Abstract
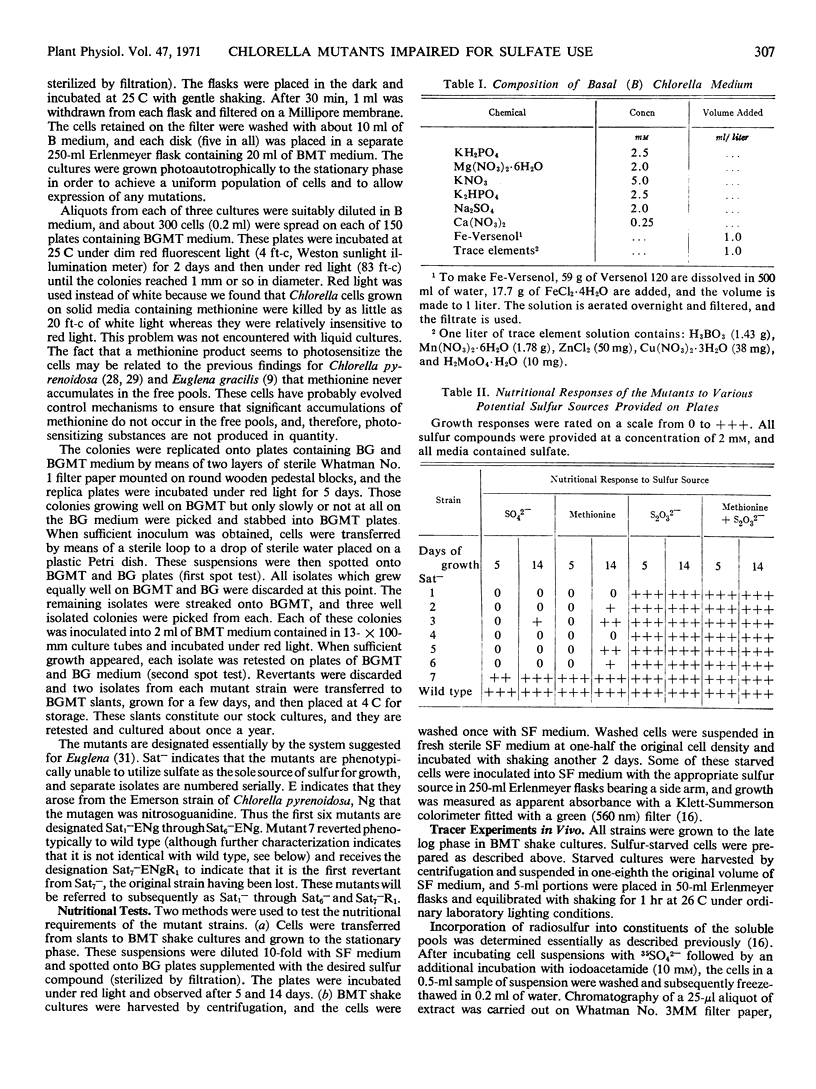
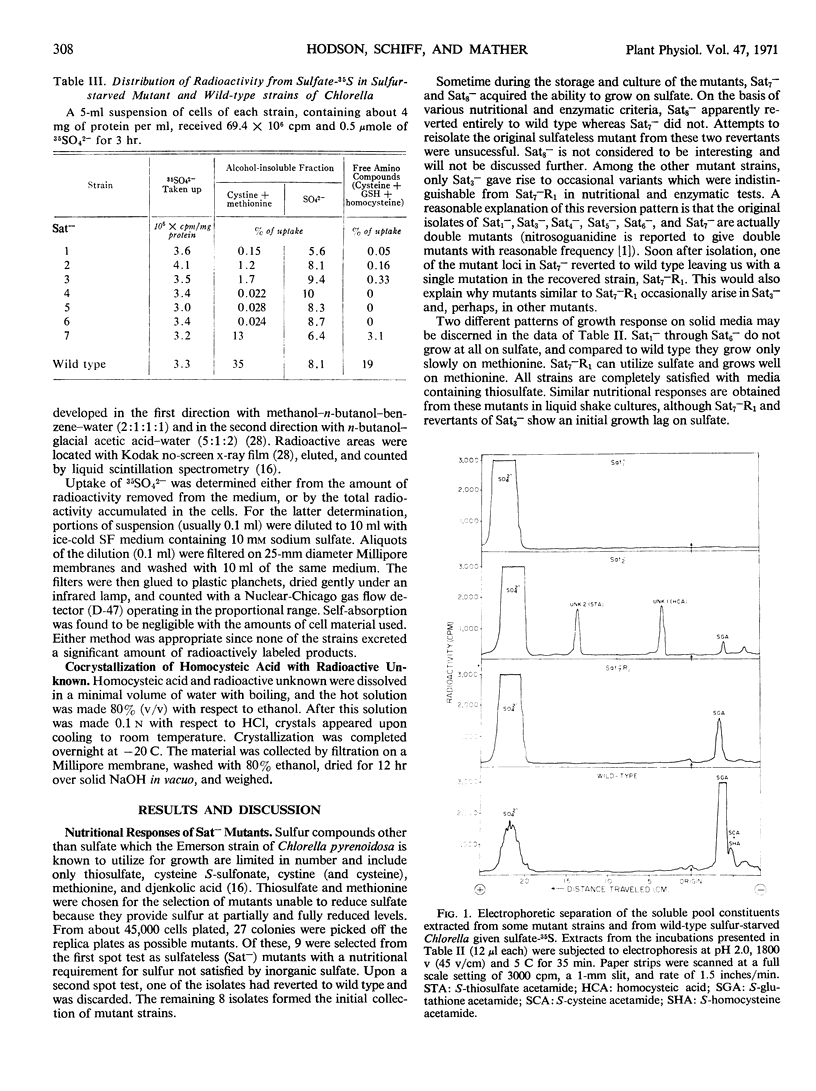
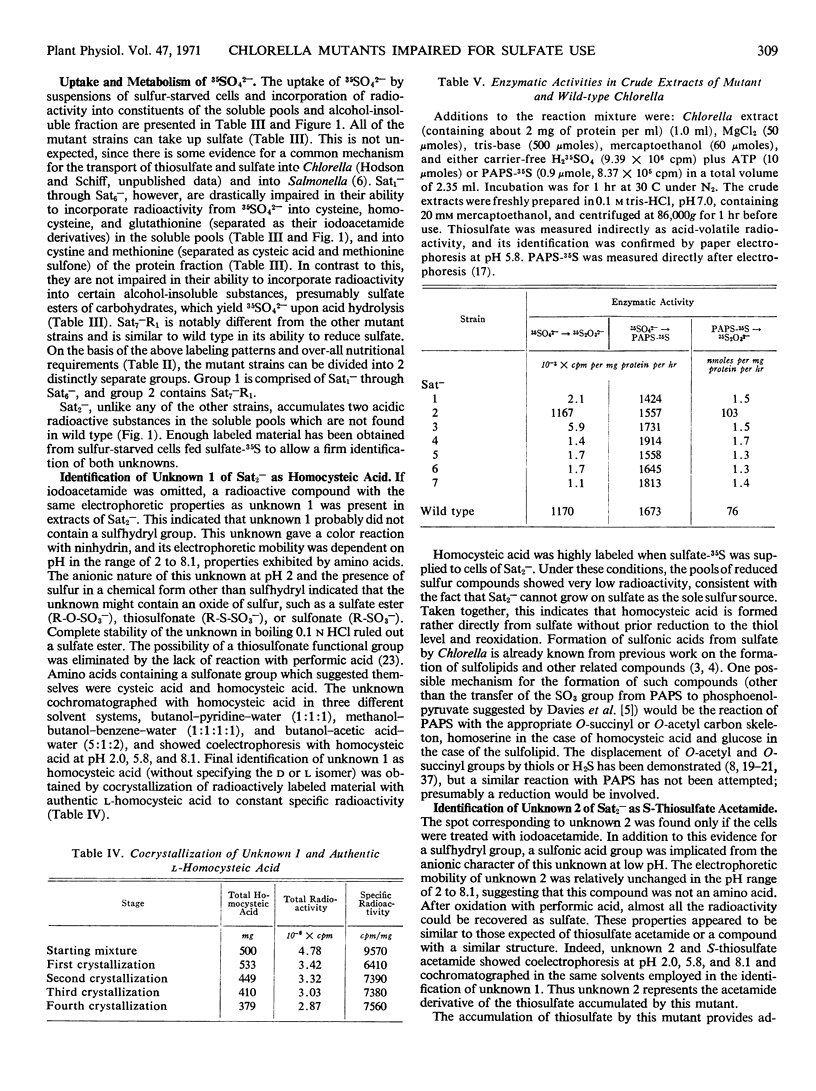
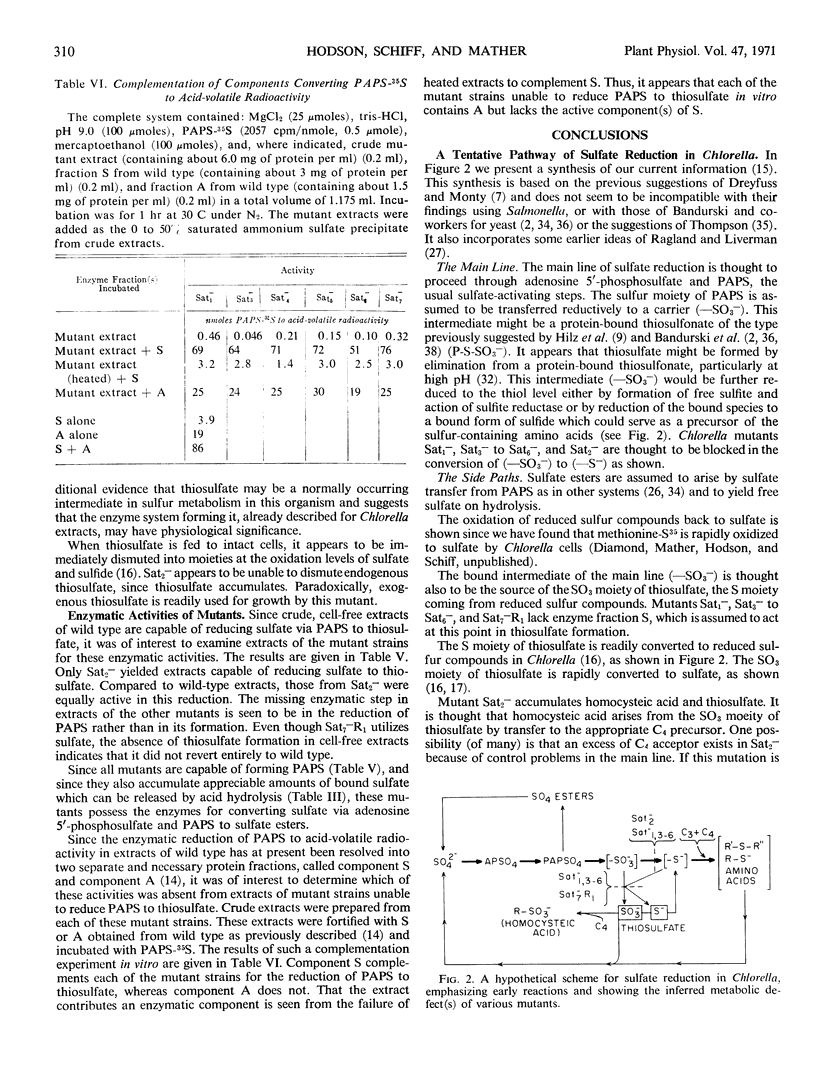
Seven mutants of Chlorella pyrenoidosa (Emerson strain 3) impaired for sulfate utilization have been isolated after treatment of the wild-type organism with nitrosoguanidine by replica plating on media containing thiosulfate and l-methionine. These mutants fall into three classes based on their ability to grow on sulfate, accumulate compounds labeled from sulfate-35S, and reduce adenosine 3′-phosphate 5′-phosphosulfate-35S (PAPS-35S) to thiosulfate-35S. Mutant Sat2− cannot grow on sulfate, but it accumulates thiosulfate-35S and homocysteic acid-35S from sulfate-35S in vivo. In addition, extracts of mutant Sat2− reduce PAPS-35S to thiosulfate-35S, indicating the possession of enzyme fractions S and A, both of which are required for thiosulfate formation. Mutants Sat1−, Sat3−, Sat4−, Sat5−, and Sat6− cannot grow on sulfate, and their extracts lack the ability to reduce PAPS-35S to thiosulfate-35S. Mutant Sat7−R1, a probable revertant, can grow on sulfate but still lacks the ability to reduce PAPS-35S to thiosulfate-35S in vitro. Complementation experiments in vitro show that the block in formation of acid-volatile radioactivity in every case is due to the absence of activity associated with fraction S. All mutants can grow on thiosulfate and all possess the activating enzymes which convert sulfate to PAPS. Through a comparison of nutritional and enzymatic characteristics, the first outlines of a branched and complicated pathway for sulfate reduction in Chlorella are beginning to emerge.