Abstract
Aging is the primary risk factor for dementia. With increasing life expectancy and aging populations worldwide, dementia is becoming one of the significant public health problems of the century. The most common pathology underlying dementia in older adults is Alzheimer’s disease. Proton magnetic resonance spectroscopy (MRS) may provide a window into the biochemical changes associated with the loss of neuronal integrity and other neurodegenerative pathology that involve the brain before the manifestations of cognitive impairment in patients who are at risk for Alzheimer’s disease. This review focuses on proton MRS studies in normal aging, mild cognitive impairment, and dementia, and how proton MRS metabolite levels may be potential biomarkers for early diagnosis of dementia-related pathologic changes in the brain.
Keywords: Alzheimer’s disease, magnetic resonance spectroscopy, mild cognitive impairment
Introduction
Biomarkers of Alzheimer’s disease (AD) are important for both early diagnoses and evaluating treatment effects. Three decades of research indicate that proton magnetic resonance spectroscopy (MRS) is a potential biochemical imaging marker in AD. The focus of this review is to discuss the role of proton MRS in Alzheimer’s disease. MRS allows regional measurement of metabolites including myo-Inositol (mI), choline (Cho), N-acetyl aspartate (NAA), and creatine (Cr). Cr is typically used as an internal reference to control for variability in measurement because it remains unchanged in AD.1–3 Other metabolites that can be measured with proton MRS with advanced MRS sequences and post-processing methods include gamma-Aminobutyric acid (GABA) and glutathione which may not be available in conventional clinical scanners and are not the focus of this review. MRS may serve to identify patients with AD before clinical symptom onset as well as help distinguish AD from other neurodegenerative disorders.
Development of metabolic biomarkers for AD
In 1992, Klunk et al4 demonstrated a decrease in the neuronal metabolite NAA on MRS on autopsy brain samples of patients with AD compared to controls. The lower NAA level correlated with the amount of plaque and tangle pathology.4 NAA is considered a neuronal marker and is synthesized in mitochondria.5 Supporting the notion that NAA levels correspond to neuronal integrity, reduced NAA levels in cortical tissue from patients with AD demonstrated a correlation between NAA concentration and neuronal density.6 Decreased NAA seen in head trauma, seizure, or coronary artery bypass surgery can resolve after recovery.5,7–9 Further, NAA levels decreased in AD show transient improvement with acetylcholinesterase inhibitor treatment.10 The neurofibrillary tangles pathology of AD follow a typical progression from limbic to neocortical areas as AD advances.11 Similarly, changes in NAA follow a regional pattern as disease advances. For example, AD patients show a regional decrease in NAA/Cr ratio in the superior temporal lobe and posterior cingulate voxels compared to controls, but mild cognitive impairment (MCI) patients do not show a decline in NAA/Cr as AD-related neurodegeneration has not yet extended to these regions.12 Medial occipital lobe AD pathology involvement typically occurs at the final stage of the disease.11 Therefore, it is not surprising that there is no regional decrease in NAA/Cr in mild AD in the occipital lobe while more advanced cases demonstrate decrease NAA/Cr ratio.12–14 Eventually, NAA changes become widespread and have been shown to involve the parietal, temporal, and frontal lobe.12,15–19 Decreased NAA/Cr ratios are non-specific and can be seen in other types of dementia including normal pressure hydrocephalus and have even been reported in cognitive decline associated with acquired immunodeficiency syndrome (AIDS).20,21
In 1993, it was demonstrated that in addition to decreased NAA levels, AD patients have elevated myo-Inositol to creatine (mI/Cr) levels.13 While NAA is a neuronal marker, mI is associated with glia and elevated levels with glial proliferation.22,23 The finding of decreased NAA and elevated mI in AD has been confirmed in several studies.12,16,24
The role of choline metabolite in AD is more controversial. A number of studies demonstrated increased choline in AD.25–27 Other studies have demonstrated no change in choline concentration in AD compared to controls.28–31 One study reported a decrease of choline/H20 in the medial temporal lobes of AD patients.32 Brain choline is concentrated in phospholipids. The choline peak in MRS represents cytosolic glycerophosphocholine and phosphocholine which are breakdown products of phosphatidylcholine.33 Therefore the larger choline peak may be due to increased membrane turnover. Also, it has been proposed that catabolism of the phospholipid membrane bilayer allows AD subjects to produce choline to compensate for declining acetylcholine.34 Administration of xanomeline, an M1 selective muscarinic cholinergic agonist, to AD patients resulted in a significant decline in Cho/Cr ratios, perhaps representing a reduction in compensatory mechanisms to produce acetylcholine through phospholipid breakdown.35 Cho/Cr increases in amnestic MCI if it progresses to AD but the ratio decreases if cognition remains stable.36
Decreased glutamate (Glu) or glutamate + glutamine/Cr ratio has also been found in the grey matter of AD patients, but not the white matter.37–40 Furthermore, increased Glu and the ratio of Glu to Cr measured from the hippocampus by MRS after galantamine treatment were associated with increased cognitive performance.41 MRS studies in transgenic mice with AD mutations have shed light on the pathological correlates of metabolite changes seen in AD. Transgenic mice with AD mutations demonstrate similar decreased NAA and increased mI as seen in human AD patients.6,42 In addition, lower NAA and glutamate levels correlate with amyloid beta (Aβ) plaque load in the frontal cortex of mice with PS2APP mutation.43 Further, the MRS metabolite changes consistent with AD precede overt cognitive dysfunctions in early-stage AD.44
The temporal progression of metabolite abnormalities in AD are characterized by an increased mI/Cr followed by a decrease in NAA/Cr and an increase in Cho/Cr.12 A recent study of pathologic correlates of MRS metabolite changes in cases of varying AD pathology demonstrated that antemortem NAA/Cr and mI/Cr levels correlate with the pathologic severity of AD, and that the strongest predictor of AD pathology was a NAA/mI ratio.45 Longitudinal studies have demonstrated that NAA/Cr and NAA/mI decrease over time compared to controls.36,46,47
