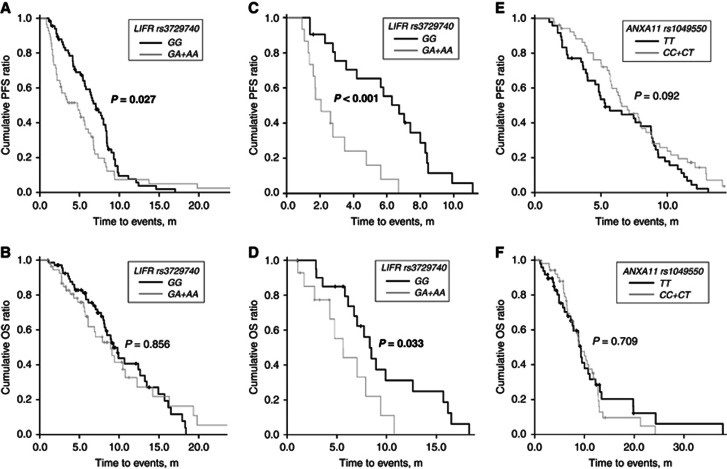
For the cetuximab regimens, patients homozygous for the wild-type alleles (GG) of LIFR rs3729740 exhibited greater ORR and DCR than those for the mutant allele (GA and AA; P=0.022 and 0.046, respectively; Table 2). Progression-free survival was also longer in the former than the latter (mean±s.e.m.: 6.8±0.4 m vs 5.4±0.7 m, P=0.027), whereas OS periods for these two groups were similar (10.3±0.7 m vs 10±1.1 m, P=0.856; Figures 1A and B). Interestingly, of the patients who had received three or more prior chemotherapy lines, the PFS and OS periods were longer for those carrying homozygous wild-type alleles than for those with the mutant allele (PFS: 6±0.6 m vs 2.8±0.5 m, P<0.001; OS: 9.6±1.2 m vs 5.9±0.9 m, P=0.033; Figures 1C and D). No specific genotypes of ISX rs361863 were related to the tumour responses and survival outcomes of the cetuximab regimens (P=0.295–0.673).
Table 2. Association of genotypes with respect to the three candidate SNPs with tumour responses in patients treated with cetuximab and bevacizumab regimensa.
| |
|
Overall response ratesb |
DCRsb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene SNPs | Genotypes | Responders/subgroups (%) | OR | 95% CI | P-valuec | Responders/subgroups (%) | OR | 95% CI | P-valuec |
|
Cetuximab regimens | |||||||||
| LIFR rs3729740 | GG | 30/68 (44.1) | 1 | 54/68 (79.4) | 1 | 0.194–0.961 | |||
| |
GA+AA |
13/56 (23.2) |
0.383 |
0.175–0.838 |
0.022 |
35/56 (62.5) |
0.432 |
|
0.046 |
| ISX rs361863 | CC | 33/92 (35.9) | 1 | 68/92 (73.9) | 1 | ||||
| |
CT+TT |
10/32 (31.3) |
0.813 |
0.344–1.921 |
0.673 |
21/32 (65.6) |
0.674 |
0.284–1.601 |
0.372 |
|
Bevacizumab regimens | |||||||||
| ANXA11 rs1049550 | CC+CT | 10/48 (20.8) | 1 | 27/48 (56.3) | 1 | ||||
| TT | 22/52 (42.3) | 2.787 | 1.147–6.77 | 0.031 | 40/52 (76.9) | 2.593 | 1.096–6.133 | 0.034 | |
Abbreviations: CI=confidence interval; CR=complete response; DCR=disease-control rate; 5-FU=5-fluorouracil; OR=odds ratio; PD=progressive disease; PR=partial response; SD=stable disease; SNP=single-nucleotide polymorphism.
Bold font, P<0.05.
Either irinotecan or oxaliplatin was combined to cetuximab with 5-FU/leucovorin (FL) or capecitabine, namely, FOLFIRI and XELIRI or FOLFOX and XELOX.