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASAHI T., BANDURSKI R. S., WILSON L. G. Yeast sulfate-reducing system. II. Enzymatic reduction of protein disulfide. J Biol Chem. 1961 Jun;236:1830–1835. [PubMed] [Google Scholar]
- Benson A. A., Daniel H., Wiser R. A SULFOLIPID IN PLANTS. Proc Natl Acad Sci U S A. 1959 Nov;45(11):1582–1587. doi: 10.1073/pnas.45.11.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREYFUSS J. CHARACTERIZATION OF A SULFATE- AND THIOSULFATE-TRANSPORTING SYSTEM IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jul;239:2292–2297. [PubMed] [Google Scholar]
- Davies W. H., Mercer E. I., Goodwin T. W. Some observations on the biosynthesis of the plant sulpholipid by Euglena gracilis. Biochem J. 1966 Feb;98(2):369–373. doi: 10.1042/bj0980369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN N. S., SCHIFF J. A. STUDIES OF SULFATE UTILIZATION BY ALGAE. 3. PRODUCTS FORMED FROM SULFATE BY EUGLENA. J Protozool. 1964 Feb;11:120–127. doi: 10.1111/j.1550-7408.1964.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Sulfuration of O-acetylhomoserine and O-acetylserine by two enzyme fractions from spinach. Biochem Biophys Res Commun. 1968 Apr 19;31(2):275–280. doi: 10.1016/0006-291x(68)90742-0. [DOI] [PubMed] [Google Scholar]
- HILZ H., KITTLER M., KNAPE G. [The reduction of sulfate in yeast]. Biochem Z. 1959;332:151–166. [PubMed] [Google Scholar]