NAA/Cr and mI/Cr ratios correlate with cognitive testing in Alzheimer’s disease.16,17,24,48–51 In one study, NAA/Cr in the medial temporal lobe, primary motor and sensory cortices correlated with Mini-Mental State Examination and the cognitive part of the Alzheimer Disease Assessment Scale scores.16 NAA/Cr, mI/Cr, NAA/mI have also been shown to correlate with verbal memory testing (Auditory Verbal Learning Test) and general cognition (Dementia Rating Scale).50
Several studies have investigated the ability of MRS to distinguish AD patients from controls with varying results depending on the anatomic area analyzed and acquisition parameters. The sensitivity was as high as 90% in the temporoparietal region and as low as 57% in the parietal lobe grey matter. The specificity was as a high as 95% in the medial occipital lobe and as low as 73% in the posterior cingulate.14,19,52–54 Furthermore, adding hippocampal volume to MRS, improves the ability to distinguish AD.19,53–56
A few studies with relatively small sample sizes have investigated MRS as a biomarker for treatment response in AD. NAA/Cr improved after acetylcholinesterase inhibitor treatment in AD.10,28 Another trial showed decreases in Cho/Cr and mI/Cr in the hippocampus in absence of clinical improvement in AD subjects, however, this study showed continued decrease in NAA/Cr in contrast to the studies mentioned above.39
MRS also correlates with psychiatric symptoms in AD patients. AD subjects with psychosis have significantly reduced cortical NAA compared to AD subjects without psychosis.57 Psychiatric and behavioral symptoms in AD including delusional thinking correlated with a decrease in NAA/Cr and an increase in mI/Cr in the anterior cingulate.58
MRS for MCI
NAA/Cr levels in MCI are mildly reduced but decline as patients with MCI progress to AD.36 Further, lower NAA/Cr in MCI patients predicts progression to AD.36,59,60 Cho/Cr and mI/Cr levels are also elevated in the posterior cingulate in MCI although higher levels of these metabolites are detected in AD.12 The Cho/Cr ratio is also useful in determining progression from MCI to AD. In MCI patients, a decline in Cho/Cr predicted stability versus an increase, which predicted conversion to AD. The changes in metabolite concentration on MRS correlate as strongly as ventricular volume in predicting cognitive decline.36
MRS is also useful in distinguishing subtypes of MCI. Amnestic MCI patients have smaller hippocampi with elevated mI/Cr ratios compared to patients with non-amnestic MCI in line with the observation that amnestic MCI patients are more likely to progress to AD than non-amnestic MCI.61
MRS based identification of biomarkers for other neurological disorders, to distinguish from AD
The main differential diagnoses of Alzheimer’s dementia are other types of dementia including dementia with Lewy bodies (DLB), frontotemporal dementia (FTD), and vascular dementia (VaD). The MRS signature of AD is decreased NAA/Cr and elevated Cho/Cr and mI/Cr metabolites.25 Several studies have investigated the metabolite patterns among different types of dementia in order to identify patterns of metabolite changes unique to each dementia.
Many patients with dementia have significant overlap in underlying pathology.62 In patients with VaD, the location of the metabolite change is important for distinguishing VaD from AD. For example, NAA levels in VaD are decreased in a similar way to those in AD patients and the decrease is greater than AD in the white matter.63–65 Unlike AD, cortical mI is normal in VaD.66,67 Further, mI/Cr is higher in AD compared to VaD.25 Therefore, mI and grey matter NAA may serve as useful markers to distinguish AD from VaD.
Similar to AD, FTD is associated with elevated mI/Cr and decreased NAA/Cr.68,69 Despite the similarities, regional differences in metabolites may help distinguish the two dementias. Compared to early AD, patients with FTD have higher mI/Cr and lower NAA/Cr in the frontal cortex.68,70 However, others have noted no difference between FTD and AD with similar levels of metabolite abnormalities outside the frontal lobes.25
Similar to the other dementias, significant overlap exists between DLB and AD. Compared to other dementia subtypes, DLB has a normal NAA/Cr ratio in the posterior cingulate.25 The normal NAA/Cr is possibly related to the relative preservation of neurons in DLB compared to AD.71 In the hippocampus of DLB patients, the NAA has been reported to be decreased although it is unclear if this decrease represents concomitant AD given the overlap in pathologies mentioned above.72 Additionally, NAA is similarly decreased in the white matter of patients with DLB.73 Therefore, NAA levels in the posterior cingulate may help distinguish AD from DLB. The relative preservation of the cingulate is in agreement with fluorodeoxyglucose (FDG) positron emission tomography (PET) which demonstrates a relative preservation of glucose metabolism in the cingulate of DLB when compared to AD.74
Similar to AD, DLB patients have an elevated Cho/Cr compared to controls.25 This finding is intriguing because both AD and DLB are characterized by a cholinergic deficit.75 Since Cho/Cr levels decrease with cholinergic agonist treatment in AD,35 the decrease raises the possibility that Cho/Cr can be used as a therapeutic biomarker in AD and DLB.
In summary, while significant overlap exists between dementias, AD has a unique metabolite pattern compared to other dementias when regional differences are taken into account.
Current place of MRS in the differential diagnosis of AD
The American Academy of Neurology practice parameter recommends against routine imaging with quantitative MRI techniques in the evaluation of dementia because of insufficient evidence.76 Nonetheless, as a research tool, MRS can provide valuable information in the differential diagnoses of dementia. AD biomarkers, such as Amyloid PET imaging and cerebrospinal fluid Aβ, provide useful information about whether AD is the pathology underlying a given dementia. MRS can provide complementary predictive information. In addition, MRS allows for identification of a metabolite signature of different dementia subtypes which can provide complementary data to the clinical impression. To date, no MRS study has used metabolite signature in conjunction with regional differences to determine the underlying pathology.