Assessment using RECIST criteria (Therasse et al, 2000). Overall response (OR=CR+PR) and disease-control (DCR=CR+PR+SD) rates.
All genotypes were compared by dominant model, except for ANXA11 rs1049550 (recessive model), using Fisher's exact test.
Figure 1.
Progression-free survival (PFS) and overall survival (OS) in all patients treated with cetuximab regimens (A and B) and in those who had received three or more chemotherapy lines (C and D), between patients carrying wild-type alleles (GG) of LIFR rs3729740 and those carrying mutant allele(s) (GA/AA). Progression-free survival and OS in all patients treated with bevacizumab regimens, between patients carrying minor alleles (TT) of ANXA11 rs1049550 and those carrying ancestral allele(s) (CC and CT; E and F). P-values using Kaplan–Meier method by a log-rank test are displayed over the bars. Bold font, P<0.05.
Association of ANXA11 rs1049550 with bevacizumab responses
For the bevacizumab regimens, patients homozygous for the minor alleles (TT) of ANXA11 rs1049550 exhibited greater ORR and DCR than those for the ancestral allele (CC and CT; P=0.031 and 0.034, respectively; Table 2). However, the PFS appeared to be different between these two groups (minor alleles vs ancestral allele(s): 7.5±0.5 m vs 6.4±0.5 m, P=0.092) without reaching statistical significance, but the OS did not (Figures 1E and F). The grade 3/4 hypertension occurred in 11% of patients treated with bevacizumab regimens, but this adverse event was not associated with response rates or survival outcome, either in patients with hypertension episode alone or together with minor alleles of ANXA11 rs1049550.
KRAS mutation and skin toxicity combined with LIFR rs3729740
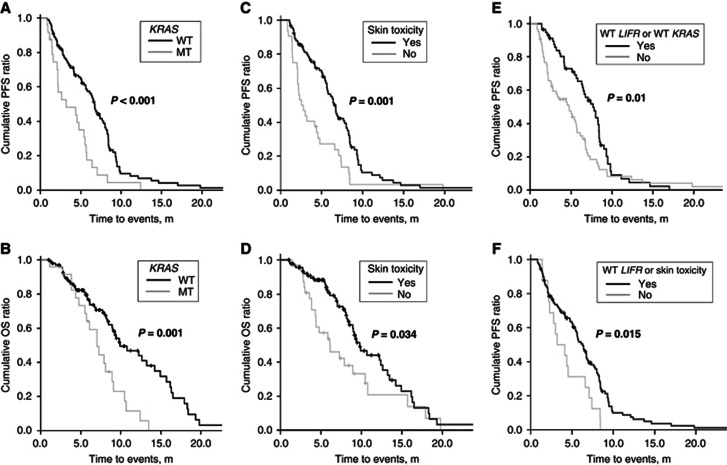
Wild-type KRAS codons 12 and 13 and skin toxicity were associated with enhanced ORR (P=0.009 and 0.032, respectively) and DCR (P=0.04 and 0.001, respectively) to cetuximab regimens (Table 3). Prolonged PFS and OS periods were strongly correlated with wild-type KRAS codons 12 and 13 (wild-type vs mutant: 6.7±0.5 m vs 4±0.6 m, P<0.001; 11.3±0.8 m vs 7.3±0.7 m, P=0.001, respectively; Figures 2A and B) and skin toxicity (yes vs no: 6.8±0.5 m vs 4.2±0.7 m, P=0.001; 10.9±0.7 m vs 8.2±1.1 m, P=0.034, respectively; Figures 2C and D). Overall response rate and DCR gains were achieved approximately 10% in patients with either the wild-type LIFR rs3729740 or wild-type KRAS and with either wild-type LIFR rs3729740 or skin toxicity, compared with wild-type KRAS or skin toxicity alone (Table 3). Significant prolonged survival was also identified in these combinations (Figures 2E and F). On the other hand, the specific genotypes of LIFR rs3729740 were not related to KRAS mutations or skin toxicity (P=0.171 and 0.543, respectively). Among 99 patients confined to wild-type KRAS, ORR was greater in patients with wild-type LIFR rs3729740 than those with mutant type (50% vs 26.8%, P=0.024), although survival gain was not prominent.