- Hodson R. C., Schiff J. A. Preparation of adenosine-3'-phosphate-51-phosphosulfate (PAPS): an improved enzymatic method using Chlorella pyrenoidosa. Arch Biochem Biophys. 1969 Jun;132(1):151–156. doi: 10.1016/0003-9861(69)90347-6. [DOI] [PubMed] [Google Scholar]
- Hodson R. C., Schiff J. A., Scarsella A. J., Levinthal M. Studies of Sulfate Utilization by Algae. 6. Adenosine-3'-Phosphate-5'-Phosphosulfate (PAPS) as an Intermediate in Thiosulfate Formation From Sulfate by Cell-Free Extracts of Chlorella. Plant Physiol. 1968 Apr;43(4):563–569. doi: 10.1104/pp.43.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson R. C., Schiff J. A., Scarsella A. J. Studies of Sulfate Utilization by Algae. 7. In vivo Metabolism of Thiosulfate by Chlorella. Plant Physiol. 1968 Apr;43(4):570–577. doi: 10.1104/pp.43.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson R. C., Schiff J. A. Studies of Sulfate Utilization by Algae: 9. Fractionation of a Cell-free System from Chlorella into Two Activities Necessary for the Reduction of Adenosine 3'-Phosphate 5'-Phosphosulfate to Acid-Volatile Radioactivity. Plant Physiol. 1971 Feb;47(2):300–305. doi: 10.1104/pp.47.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson R. C., Schiff J. A. Studies of sulfate utilization by algae: 8. The ubiquity of sulfate reduction to thiosulfate. Plant Physiol. 1971 Feb;47(2):296–299. doi: 10.1104/pp.47.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. Isolation, characterization and mapping oc cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):589–595. doi: 10.1042/bj1100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M., Flavin M. Cystathionine gamma-synthetase of Salmonella. Structural properties of a new enzyme in bacterial methionine biosynthesis. J Biol Chem. 1966 Dec 25;241(24):5781–5789. [PubMed] [Google Scholar]