Utility of MRS along with other AD biomarkers
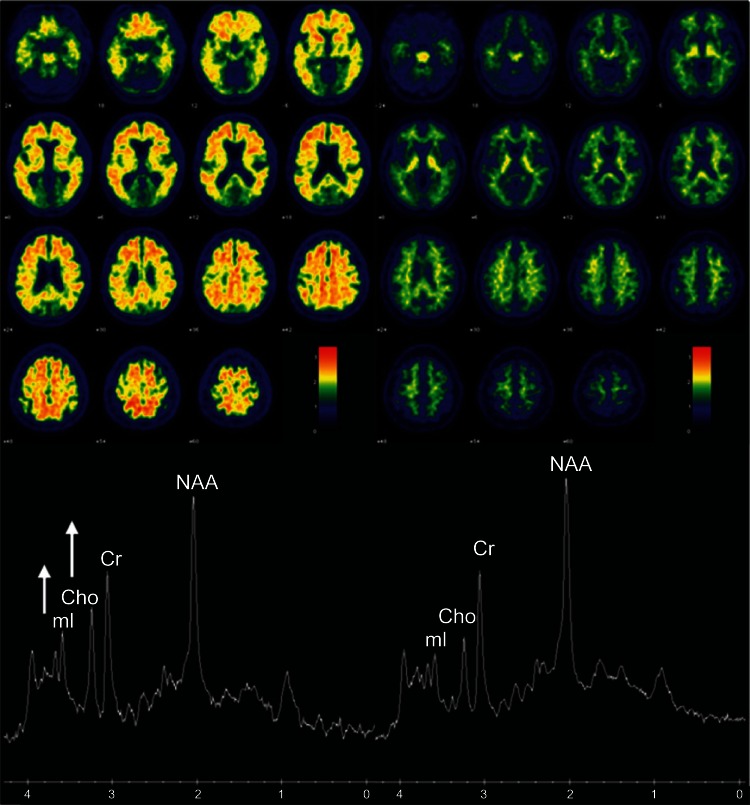
In 2011, the National Institute on Aging and Alzheimer’s Association revised criteria for preclinical, MCI, and Alzheimer’s disease.77–80 The pathology that contributes to AD begins to accumulate years before clinical symptoms. Therefore, identifying the population at high risk of developing symptoms has become important. Principal AD imaging biomarkers include Aβ imaging with PET, FDG-PET, and structural MRI. In cognitively normal older adults, mI/Cr and Cho/Cr correlate with Aβ load in amyloid PET imaging as demonstrated in two example cases with high and low Aβ load in their brains in Figure 1.81
Figure 1.
Association between MRS metabolite ratios and cortical Pittsburgh compound-B (PiB) retention ratio on PET.
Notes: The left panel shows the PiB retention ratio PET images in an 82-year-old man with a PiB retention ratio of 2.42, NAA/Cr ratio of 1.51, mi/Cr ratio of 0.58, and Cho/Cr ratio of 0.76. The right panel shows the PiB retention ratio in a 78 year-old woman with a PiB retention ratio PET images of 1.27, NAA/Cr ratio of 1.69, mi/Cr ratio of 0.45, and Cho/Cr ratio of 0.54. The spectra are scaled to the Cr peak. High PiB retention is associated with significantly elevated mI/Cr and Cho/Cr ratio and a trend of lower NAA/Cr ratio.
Abbreviations: Cho, choline; Cr, creatine; mi, myo-inositol; MRS, magnetic resonance spectroscopy; NAA, N-acetyl aspartate; PET, positron emission tomography.
According to one model of biomarkers in AD, amyloid pathology accumulates before evidence of neurodegeneration.82 Amyloid PET imaging serves as a surrogate for brain amyloid load and FDG-PET and Structural MRI serve as markers of neurodegeneration. While these imaging markers are well validated measures of amyloid and neurofibrillary tangle pathology, they do not measure microglial activation. MRS mI is a potential surrogate marker for glial activity. A recent 13-carbon MRS and 1H MRS linked glial and microglial activity to mI elevation in AD.83 This raises the possibility that elevated mI represents inflammation which is an early event in the evolution of AD pathology.2
While hippocampal atrophy predicts AD pathology and is an imaging marker of neurodegeneration in the National Institute on Aging – Alzheimer’s Association (criteria for preclinical AD),84,85 up to 11% of AD cases are hippocampal sparing, with corresponding preserved hippocampal volumes on MRI.86,87 Subjects with at least one type of hippocampal sparing AD (posterior cortical atrophy), have been demonstrated to have decreased NAA/Cr in the posterior cingulate.88 Therefore, MRS may serve a role as a critical marker of AD pathology in a significant minority of AD cases where the hippocampus is relatively preserved. Other imaging markers that may provide important information in atypical AD cases include resting state functional MRI, and diffusion tensor imaging (DTI). Cognitive testing may provide additional information.
MRS can serve as a predictor of the degree of AD pathology in clinical trial design. For example, mI/Cr is elevated in MCI and mild AD even with normal NAA/Cr.12,24,89 In addition, isolated mI/Cr elevation is associated with earlier stage AD pathology compared to elevated mI and decrease in NAA/Cr which is associated with a later stage AD pathology. Isolated elevation in mI/Cr can be seen prior to structural MRI changes in individuals with familial dementia prior to symptom onset.90 Therefore, MRS could potentially serve as an adjunct to help select patients for intervention trials based on degree of AD pathology.
In addition to serving as a potential marker of glial activity, MRS can be used along with other biomarkers as a tool to predict cognitive decline. In a group of cognitively normal individuals, elevated Cho/Cr in the white matter predicts progression to dementia.91 The metabolite formula changes in preclinical familial AD families with amyloid precursor protein, presenilin 1 or 2 mutations. Asymptomatic mutation carriers demonstrated elevated mI/Cr and decreased NAA/Cr with reduction in NAA/mI correlating with nearness to age of onset.92 In addition to serving as a marker of preclinical disease, MRS has utility in monitoring disease progression. NAA/Cr levels predict cognitive decline in individuals with AD.36,46,93
Future predictions for the use of MRS in the differential diagnosis of AD
While much progress has been made in understanding the role of MRS in Alzheimer’s disease, MRS is still not routinely used clinically in the assessment of dementia. Reasons for ineffective translation of technology to clinical practice or patient-oriented research are twofold: (1) Lack of standardization for multi-site applications and normative data; and (2) insufficient understanding of the pathologic basis of 1H MRS metabolite changes.94 Although metabolite abnormalities in AD have been demonstrated in different samples and in pathologically confirmed cases, the pathological substrates for these metabolite abnormalities are not fully understood. Future studies are needed to elucidate the pathological significance of these metabolite changes in AD. As we learn more about the pathophysiologic underpinnings of the metabolite abnormalities, the routine use of MRS as a biomarker will become more prevalent.
Table 1.