Table 3. Association of various predictive parameters and their combinations with tumour responses in patients treated with cetuximab regimensa.
| |
|
Overall response ratesb |
DCRsb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictive parameters | Subgroups | Responders/subgroups (%) | OR | 95% CI | P-valuec | Responders/subgroups (%) | OR | 95% CI | P-valuec |
| KRAS | WT | 40/99 (40.4) | 1 | 76/99 (76.8) | 1 | ||||
| |
MT |
3/24 (12.5) |
0.211 |
0.059–0.754 |
0.009 |
12/24 (54.2) |
0.358 |
0.141–0.905 |
0.04 |
| Skin toxicityd | Yes | 37/92 (40.2) | 1 | 0.129–0.915 | 74/92 (80.4) | 1 | |||
| |
No |
6/32 (18.8) |
0.343 |
|
0.032 |
15/32 (46.9) |
0.215 |
0.09–0.509 |
0.001 |
| WT LIFR rs3729740 |
Yes |
29/58 (50) |
1 |
|
|
48/58 (82.8) |
1 |
|
|
| or WT KRAs |
No |
14/66 (21.2) |
0.269 |
0.123–0.589 |
0.001 |
41/66 (62.1) |
0.342 |
0.147–0.794 |
0.016 |
| WT LIFR rs3729740 |
Yes |
27/52 (51.9) |
1 |
0.122–0.576 |
|
47/52 (90.4) |
1 |
|
|
| or skin toxicity | No | 16/72 (22.2) | 0.265 | 0.001 | 42/72 (58.3) | 0.149 | 0.053–0.419 | <0.001 | |
Abbreviations: CI=confidence interval; CR=complete response; DCR=disease-control rate; 5-FU=5-fluorouracil; OR=odds ratio; MT= mutant; PD=progressive disease; PR=partial response; SD=stable disease; WT=wild-type.
Bold font, P<0.05.
Either irinotecan or oxaliplatin was combined to cetuximab with 5-FU/leucovorin (FL) or capecitabine, namely, FOLFIRI and XELIRI or FOLFOX and XELOX.
Assessment using RECIST criteria (Therasse et al, 2000). Overall response (OR=CR+PR) and disease-control (DCR=CR+PR+SD) rates. .
Predictive parameters and their combinations were compared between the two subgroups, using Fisher's exact test.
Skin toxicity confined to acneform rash, nail toxicity and dry skin.
Figure 2.
Progression-free survival (PFS) and overall survival (OS) for all patients treated with cetuximab regimens, between patients carrying wild-type alleles of KRAS and those carrying mutant allele (A and B), and between patients suffering with or without skin toxicity (C and D). Progression-free survival for all patients treated with cetuximab regimens, between patients carrying either wild-type alleles of LIFR rs3729740 or KRAS and their mutant allele (E), and between patients carrying either wild-type alleles of LIFR rs3729740 or skin toxicity and mutant allele(s) or no skin toxicity (F). P-values using Kaplan–Meier method by a log-rank test are displayed over the bars. Bold font, P<0.05. MT, mutant; WT, wild-type.
Clinicopathological features correlated with three proposed SNPs
The three candidate SNPs were investigated in a separate cohort without targeted therapy, to see if they were associated with recurrence or survival outcomes and clinicopathological parameters (Supplementary Table 1). The three candidate SNPs did not affect recurrence rates either in univariate analysis (P=0.227–0.875) or multivariate analysis using these potential confounders (P=0.116–0.875). Similarly, these SNPs were not associated with 5-year DFS and 5-year OS in either univariate analysis (P=0.069–0.975) or multivariate analysis, using the potential confounders (P=0.11–0.949).