- Kerr D. S., Flavin M. Synthesis of cystathionine from O-acetylhomoserine in Neurospora: a step in methionine biosynthesis. Biochem Biophys Res Commun. 1968 Apr 5;31(1):124–130. doi: 10.1016/0006-291x(68)90041-7. [DOI] [PubMed] [Google Scholar]
- Levinthal M., Schiff J. A. Studies of sulfate utilization by algae. 5. Identification of thiosulfate as a major Acid-volatile product formed by a cell-free sulfate-reducing system from chlorella. Plant Physiol. 1968 Apr;43(4):555–562. doi: 10.1104/pp.43.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA T., SATO R. Synthesis from sulphate and accumulation of S-sulphocysteine by a mutant strain of Aspergillus nidulans. Biochem J. 1963 Feb;86:328–335. doi: 10.1042/bj0860328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PECK H. D., Jr Symposium on metabolism of inorganic compounds. V. Comparative metabolism of inorganic sulfur compounds in microorganisms. Bacteriol Rev. 1962 Mar;26:67–94. doi: 10.1128/br.26.1.67-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAGLAND J. B., LIVERMAN J. L. Sulfate inhibition of thiosulfate utilization by certain strains of Neurospora crassa. Arch Biochem Biophys. 1958 Aug;76(2):496–508. doi: 10.1016/0003-9861(58)90175-9. [DOI] [PubMed] [Google Scholar]
- SHEPHERD C. J. Pathways of cysteine synthesis in Aspergillus nidulans. J Gen Microbiol. 1956 Aug;15(1):29–38. doi: 10.1099/00221287-15-1-29. [DOI] [PubMed] [Google Scholar]
