Abstract
Background
Autism spectrum disorder (ASD) is a neurodevelopmental disorder of complex etiology. Whereas strong evidence supports the causal role of genetic factors, a number of environmental risk factors have also been implicated. This study employed a cotwin-control design to investigate low birth weight as a risk factor for ASD.
Methods
A population-based sample of 3,715 same-sex twin-pairs participating in the Child and Adolescent Twin Study of Sweden were studied. ASD was assessed using a structured parent interview for screening of ASD and related developmental disorders, based on DSM-IV criteria. Birth weight was obtained from medical birth records maintained by the Swedish Medical Birth Registry.
Results
Lighter twins in birth weight and ASD discordant twin-pairs (n= 34) were over three times more likely to meet criteria for ASD than heavier twins (OR = 3.25). Analyses of birth weight as a continuous risk factor showed a 13% reduction in risk of ASD per 100 gram increase in birth weight (n=78). Analysis of the effect of birth weight on ASD symptoms in the entire population (most of whom did not have ASD) showed a modest association. That is, for every 100 gram increase in birth weight a 2% decrease in severity of ASD indexed by A-TAC scores would be expected in the sample as a whole.
Conclusions
Data are consistent with the hypothesis that low birth weight confers risk to ASD. Thus, even though genetic effects are of major importance, non-genetic influence associated with birth weight may contribute to the development of ASDs.
Keywords: Autism, Twin, Birth weight
Autism spectrum disorder (ASD) is a neurodevelopmental disorder affecting approximately 1 in 100 children (CDC, 2009). Although the cause of ASD is not definitively known, evidence for genetic contribution comes from studies implicating a range of copy number variants and single nucleotide polymorphisms (SNPs) associated with ASD (e.g., Weiss et al., 2008; 2009), as well as twin and family studies indicating considerably higher concordance rates among monozygotic than dizygotic twins (Folstein & Rutter, 1977; Lichtenstein et al., 2010), and over a 20-fold relative risk for siblings of individuals with ASD (Lauritsen et al., 2005; Constantino et al., 2010; Ozonoff et al., 2011), respectively. A genetic contribution to autism is further supported by evidence of subtle differences in social interaction, communication, and flexibility among family members of individuals with ASD, which have been hypothesized to reflect genetic liability (Bolton et al., 1994; Piven et al., 1997; Losh et al., 2008). Such findings offer compelling evidence for genetic etiology, yet because the concordance among monozygotic twins is far from 100%, and given recent evidence from a large-scale twin study that concordance for ASD in dizygotic twins is considerably higher than had been estimated previously (Hallmayer et al., 2011), environmental risk factors are also implicated (Szatmari, 2011). Potential environmental risk factors have included prenatal viral exposure (Chess, 1971; 1977; Markowitz, 1983), maternal stress during pregnancy (Ward, 1990; Beversdorf et al., 2005), paternal and maternal age (Larsson et al., 2005; Reichenberg et al., 2006; Lundstrom et al., 2010), parity (Piven et al., 1993; Bolton & Griffiths, 1997; Juul-Dam et al., 2001), and indices of pre- and perinatal suboptimality, such as prematurity and low birth weight.
Interestingly, the landmark British twin study of Folstein and Rutter (1977), which provided the first empirical evidence for a genetic basis of autism, also reported evidence that perinatal hazards (including low birth weight) may predispose to autism. Examining characteristics of twin pairs discordant for autism, investigators found that in all 11 discordant cases the affected twin experienced some sort of perinatal hazard, and in three of these cases the affected twin was at least one pound lighter than the unaffected twin. A subsequent Scandinavian twin study also reported lower perinatal optimality among affected twins in discordant pairs, but did not report birth weight (Steffenburg et al., 1989). A study following up on the original British twin sample, and including an additional 28 twin-pairs (Bailey et al., 1995), also reported substantial weight differences (>500 grams) in six of 29 discordant pairs with reliable obstetric data available, with the autistic twin being the lighter twin in five of the six cases (although only one of these twin-pairs was monozygotic). A recent twin study of dimensionally measured ASD-like traits in the general population reported an association between differences in monozygotic twins’ birth weights and their differences in social ASD-like traits, although overall, investigators concluded that there was weak support for a causal role of pre- and neonatal features in ASD-like traits (Ronald et al., 2010).
An association between birth weight and ASD has also been reported in studies of singletons. The majority of such studies have focused on infants with very low (<1,500 g) and extremely low (<1,000 g) birth weight (most often involving preterm births) (Indredavik et al., 2004; Skranes et al,. 2007; Schendel & Bhasin, 2008; Hack et al., 2009; Kuban et al., 2009; Limperpoulos, 2009); however, associations between ASD and less extreme values of low birth weight (<2,500 g) have been detected (Bryson et al., 1988; Burd et al., 1999; Maimburg & Vaeth, 2006; Kolevzon et al., 2007; Burstyn et al., 2010; Itzchak et al., 2011). Contrary findings have also been reported (Levy et al., 1988; Mason-Brothers et al., 1990; Cryan et al., 1996; Deb et al., 1997; Larsson et al., 2005; Kolevzon et al., 2007).