In vivo studies with MRS in AD and MCI
| Authors | Disease | Acquisition parameters | Magnet field strength | Single voxel locations | Key findings |
|---|---|---|---|---|---|
| Schuff et al17 | AD | TR/TE= 1800/135 ms | 1.5 T | Frontal, middle, posterior mesial cortex | Decreased NAA frontal and posterior mesial cortex. |
| Zhu et al19 | AD | TR/TE= 1800/25 ms | 1.5 T | Parietal GM, frontal GM, parietal WM, and frontal WM | Decreased NAA/Cr parietal/frontal GM and parietal WM. Increased ml/Cr in parietal GM. |
| Schuff et al31 | AD | TR/TE= 1800/135 ms | 1.5 T | Hippocampus | Decreased NAA/Cr hippocampus. |
| Frederick et al15 | AD | TR/TE = 2000/272 ms | Not reported | Parietal, temporal lobes | Decreased NAA/Cr temporal lobe compared to controls. |
| Jessen et al16 | AD | TR/TE = 2000/272 ms | 1.5 T | Medial temporal lobe and the primary motor and sensory cortex | Decreased NAA/Cr and Cho/Cr medial temporal lobe. |
| Huang et al24 | AD | TR/TE = 3000/30 ms | 1.5 T | Occipital and parietal regions | Decreased NAA/Cr in occipital and parietal regions in AD which changes with severity. Increased ml/Cr in occipital and parietal regions even in mild AD. |
| Chantal et al95 | AD and MCI | TR/TE = 1200/50 ms | 1.5 T | Medial temporal lobes, parietotemporal cortices and prefrontal cortices | AD and MCI demonstrated reductions of NAA/H2O and Cho/H2O in the left MTL, ml/Cr increased in AD relative to MCI and controls in medial temporal lobe. |
| Kantarci et al50 | AD and MCI | TR/TE = 2000/30 ms | 1.5 T | Posterior cingulate | NAA/Cr AD < MCI = control. ml/Cr AD > Controls. |
| Shinno et al58 | AD | TR/TE = 1500/30 ms | 1.5 T | Posterior cingulate, anterior cingulate | NAA/Cr positively correlated and ml/Cr negatively correlated to cognitive tests in the posterior but not anterior cingulate. Behavioral symptoms of dementia negatively related with NAA/Cr and positively related with ml/Cr in the anterior cingulate gyrus, but not in the posterior cingulate. |
| Modrego et al10 | AD | TR/TE = 2500/30 ms | 1.5 T | Frontal, parietal and occipital cortices | Rivastigmine treatment reversed NAA/Cr decrease in frontal cortex only. |
| Miller et al13 | AD | TR/TE = 1500/30 ms | 1.5 T | Parietal WM and occipital GM | Decreased NAA/Cr in occipital GM and parietal WM in AD. Increased ml/Cr in occipital GM and parietal WM. |
| Shonk et al14 | AD | TR/TE = 1500/30 ms | Not reported | Occipital grey matter | Reduced NAA/Cr and increase in ml/Cr in occipital grey matter in AD. |
| Tedeschi et al18 | AD | TR/TE = 2200/272 ms | 1.5 T | Frontal, temporal, parietal, occipital, and insular cortices, subcortical WM, and thalamus | Reduction of NAA/Cr in the frontal, temporal, parietal cortices in AD. Reduction of Cho/Cr in the WM. |
| Kantarci et al25 | AD | TR/TE = 2000/30 ms | 1.5 T | Posterior cingulate | Reduced NAA/Cr and increase in ml/Cr and elevated Cho/Cr in AD compared to controls. |
| Meyerhoff et al26 | AD | Not reported | 2T | Centrum semiovale containing white and mesial grey matter | Reduced NAA/Cr in WM of AD. Posterior section of the centrum semiovale in patients showed increased Cho/Cr compared to controls. |
| Krishnan et al28 | AD | TR/TE = 1200/35 ms | 1.5 T | Subcortical gray, periventricular, cortical, and WM at the level of the third ventricle | Elevated NAA in the donepezil-treated compared to placebo controls. |
| Rose et al30 | AD | TR/TE = 2000/30 ms | 1.5 T | Parietal lobes | NA/Cr was significantly reduced in the AD group with increase in ml/Cr. Metabolite measures also correlated with atrophy. |
| Satlin et al35 | AD | TR/TE = 2 sec/272 ms | 1.5 T | Parietal lobe | For patients taking xanomeline, there was a decrease in Cho/Cr. |
| Kantarci et al36 | AD and MCI | TR/TE = 2000/30 ms | 1.5 T | Posterior cingulate | NAA/Cr declined in MCI and AD patients compared to controls. Cho/Cr declined in stable MCI, compared to converter MCI patients. |
| Hattori et al38 | AD | TR/TE = 6 s/25 ms | 3T | Posterior cingulate gyrus and the precuneus and parietoccipital WM | NA/Cr decreased in both regions in AD. Decrease in the glutamate+Glu/Cr and were detected in the grey matter, but not in the white matter. |
| Bartha et al39 | AD | TR/TE = 3200/46 ms | 4T | Hippocampus | Decreased NAA/Cr, Cho/Cr, and ml/Cr were observed in AD patients after 4 months of donepezil treatment. |
| Rupsingh et al40 | AD | TR/TE = 2.2 s/46 ms | 4T | Hippocampus | Subjects with AD had decreased glutamate as well as decreased Glu/Cr, Glu/ml, Glu/NAA, and NAA/Cr ratios compared to controls. |
| Penner et al41 | AD | TR/TE = 3200/46 ms | 4T | Right hippocampus | Levels of Glu, Glu/Cr, and Glu/NAA increased after 4 months of treatment with galantamine compared to controls. |
| Schott et al47 | AD | TR/TE = 2000/30 ms | 1.5 T | Posterior cingulate | Decreased NAA/Cr in post cingulate. Increased ml/Cr in post cingulate. NAA/ml declines over time in AD compared to controls. |
| Ackl et al48 | AD and MCI | TR/TE = 2000/35 ms for the parietal white and grey matter and TE = 70 ms for the hippocampus on the left cerebral hemisphere | 1.5 T | Parietal gray and white matter and the hippocampus | NAA/Cr was reduced in the hippocampus of MCI and AD. AD had elevated ml/Cr PGM, reduced NAA/Cr in PWM. |
| Schuff et al56 | AD | TR/TE = 1800/135 ms | 1.5 T | Medial temporal, frontal, parietal gray and white matter | NAA concentration was less in AD in the medial temporal lobe and parietal grey matter, but not white matter and frontal lobe GM. Lower NAA AD patients had smaller hippocampi and less cortical GM. |
| Metastasio et al60 | MCI | TR/TE = 2000/40 ms | 1.5 T | Paratrigonal WM | Patients with MCI who progressed to dementia had lower NAA/Cr at baseline than stable MCI. |
| Kantarci et al61 | MCI | TR/TE = 2000/30 ms | 1.5 T | Posterior cingulate | NAA/Cr can predict conversion from MCI to dementia. |
| Mckay et al64 | AD and VaD | TR/TE = 3000/30 and 80 ms | 2T | Anterior, medial, and posterior GM and WM | Lower NAA/Cr compared to controls. High Cho/Cr in posterior regions compared to controls. Lower NAA/Cr in VaD compared to controls. |
| Schuff et al65 | AD and VaD | TR/TE = 1800/135 ms | 1.5 T | Frontal, parietal, temporal cortex | Compared to controls, VaD had lower NAA in frontal and parietal cortex. Compared to patients with AD, VaD had lower NAA in frontal cortex and parietal cortex. |
| Waldman et al67 | AD, VaD | TR/TE = 1500/30 ms | 1 T | Occipital GM | AD had higher ml/Cr compared to those with VaD and normal subjects. |
| Mihara et al68 | AD, FTD | TR/TE = 6000/25 ms | 3T | Posterior and anterior cingulate cortices and the parietoccipital and frontal white matter | NAA/Cr reduced in the posterior cingulate cortex in AD and FTD. AD showed a posterior dominant decrease, whereas FTD showed a frontal decrease. |
| Ernst et al70 | AD, FTD | TR/TE = 3000/30 ms | 1.5 T | Midfrontal and temporoparietal gray matter | FTD showed reduced NAA and glutamate plus glutamine and increased ml. In AD, no abnormalities were observed in the frontal region, but ml was elevated in the temporoparietal region. |
Abbreviations: AD, Alzheimer’s disease; Cho, choline, Cr, creatine; FTD, frontotemporal dementia; Glu, glutamate; GM, grey matter; ml/, myo-inositol; MCI, mild cognitive impairment; NAA, N-acetyl aspartate; PGM, parietal grey matter; PWM, parietal white matter; T, tesla; TE, echo time; TR, repetition time; WM, white matter; VaD, vascular dementia.