Biological correlations with LIFR and ANXA11 regulatory pathway
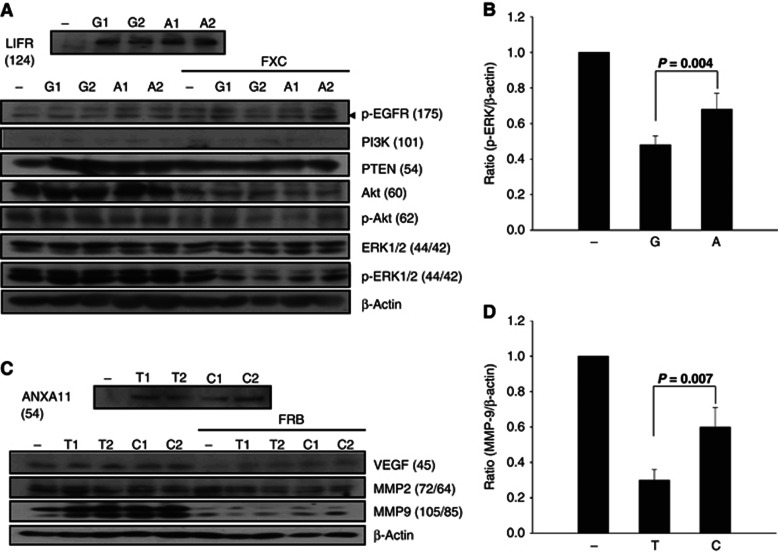
Relative mRNA expression ratios of the stably transfected plasmids did not differ between RKO cells carrying wild-type (or ancestral) allele and those carrying mutant-type (minor) allele (14.44±4.25 vs 13.49±2.78 for LIFR rs3729740, P=0.868; 2.64±0.07 vs 2.4±0.07 for ANXA11 rs1049550, P=0.133) after treatment with cetuximab and bevacizumab regimens, respectively. Protein expressions in RKO clones were shown in Figures 3A and B. Clones treated with cetuximab regimen showed 27%–66.7% reduced expression of Akt, p-Akt and active p-ERK (42 kD) compared with those without treatment, regardless of allelotype of LIFR rs3729740 (P⩽0.001–0.006). On the other hand, clones treated with bevacizumab regimen showed 40.9%–72.7% reduced expression of VEGF and active MMP-9 (85 kD) compared with those without treatment, regardless of allelotype of ANXA11 rs1049550 (P⩽0.001–0.007). Specifically in clones treated with relevant targeted regimens, active p-ERK and MMP-9 expressions were significantly reduced in clones expressing wild-type LIFR rs3729740 by 29.4% (P=0.044; Figure 3C) and in those expressing minor-type ANXA11 rs1049550 by 50% (P=0.007; Figure 3D), respectively, compared with their mutant- and ancestral-type clones.
Figure 3.
Expressions of LIFR, ANXA11, and their regulatory proteins by the western blot in clones with LIFR rs3729740 wild-type allele (G allele, G1 and G2 clones), mutant allele (A allele, A1 and A2 alleles), and control RKO cells (−); ANXA11 expression in clones with ANXA11 rs1049550 minor allele (T allele, T1 and T2 clones), ancestral allele (C allele, C1 and C2 alleles), and control RKO cells (−), respectively (A and B). Empty vectors were used for control RKO cells, that is, HA-tagged pcDNA3 for LIFR and Myc/His-tagged pcDNA3 for ANXA11, respectively. RKO clones stably expressing wild-type (or ancestral) and mutant (or minor) alleles (upper rows) were incubated for 24 h with FXC for LIFR and FRB for ANXA11, respectively. β-Actin was used as a loading control. Values in each parenthesis indicate the molecular weight (kD) of relevant protein. Relative expression ratios of p-ERK and MMP-9 to β-actin were calculated between clones expressing respective alleles and control RKO cells (C and D, respectively). Values are means±s.e.m. of quadruplicates. P-values using Student's t-test are displayed over the bars. Bold font, P<0.05.