Taken together, previous reports could support an association between birth weight and ASD; however, the mechanisms underlying this association are not straightforward. Low birth weight (and other environmental factors) could act independently, or interact with underlying genetic predisposition as part of a liability threshold model of complex disorders (Falconer, 1981). It is also possible that low birth weight may result from underlying genetic liability to ASD. For instance, studies have reported that obstetric complications appear to be more common among probands from families with a strong family history of ASD and ASD-like traits (who presumably represent cases of higher genetic loading) (Bolton et al., 1997; Zwaigenbaum et al., 2002). Both ASD and birth weight have also been shown to be heritable (Clausson et al., 2000; Magnus et al., 2001; Gielen et al., 2008), raising the possibility that shared genetic variants could act as confounders influencing both characteristics.
In an attempt to address these confounds, this study examined the association between birth weight and ASD using a cotwin-control design in a large population based sample of twins. Examining differences in discordant monozygotic twins in a cotwin-control design can provide an estimate of the “non-shared” or “causal” effect, and also yield an estimate of the strength of the association, controlling for shared genetic (including maternal genetic) and environmental factors (e.g., Hrubec & Robinette, 1984). Additionally, because dizygotic (DZ) and monozygotic (MZ) twins share 50% or 100% of their segregating genes, respectively, stratifying analyses by zygosity allows for varying degrees of control for genetic factors. Birth weight was examined in same-sex MZ and DZ twin-pairs enrolled in the Child and Adolescent Twin Study in Sweden (CATSS) (Lichtenstein et al., 2006; 2010; Anckarsäter et al., 2008). Because sex differences have been documented in birth weight and ASD, same-sex twin-pairs were studied in order to reduce confounding effects of sex.
METHODS
Sample
Participants included a population-based cohort of twin-pairs enrolled in CATSS (Anckarsäter et al., 2008; Lichtenstein et al., 2010), a nation-wide cohort that includes all Swedish twins turning 9 or 12 years between July 1992 and 1998, where both twins were alive and residing in Sweden in 1994 (N = 11,400 individuals, 5,700 twin-pairs). CATSS has an 80% response rate, making it a highly representative population sample. Parents of 9 (born July 1995-) and 12 year-old (born July 1992-June 1995) Swedish twins identified through the Swedish Twin Registry were contacted and, once enrolled in the study, interviewed by trained interviewers employed by a professional company, “Intervjubolaget”. Interviewers were given a brief introduction to child and adolescent psychiatry and twin research, and trained on the administration of the structured interview used to assess ASD symptoms, the Autism - Tics, ADHD, and other Comorbidities Inventory (A-TAC; (Hansson et al., 2005), described below. Interviews took place during the month of the child’s birthday (for further details see (Lichtenstein et al., 2010).
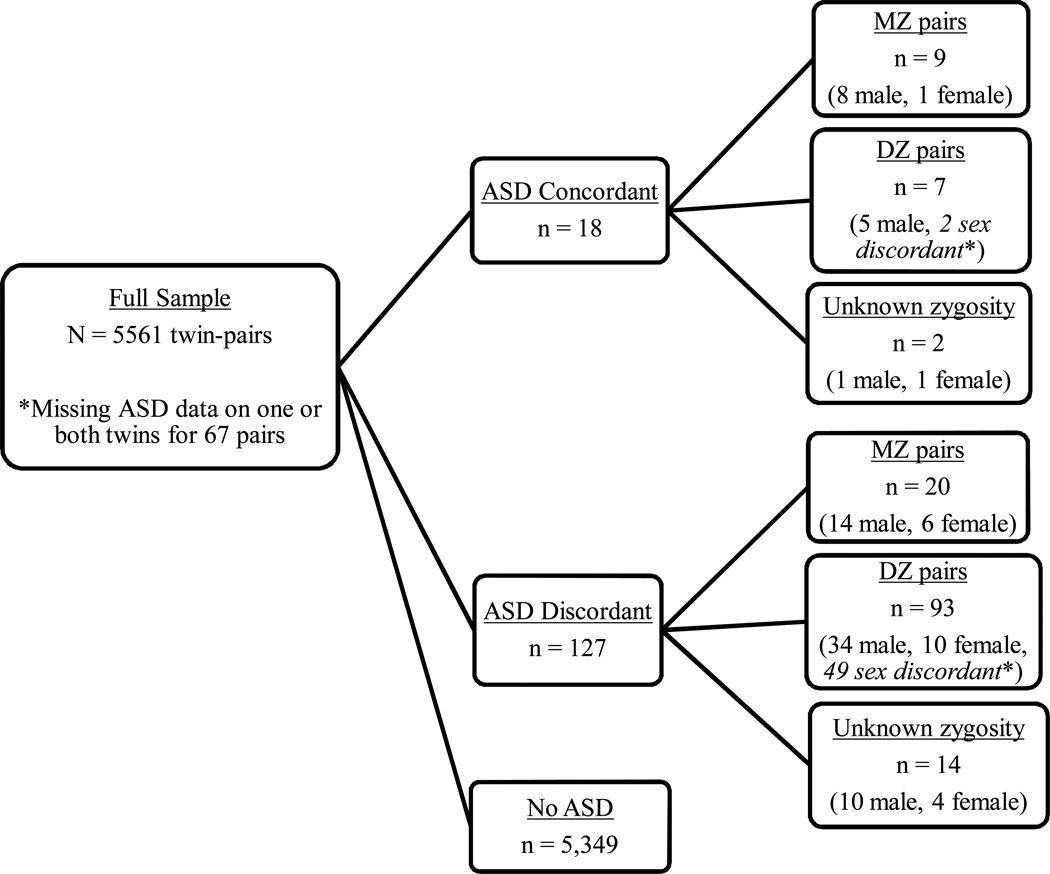