Acknowledgment
Grant support: Dr Kantarci’s research program is supported by the National Institutes of Health: R01 AG40042, P50 AG16574/Project1, P50AG044170/Project 2, and R21 NS066147. The authors would like to acknowledge Samantha Wille for manuscript preparation.
Footnotes
Disclosure
Dr Kantarci serves on the data safety monitoring board for Pfizer Inc, and Janssen Amyloid Immunotherapy and Takeda Global Research and Development Center, Inc; she is funded by the National Institutes of Health (R01AG040042 [PI], R21 NS066147 [PI], P50 AG44170/Project 2 [PI], P50 AG16574/Project 1 [PI], and R01 AG11378 [Co-I]).
Dr Graff-Radford reports no conflicts of interest in this work.
References
- 1.Kantarci K.1H magnetic resonance spectroscopy in dementia Br J Radiol 200780Spec No 2:S146–S152. [DOI] [PubMed] [Google Scholar]
- 2.Ross BD, Bluml S, Cowan R, Danielsen E, Farrow N, Tan J. In vivo MR spectroscopy of human dementia. Neuroimaging Clin N Am. 1998;8(4):809–822. [PubMed] [Google Scholar]
- 3.Valenzuela MJ, Sachdev P. Magnetic resonance spectroscopy in AD. Neurology. 2001;56(5):592–598. doi: 10.1212/wnl.56.5.592. [DOI] [PubMed] [Google Scholar]
- 4.Klunk WE, Panchalingam K, Moossy J, McClure RJ, Pettegrew JW. N-acetyl-L-aspartate and other amino acid metabolites in Alzheimer’s disease brain: a preliminary proton nuclear magnetic resonance study. Neurology. 1992;42(8):1578–1585. doi: 10.1212/wnl.42.8.1578. [DOI] [PubMed] [Google Scholar]
- 5.Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7(8):1397–1400. [PubMed] [Google Scholar]
- 6.Cheng LL, Newell K, Mallory AE, Hyman BT, Gonzalez RG. Quantification of neurons in Alzheimer and control brains with ex vivo high resolution magic angle spinning proton magnetic resonance spectroscopy and stereology. Magn Reson Imaging. 2002;20(7):527–533. doi: 10.1016/s0730-725x(02)00512-x. [DOI] [PubMed] [Google Scholar]
- 7.Brooks WM, Stidley CA, Petropoulos H, et al. Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J Neurotrauma. 2000;17(8):629–640. doi: 10.1089/089771500415382. [DOI] [PubMed] [Google Scholar]
- 8.Bendszus M, Reents W, Franke D, et al. Brain damage after coronary artery bypass grafting. Arch Neurol. 2002;59(7):1090–1095. doi: 10.1001/archneur.59.7.1090. [DOI] [PubMed] [Google Scholar]
- 9.Hugg JW, Kuzniecky RI, Gilliam FG, Morawetz RB, Fraught RE, Hetherington HP. Normalization of contralateral metabolic function following temporal lobectomy demonstrated by 1H magnetic resonance spectroscopic imaging. Ann Neurol. 1996;40(2):236–239. doi: 10.1002/ana.410400215. [DOI] [PubMed] [Google Scholar]
- 10.Modrego PJ, Pina MA, Fayed N, Diaz M. Changes in metabolite ratios after treatment with rivastigmine in Alzheimer’s disease: a nonrandomised controlled trial with magnetic resonance spectroscopy. CNS Drugs. 2006;20(10):867–877. doi: 10.2165/00023210-200620100-00006. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 12.Kantarci K, Jack CR, Jr, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: A 1H MRS study. Neurology. 2000;55(2):210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller BL, Moats RA, Shonk T, Ernst T, Woolley S, Ross BD. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology. 1993;187(2):433–437. doi: 10.1148/radiology.187.2.8475286. [DOI] [PubMed] [Google Scholar]
- 14.Shonk TK, Moats RA, Gifford P, et al. Probable Alzheimer disease: diagnosis with proton MR spectroscopy. Radiology. 1995;195(1):65–72. doi: 10.1148/radiology.195.1.7892497. [DOI] [PubMed] [Google Scholar]
- 15.Frederick BB, Satlin A, Yurgelun-Todd DA, Renshaw PF. In vivo proton magnetic resonance spectroscopy of Alzheimer’s disease in the parietal and temporal lobes. Biol Psychiatry. 1997;42(2):147–150. doi: 10.1016/s0006-3223(97)00242-4. [DOI] [PubMed] [Google Scholar]
- 16.Jessen F, Block W, Traber F, et al. Proton MR spectroscopy detects a relative decrease of N-acetylaspartate in the medial temporal lobe of patients with AD. Neurology. 2000;55(5):684–688. doi: 10.1212/wnl.55.5.684. [DOI] [PubMed] [Google Scholar]
- 17.Schuff N, Amend DL, Meyerhoff DJ, et al. Alzheimer disease: quantitative H-1 MR spectroscopic imaging of frontoparietal brain. Radiology. 1998;207(1):91–102. doi: 10.1148/radiology.207.1.9530304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedeschi G, Bertolino A, Lundbom N, et al. Cortical and subcortical chemical pathology in Alzheimer’s disease as assessed by multislice proton magnetic resonance spectroscopic imaging. Neurology. 1996;47(3):696–704. doi: 10.1212/wnl.47.3.696. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, Schuff N, Kornak J, et al. Effects of Alzheimer disease on fronto-parietal brain N-acetyl aspartate and myo-inositol using magnetic resonance spectroscopic imaging. Alzheimer Dis Assoc Disord. 2006;20(2):77–85. doi: 10.1097/01.wad.0000213809.12553.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiino A, Matsuda M, Morikawa S, Inubushi T, Akiguchi I, Handa J. Proton magnetic resonance spectroscopy with dementia. Surg Neurol. 1993;39(2):143–147. doi: 10.1016/0090-3019(93)90093-g. [DOI] [PubMed] [Google Scholar]
- 21.Barker PB, Lee RR, McArthur JC. AIDS dementia complex: evaluation with proton MR spectroscopic imaging. Radiology. 1995;195(1):58–64. doi: 10.1148/radiology.195.1.7892496. [DOI] [PubMed] [Google Scholar]
- 22.Bitsch A, Bruhn H, Vougioukas V, et al. Infammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol. 1999;20(9):1619–1627. [PMC free article] [PubMed] [Google Scholar]