Discussion
We chose to study three novel SNPs that might be associated with chemosensitivity to cetuximab and bevacizumab regimens in terms of in vitro cytotoxicity according to our previous work (Kim et al, 2011). Additional screening analyses showed that these three SNPs were not associated with chemosensitivity to 5-FU and capecitabine with irinotecan, or oxalipaltin, which were present in the cetuximab or bevacizumab regimens (Kim et al, 2010, 2011). In this study, the lowest minor allele frequency of >18% for ISX rs361863 was used to estimate the smallest sample size (170 patients) for the clinical association analysis to have a 98% chance of identifying susceptibility alleles. On the other hand, among a separate cohort of 314 CRC patients without targeted chemotherapy, the three SNPs were not associated with recurrence or survival outcomes, nor were any of the clinicopathological parameters found to be related to prognosis in a multivariate analysis.
As first- or second-line chemotherapy of our patients, the bevacizumab regimens were administered 1.5 times more often than the cetuximab regimens. Cetuximab requires an examination for KRAS mutational status, while bevacizumab does not need any marker tests that possibly leads to popular use of bevacizumab as a first-line treatment. Skin toxicity, known as acneform rash, overwhelmingly occurred in patients with cetuximab regimens, similar to the 80% incidence reported recently (Custidio and Feliu, 2013).
In this study, patients homozygous for wild-type alleles of LIFR rs3729740 exhibited an ORR about twice as high and a PFS period about 26% longer than those with mutant allele, suggesting that this SNP could be a predictive marker for patients treated with cetuximab regimens. Interestingly, longer OS as well as longer PFS period in patients with wild-type alleles were identified in a subset of patients who received three or more prior chemotherapy lines, which was generally known to reduce chemo-responsiveness. One meta-analysis using 14 eligible RCTs recently demonstrated clear benefits of anti-EGFR treatment when used alongside infusional 5-FU-based regimens in patients without KRAS mutations, regardless of pre-treatment lines (Vale et al, 2012). Intracellular signalling activated by EGFR includes PI3K (PTEN/PI3K-Akt) and MAPK (Ras-Raf-MEK-ERK) pathways (Lemmon and Schlessinger, 2010). In RKO clones treated with cetuximab regimen, we found remarkable downregulation of EGFR, Akt, p-Akt and active p-ERK compared with untreated clones. Specifically in clones treated with cetuximab, active p-ERK expression was significantly less in clones expressing wild-type LIFR rs3729740 than mutant clones. Several studies suggest that LIF signalling is mediated mainly by the JAK/STAT3, p44/42 kinase and PI3K/Akt pathways in a variety of cells and tissues (Kritikou et al, 2003; Carbia-Nagashima and Arzt, 2004). Taken together, these findings suggest that LIFR expression and cetuximab chemosensitivity may affect the downstream of EGFR signalling mediated by MAPK as well as PI3K/Akt and JAK/STAT3 pathways. The SNP ISX rs361863 is less likely to be a chemosensitivity marker for cetuximab regimens as it did not lead to any significant differences.
Although several biomarkers associated with angiogenesis have been shown to have prognostic value, few markers have been proven to predict chemosensitivity to bevacizumab (Gerger et al, 2011). We found that patients homozygous for the minor alleles of ANXA11 rs1049550 had ORR twice as high as those with the ancestral allele, but the survival curves of the two sets of patients were not significantly different. ANXA11 rs1049550*T, located in the first conserved annexin domain, has been reported to be associated with a variant form that protects against sarcoidosis in European Caucasians (Gerke and Moss, 2002; Draeger et al, 2011). According to our biological correlation assay, we found greater downregulation of VEGF and active MMP-9 in RKO clones treated with bevacizumab regimen than those without treatment. Specifically in clones treated with bevacizumab, active MMP-9 expression was more greatly reduced in clones expressing minor-type ANXA11 rs1049550 (T allele) than ancestral-type (C allele). MMP-9 has been reported to have potential importance for reversing vascular basement membrane thickness of glioblastoma in the process of vascular normalisation (Winkler et al, 2004). Taken together with our finding, active MMP-9 appears to be partly downregulated by specific ANXA11 genotype during the process of anti-VEGF treatment.