- SUZUKI S., STROMINGER J. L. Enzymatic sulfation of mucopolysaccharides in hen oviduct. I. Transfer of sulfate from 3'-phosphoadenosine 5'-phosphosulfate to mucopolysaccharides. J Biol Chem. 1960 Feb;235:257–266. [PubMed] [Google Scholar]
- Schiff J. A., Levinthal M. Studies of sulfate utilization by algae. 4. Properties of a cell-free sulfate-reducing system from chlorella. Plant Physiol. 1968 Apr;43(4):547–554. doi: 10.1104/pp.43.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff J. A. Studies on Sulfate Utilization by Chlorella pyrenoidosa using Sulfate-S; the Occurrence of S-Adenosyl Methionine. Plant Physiol. 1959 Jan;34(1):73–80. doi: 10.1104/pp.34.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. F., Westley J. Metabolic interrelations of sulfur in proteins, thiosulfate, and cystine. J Biol Chem. 1969 Oct 25;244(20):5735–5744. [PubMed] [Google Scholar]
- Torii K., Bandurski R. S. Yeast sulfate-reducing system. 3. An intermediate in the reduction of 3'-phosphoryl-5'-adenosinephosphosulfate to sulfite. Biochim Biophys Acta. 1967 Mar 22;136(2):286–295. doi: 10.1016/0304-4165(67)90074-8. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., ASAHI T., BANDURSKI R. S. Yeast sulfate-reducing system. I. Reduction of sulfate to sulfite. J Biol Chem. 1961 Jun;236:1822–1829. [PubMed] [Google Scholar]
- Wiebers J. L., Garner H. R. Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast, and Escherichia coli. J Biol Chem. 1967 Dec 10;242(23):5644–5649. [PubMed] [Google Scholar]