- 23.Glanville NT, Byers DM, Cook HW, Spence MW, Palmer FB. Differences in the metabolism of inositol and phosphoinositides by cultured cells of neuronal and glial origin. Biochimica et Biophysica Acta. 1989;1004(2):169–179. doi: 10.1016/0005-2760(89)90265-8. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Alexander GE, Chang L, et al. Brain metabolite concentration and dementia severity in Alzheimer’s disease: a (1)H MRS study. Neurology. 2001;57(4):626–632. doi: 10.1212/wnl.57.4.626. [DOI] [PubMed] [Google Scholar]
- 25.Kantarci K, Petersen RC, Boeve BF, et al. 1H MR spectroscopy in common dementias. Neurology. 2004;63(8):1393–1398. doi: 10.1212/01.wnl.0000141849.21256.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyerhoff DJ, MacKay S, Constans JM, et al. Axonal injury and membrane alterations in Alzheimer’s disease suggested by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol. 1994;36(1):40–47. doi: 10.1002/ana.410360110. [DOI] [PubMed] [Google Scholar]
- 27.Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan E V, Lim KO. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magn Reson Med. 1999;41(2):276–284. doi: 10.1002/(sici)1522-2594(199902)41:2<276::aid-mrm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan KR, Charles HC, Doraiswamy PM, et al. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s disease. Am J Psychiatry. 2003;160(11):2003–2011. doi: 10.1176/appi.ajp.160.11.2003. [DOI] [PubMed] [Google Scholar]
- 29.Moats RA, Ernst T, Shonk TK, Ross BD. Abnormal cerebral metabolite concentrations in patients with probable Alzheimer disease. Magn Reson Med. 1994;32(1):110–115. doi: 10.1002/mrm.1910320115. [DOI] [PubMed] [Google Scholar]
- 30.Rose SE, de Zubicaray GI, Wang D, et al. A 1H MRS study of probable Alzheimer’s disease and normal aging: implications for longitudinal monitoring of dementia progression. Magn Reson Imaging. 1999;17(2):291–299. doi: 10.1016/s0730-725x(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 31.Schuff N, Amend D, Ezekiel F, et al. Changes of hippocampal N-acetyl aspartate and volume in Alzheimer’s disease. A proton MR spectroscopic imaging and MRI study. Neurology. 1997;49(6):1513–1521. doi: 10.1212/wnl.49.6.1513. [DOI] [PubMed] [Google Scholar]
- 32.Chantal S, Labelle M, Bouchard RW, Braun CM, Boulanger Y. Correlation of regional proton magnetic resonance spectroscopic metabolic changes with cognitive deficits in mild Alzheimer disease. Arch Neurol. 2002;59(6):955–962. doi: 10.1001/archneur.59.6.955. [DOI] [PubMed] [Google Scholar]
- 33.Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm. 2000;107(8–9):1027–1063. doi: 10.1007/s007020070051. [DOI] [PubMed] [Google Scholar]
- 34.Wurtman RJ, BJ, Marie JC. “Autocannibalism” of choline-containing membrane phospholipids in the pathogenesis of Alzheimer’s disease. Neurochem Int. 1985;7:369–372. doi: 10.1016/0197-0186(85)90127-5. [DOI] [PubMed] [Google Scholar]
- 35.Satlin A, Bodick N, Offen WW, Renshaw PF. Brain proton magnetic resonance spectroscopy (1H-MRS) in Alzheimer’s disease: changes after treatment with xanomeline, an M1 selective cholinergic agonist. Am J Psychiatry. 1997;154(10):1459–1461. doi: 10.1176/ajp.154.10.1459. [DOI] [PubMed] [Google Scholar]
- 36.Kantarci K, Weigand SD, Petersen RC, et al. Longitudinal 1H MRS changes in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2007;28(9):1330–1339. doi: 10.1016/j.neurobiolaging.2006.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antuono PG, Jones JL, Wang Y, Li SJ. Decreased glutamate + glutamine in Alzheimer’s disease detected in vivo with (1)H-MRS at 0.5 T. Neurology. 2001;56(6):737–742. doi: 10.1212/wnl.56.6.737. [DOI] [PubMed] [Google Scholar]
- 38.Hattori N, Abe K, Sakoda S, Sawada T. Proton MR spectroscopic study at 3 Tesla on glutamate/glutamine in Alzheimer’s disease. Neuroreport. 2002;13(1):183–186. doi: 10.1097/00001756-200201210-00041. [DOI] [PubMed] [Google Scholar]
- 39.Bartha R, Smith M, Rupsingh R, Rylett J, Wells JL, Borrie MJ. High field (1)H MRS of the hippocampus after donepezil treatment in Alzheimer disease. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):786–793. doi: 10.1016/j.pnpbp.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging. 2011;32(5):802–810. doi: 10.1016/j.neurobiolaging.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Penner J, Rupsingh R, Smith M, Wells JL, Borrie MJ, Bartha R. Increased glutamate in the hippocampus after galantamine treatment for Alzheimer disease. Prog Neuro-Psychopharmacol Biol Psychiatry. 2010;34(1):104–110. doi: 10.1016/j.pnpbp.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Chen SQ, Wang PJ, Ten GJ, Zhan W, Li MH, Zang FC. Role of myo-inositol by magnetic resonance spectroscopy in early diagnosis of Alzheimer’s disease in APP/PS1 transgenic mice. Dement Geriatr Cogn Disord. 2009;28(6):558–566. doi: 10.1159/000261646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.von Kienlin M, Kunnecke B, Metzger F, et al. Altered metabolic profile in the frontal cortex of PS2 APP transgenic mice, monitored throughout their life span. Neurobiol Dis. 2005;18(1):32–39. doi: 10.1016/j.nbd.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Chen SQ, Cai Q, Shen YY, et al. Age-related changes in brain metabolites and cognitive function in APP/PS1 transgenic mice. Behav Brain Res. 2012;235(1):1–6. doi: 10.1016/j.bbr.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 45.Kantarci K, Knopman DS, Dickson DW, et al. Alzheimer disease: postmortem neuropathologic correlates of antemortem 1H MR spectroscopy metabolite measurements. Radiology. 2008;248(1):210–220. doi: 10.1148/radiol.2481071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jessen F, Block W, Traber F, et al. Decrease of N-acetylaspartate in the MTL correlates with cognitive decline of AD patients. Neurology. 2001;57(5):930–932. doi: 10.1212/wnl.57.5.930. [DOI] [PubMed] [Google Scholar]