Wild-type KRAS and skin toxicity are generally known to correlate with greater chemosensitivity and survival in cetuximab regimens, and this was also evident in the current study (Bass et al, 2012; Bokemeyer et al, 2012; Peeters and Price, 2012; Custidio and Feliu, 2013). At present, KRAS mutation is considered the most specific biomarker of poor chemosensitivity, allowing patients to avoid unnecessary and expensive therapy, but its positive predictive value is somewhat limited. The basis of skin toxicity and its correlation with tumour response is still poorly understood. As acneform rash cannot be evaluated until at least 1 week after administration, its predictive value before cetuximab treatment is limited. We examined codons 12 and 13 for KRAS mutations, as approximately 90% of activating mutations are reported in codons 12 (82%–87%) and 13 (13%–18% Bazan et al, 2005). Patients with wild-type KRAS and suffering skin toxicity had ORR periods higher by factors of two and three, respectively. As the genotypes of LIFR rs3729740 were not associated with those of KRAS or skin toxicity, their independent effects on ORR could be evaluated by combining them; ORR was increased by up to 10% in patients homozygous for wild-type LIFR rs3729740 in combination with either wild-type KRAS or skin toxicity (P=0.001). This additive predictability has been further identified in our limited patients carrying wild-type KRAS, showing greater ORR in patients homozygous for wild-type LIFR rs3729740 than those with mutant-type.
This study has unavoidable limitation of the low patient enrolment, mainly attributable to costly targeted regimens without any social insurance coverage in Korea, which resulted in non-RCT including patients treated with various chemotherapy lines and combinations. An efficient predictive marker for cetuximab treatment is urgently needed for the 60%–70% of unresponsive patients with wild-type KRAS, as well as those with mutant KRAS. Moreover, few studies have clinically identified an efficient predictive marker associated with chemosensitivity to bevacizumab. We hope that the two suggested markers in this study, LIFR rs3729740 and putatively ANXA11 rs1049550, will improve the identification of mCRC patients sensitive to cetuximab and bevacizumab regimens, respectively. Prediction of cetuximab responses appears also to be enhanced in patients with wild-type LIFR rs3729740 combined with wild-type KRAS or skin toxicity. Our results point to a need for further validation of chemosensitivity SNPs in large cohorts.
Acknowledgments
This study was supported by grants (to JC Kim) from the Asan Institute for Life Sciences (2012-069) and the Korea Health 21 R&D Project (A062254), Ministry of Health, Welfare, and Family Affairs, Republic of Korea.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, McAllister PK, Morton RF, Schilsky RL. American Society of Clinical Oncology Provisional Clinical Opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- Baas JM, Krens LL, Guchelaar HJ, Ouwerkerk J, de Jong FA, Lavrijsen AP, Gelderblom H. Recommendations on management of EGFR inhibitor-induced skin toxicity: a systematic review. Cancer Treat Rev. 2012;38:505–514. doi: 10.1016/j.ctrv.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Bazan V, Agnese V, Corsale S, Calò V, Valerio MR, Latteri MA, Vieni S, Grassi N, Cicero G, Dardanoni G, Tomasino RM, Colucci G, Gebbia N, Russo A. Specific TP53 and/or Ki-ras mutations as independent predictors of clinical outcome in sporadic colorectal adenocarcinomas: results of a 5-year gruppo oncologico dell'Italia meridionale (GOIM) prospective study. Ann Oncol. 2005;16 (Suppl 4:iv50–iv55. doi: 10.1093/annonc/mdi908. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Köhne CH. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48:1466–1475. doi: 10.1016/j.ejca.2012.02.057. [DOI] [PubMed] [Google Scholar]