- 47.Schott JM, Frost C, MacManus DG, Ibrahim F, Waldman AD, Fox NC. Short echo time proton magnetic resonance spectroscopy in Alzheimer’s disease: a longitudinal multiple time point study. Brain. 2010;133(11):3315–3322. doi: 10.1093/brain/awq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP. Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2005;384(1–2):23–28. doi: 10.1016/j.neulet.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 49.Doraiswamy PM, Charles HC, Krishnan KR. Prediction of cognitive decline in early Alzheimer’s disease. Lancet. 1998;352(9141):1678. doi: 10.1016/S0140-6736(05)61449-3. [DOI] [PubMed] [Google Scholar]
- 50.Kantarci K, Smith GE, Ivnik RJ, et al. 1H magnetic resonance spectroscopy, cognitive function, and apolipoprotein E genotype in normal aging, mild cognitive impairment and Alzheimer’s disease. J Int Neuropsychol Soc. 2002;8(7):934–942. doi: 10.1017/s1355617702870084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwo-On-Yuen PF, Newmark RD, Budinger TF, Kaye JA, Ball MJ, Jagust WJ. Brain N-acetyl-L-aspartic acid in Alzheimer’s disease: a proton magnetic resonance spectroscopy study. Brain Res. 1994;667(2):167–174. doi: 10.1016/0006-8993(94)91494-x. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez A, Garcia-Segura JM, Ortiz T, et al. Proton magnetic resonance spectroscopy and magnetoencephalographic estimation of delta dipole density: a combination of techniques that may contribute to the diagnosis of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;20(2–3):169–177. doi: 10.1159/000087094. [DOI] [PubMed] [Google Scholar]
- 53.Kantarci K, Xu Y, Shiung MM, et al. Comparative diagnostic utility of different MR modalities in mild cognitive impairment and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;14(4):198–207. doi: 10.1159/000066021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez-Bisbal MC, Arana E, Marti-Bonmati L, Molla E, Celda B. Cognitive impairment: classification by 1H magnetic resonance spectroscopy. Eur J Neurol. 2004;11(3):187–193. doi: 10.1046/j.1468-1331.2003.00746.x. [DOI] [PubMed] [Google Scholar]
- 55.MacKay S, Ezekiel F, Di Sclafani V, et al. Alzheimer disease and subcortical ischemic vascular dementia: evaluation by combining MR imaging segmentation and H-1 MR spectroscopic imaging. Radiology. 1996;198(2):537–545. doi: 10.1148/radiology.198.2.8596863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuff N, Capizzano AA, Du AT, et al. Selective reduction of N- acetylaspartate in medial temporal and parietal lobes in AD. Neurology. 2002;58(6):928–935. doi: 10.1212/wnl.58.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sweet RA, Panchalingam K, Pettegrew JW, et al. Psychosis in Alzheimer disease: postmortem magnetic resonance spectroscopy evidence of excess neuronal and membrane phospholipid pathology. Neurobiol Aging. 2002;23(4):547–553. doi: 10.1016/s0197-4580(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 58.Shinno H, Inagaki T, Miyaoka T, et al. A decrease in N-acetylaspartate and an increase in myoinositol in the anterior cingulate gyrus are associated with behavioral and psychological symptoms in Alzheimer’s disease. J Neurol Sci. 2007;260(1–2):132–138. doi: 10.1016/j.jns.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 59.Chao LL, Schuff N, Kramer JH, et al. Reduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patients. Neurology. 2005;64(2):282–289. doi: 10.1212/01.WNL.0000149638.45635.FF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Metastasio A, Rinaldi P, Tarducci R, et al. Conversion of MCI to dementia: role of proton magnetic resonance spectroscopy. Neurobiol Aging. 2006;27(7):926–932. doi: 10.1016/j.neurobiolaging.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Kantarci K, Weigand SD, Przybelski SA, et al. Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology. 2009;72(17):1519–1525. doi: 10.1212/WNL.0b013e3181a2e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 63.Kattapong VJ, Brooks WM, Wesley MH, Kodituwakku PW, Rosenberg GA. Proton magnetic resonance spectroscopy of vascular-and Alzheimer-type dementia. Arch Neurol. 1996;53(7):678–680. doi: 10.1001/archneur.1996.00550070116019. [DOI] [PubMed] [Google Scholar]
- 64.MacKay S, Meyerhoff DJ, Constans JM, Norman D, Fein G, Weiner MW. Regional gray and white matter metabolite differences in subjects with AD, with subcortical ischemic vascular dementia, and elderly controls with 1H magnetic resonance spectroscopic imaging. Arch Neurol. 1996;53(2):167–174. doi: 10.1001/archneur.1996.00550020079018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schuff N, Capizzano AA, Du AT, et al. Different patterns of N- acetylaspartate loss in subcortical ischemic vascular dementia and AD. Neurology. 2003;61(3):358–364. doi: 10.1212/01.wnl.0000078942.63360.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shiino A, Watanabe T, Shirakashi Y, et al. The profile of hippocampal metabolites differs between Alzheimer’s disease and subcortical ischemic vascular dementia, as measured by proton magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2012;32(5):805–815. doi: 10.1038/jcbfm.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waldman AD, Rai GS, McConnell JR, Chaudry M, Grant D. Clinical brain proton magnetic resonance spectroscopy for management of Alzheimer’s and sub-cortical ischemic vascular dementia in older people. Arch Gerontol Geriatr. 2002;35(2):137–142. doi: 10.1016/s0167-4943(02)00014-6. [DOI] [PubMed] [Google Scholar]