- Carbia-Nagashima A, Arzt E. Intracellular proteins and mechanisms involved in the control of gp130/JAK/STAT cytokine signaling. IUBMB Life. 2004;56:83–88. doi: 10.1080/15216540410001668064. [DOI] [PubMed] [Google Scholar]
- Cho DH, Jo YK, Roh SA, Na YS, Kim TW, Jang SJ, Kim YS, Kim JC. Upregulation of SPRR3 promotes colorectal tumorigenesis. Mol Med. 2010;16:271–277. doi: 10.2119/molmed.2009.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custodio A, Feliu J. Prognostic and predictive biomarkers for epidermal growth factor receptor-targeted therapy in colorectal cancer: beyond KRAS mutations. Crit Rev Oncol Hematol. 2013;85:45–81. doi: 10.1016/j.critrevonc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P, Penault-Llorca F, Rougier P, Vincenzi B, Santini D, Tonini G, Cappuzzo F, Frattini M, Molinari F, Saletti P, De Dosso S, Martini M, Bardelli A, Siena S, Sartore-Bianchi A, Tabernero J, Macarulla T, Di Fiore F, Gangloff AO, Ciardiello F, Pfeiffer P, Qvortrup C, Hansen TP, Van Cutsem E, Piessevaux H, Lambrechts D, Delorenzi M, Tejpar S. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, Bastit L, Killian A, Sesboüé R, Tuech JJ, Queuniet AM, Paillot B, Sabourin JC, Michot F, Michel P, Frebourg T. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166–1169. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draeger A, Monastyrskaya K, Babiychuk EB. Plasma membrane repair and cellular damage control: the annexin survival kit. Biochem Pharmacol. 2011;81:703–712. doi: 10.1016/j.bcp.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Gerger A, LaBonte M, Lenz HJ. Molecular predictors of response to antiangiogenesis therapies. Cancer J. 2011;17:134–141. doi: 10.1097/PPO.0b013e318212db3c. [DOI] [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- Kim JC, Kim SY, Cho DH, Ha YJ, Choi EY, Kim CW, Roh SA, Kim TW, Ju H, Kim YS. Novel chemosensitive single-nucleotide polymorphism markers to targeted regimens in metastatic colorectal cancer. Clin Cancer Res. 2011;17:1200–1209. doi: 10.1158/1078-0432.CCR-10-1907. [DOI] [PubMed] [Google Scholar]
- Kim JC, Kim SY, Cho DH, Roh SA, Choi EY, Jo YK, Jung SH, Na YS, Kim TW, Kim YS. Genome-wide identification of chemosensitive single nucleotide polymorphism markers in colorectal cancers. Cancer Sci. 2010;101:1007–1013. doi: 10.1111/j.1349-7006.2009.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritikou EA, Sharkey A, Abell K, Came PJ, Anderson E, Clarkson RW, Watson CJ. A dual, non-redundant, role for LIF as a regulator of development and STAT3-mediated cell death in mammary gland. Development. 2003;30:3459–3468. doi: 10.1242/dev.00578. [DOI] [PubMed] [Google Scholar]
- Kuramochi H, Nakajima G, Kaneko Y, Nakamura A, Inoue Y, Yamamoto M, Hayashi K. Amphiregulin and epiregulin mRNA expression in primary colorectal cancer and corresponding liver metastases. BMC Cancer. 2012;12:88. doi: 10.1186/1471-2407-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters M, Price T. Biologic therapies in the metastatic colorectal cancer treatment continuum- applying current evidence to clinical practice. Cancer Treat Rev. 2012;38:397–406. doi: 10.1016/j.ctrv.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Roh SA, Choi EY, Cho DH, Yoon YS, Kim TW, Kim YS, Kim JC. Characterization of biological responses of colorectal cancer cells to anticancer regimens. J Korean Surg Soc. 2012;83:21–29. doi: 10.4174/jkss.2012.83.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Vale CL, Tierney JF, Fisher D, Adams RA, Kaplan R, Maughan TS, Parmar MK, Meade AM. Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat Rev. 2012;38:618–625. doi: 10.1016/j.ctrv.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.