- 68.Mihara M, Hattori N, Abe K, Sakoda S, Sawada T. Magnetic resonance spectroscopic study of Alzheimer’s disease and frontotemporal dementia/Pick complex. Neuroreport. 2006;17(4):413–416. doi: 10.1097/01.wnr.0000203353.52622.05. [DOI] [PubMed] [Google Scholar]
- 69.Shonk TK, Moats RA, Gifford P, et al. Probable Alzheimer disease: diagnosis with proton MR spectroscopy. [see comment] Radiology. 1995;195(1):65–72. doi: 10.1148/radiology.195.1.7892497. [DOI] [PubMed] [Google Scholar]
- 70.Ernst T, Chang L, Melchor R, Mehringer CM. Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology. 1997;203(3):829–836. doi: 10.1148/radiology.203.3.9169712. [DOI] [PubMed] [Google Scholar]
- 71.Gomez-Isla T, Growdon WB, McNamara M, et al. Clinicopathologic correlates in temporal cortex in dementia with Lewy bodies. Neurology. 1999;53(9):2003–2009. doi: 10.1212/wnl.53.9.2003. [DOI] [PubMed] [Google Scholar]
- 72.Xuan X, Ding M, Gong X. Proton magnetic resonance spectroscopy detects a relative decrease of N-acetylaspartate in the hippocampus of patients with dementia with Lewy bodies. J Neuroimaging. 2008;18(2):137–141. doi: 10.1111/j.1552-6569.2007.00203.x. [DOI] [PubMed] [Google Scholar]
- 73.Molina JA, Garcia-Segura JM, Benito-Leon J, et al. Proton magnetic resonance spectroscopy in dementia with Lewy bodies. Eur Neurol. 2002;48(3):158–163. doi: 10.1159/000065520. [DOI] [PubMed] [Google Scholar]
- 74.Lim SM, Katsifs A, Villemagne VL, et al. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50(10):1638–1645. doi: 10.2967/jnumed.109.065870. [DOI] [PubMed] [Google Scholar]
- 75.Tiraboschi P, Hansen LA, Alford M, et al. Early and widespread cholinergic losses differentiate dementia with Lewy bodies from Alzheimer disease. Arch Gen Psychiatry. 2002;59(10):946–951. doi: 10.1001/archpsyc.59.10.946. [DOI] [PubMed] [Google Scholar]
- 76.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. [see comment] Neurology. 2001;56(9):1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 77.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ross AJ, Sachdev PS, Wen W, Valenzuela MJ, Brodaty H. 1H MRS in stroke patients with and without cognitive impairment. Neurobiol Aging. 2005;26(6):873–882. doi: 10.1016/j.neurobiolaging.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 81.Kantarci K, Lowe V, Przybelski SA, et al. Magnetic resonance spectroscopy, beta-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology. 2011;77(10):951–958. doi: 10.1212/WNL.0b013e31822dc7e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sailasuta N, Harris K, Tran T, Ross B. Minimally invasive biomarker confirms glial activation present in Alzheimer’s disease: a preliminary study. Neuropsychiatr Dis Treat. 2011;7:495–499. doi: 10.2147/NDT.S23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jack CR, Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58(5):750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 2011;10(9):785–796. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitwell JL, Dickson DW, Murray ME, et al. Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol. 2012;11(10):868–877. doi: 10.1016/S1474-4422(12)70200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whitwell JL, Jack CR, Jr, Kantarci K, et al. Imaging correlates of posterior cortical atrophy. Neurobiol Aging. 2007;28(7):1051–1061. doi: 10.1016/j.neurobiolaging.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Catani M, Cherubini A, Howard R, et al. (1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport. 2001;12(11):2315–2317. doi: 10.1097/00001756-200108080-00007. [DOI] [PubMed] [Google Scholar]
- 90.Kantarci K, Boeve BF, Wszolek ZK, et al. MRS in presymptomatic MAPT mutation carriers: a potential biomarker for tau-mediated pathology. Neurology. 2010;75(9):771–778. doi: 10.1212/WNL.0b013e3181f073c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.den Heijer T, Sijens PE, Prins ND, et al. MR spectroscopy of brain white matter in the prediction of dementia. Neurology. 2006;66(4):540–544. doi: 10.1212/01.wnl.0000198256.54809.0e. [DOI] [PubMed] [Google Scholar]
- 92.Godbolt AK, Waldman AD, MacManus DG, et al. MRS shows abnormalities before symptoms in familial Alzheimer disease. Neurology. 2006;66(5):718–722. doi: 10.1212/01.wnl.0000201237.05869.df. [DOI] [PubMed] [Google Scholar]
- 93.Adalsteinsson E, Sullivan EV, Kleinhans N, Spielman DM, Pfefferbaum A. Longitudinal decline of the neuronal marker N-acetyl aspartate in Alzheimer’s disease. Lancet. 2000;355(9216):1696–1697. doi: 10.1016/s0140-6736(00)02246-7. [DOI] [PubMed] [Google Scholar]
- 94.Kantarci K. Proton MRS in mild cognitive impairment. J Magn Reson Imaging. 2013;37(4):770–777. doi: 10.1002/jmri.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chantal S, Braun CM, Bouchard RW, Labelle M, Boulanger Y. Similar 1H magnetic resonance spectroscopic metabolic pattern in the medial temporal lobes of patients with mild cognitive impairment and Alzheimer disease. Brain Res. 2004;1003(1–2):26–35. doi: 10.1016/j.brainres.2003.11.074. [DOI] [PubMed] [Google Scholar]