Fifty-two percent of the sample was male. One hundred thirty children (76 males, 54 females) were excluded from the analyses due to known brain injury (n = 118) or a chromosomal syndrome (n = 12). Eighty individuals (40 pairs) were missing information on sex, birth order, and/or birth weight on at least one of the twins in the pair, and so were removed from the data set. Discrepancies with birth weight and/or birth order led to removal of an additional 198 individuals. The final working data set consisted of 5,561 twin-pairs, 3,715 of whom were same-sex, and this subset served as the primary focus of analyses. Figure 1 provides an overview of twins examined in analyses, grouped by ASD status and zygosity.
Figure 1.
Flow chart illustrating twin-pairs examined in analyses of birth weight and ASD. ASD defined as score of ≥4.5 on the ASD module of the A-TAC.
*Note: Sex-discordant pairs were not included in analyses.
Zygosity
Zygosity was determined by an algorithm based on 5 items concerning twin similarity and confusion, validated in twins for whom we had DNA on both twins based on a panel of 48 SNPs derived for zygosity analyses (Lichtenstein et al., 2010). Twins with more than 95% probability of being correctly classified were assigned a zygosity. Those with uncertain scores were classified as unknown zygosity. Same-sex twin-pairs included in analyses consisted of 811 pairs of MZ males, 855 pairs of DZ males, 822 pairs of MZ females, 719 pairs of DZ females, and 508 pairs with unknown zygosity (257 males and 251 females). Pairs with unknown zygosity were included in overall analyses and male-female comparisons, but excluded from analyses of zygosity.
Measures
Autism Spectrum Disorder (ASD)
The presence of ASD was assessed through the Autism - Tics, ADHD, and other Comorbidities Inventory (A-TAC; Hansson et al., 2005). The A-TAC is a comprehensive structured interview designed as a tool for assigning research proxies for clinical diagnosis of ASD and other targeted disorders, based on DSM-IV criteria.. It has been validated in two formal studies comparing children later diagnosed at a specialized neuropsychiatric clinic to controls, using blind lay interviewers who conducted assessments by phone (Hansson et al., 2005; Larson et al., 2010). Additionally, intra-class correlations conducted for 200 families participating in the present study, who had been re-assessed with the A-TAC revealed good test-retest (.89) and inter-rater (.80) reliability for ASD.
Continuous scores on the ASD module of the A-TAC have been shown to be useful as an index of the probability of a clinical diagnosis among children demonstrating clinically significant ASD symptoms (areas under Receiver Operating Characteristics (ROC) curves 0.88 and 0.96 in the two independent validation studies. Using the established cut-off for ASD at 4.5 points by the twelve DSM-IV based items assessing ASD, sensitivity was 0.83 and specificity 0.94 for the identification of ASD in the latest validation study (Larson et al., 2010). Further details on the psychometric properties of the A-TAC are provided elsewhere (Hansson et al., 2005; Anckarsäter et al., 2007; Larson et al., 2010).
The A-TAC assesses ASD-like traits comprising the principal symptom domains of ASD (i.e., language impairment, social dysfunction, and restricted interests/repetitive behaviors) separately, yielding summary scores for each and a total score for ASD. This dimensional approach has been shown to capture severity by indexing the number of symptoms endorsed and the problem load in each symptom module (Anckarsäter et al., 2008). Scores on the A-TAC were examined as both categorical (affected vs. unaffected) and continuous/dimensional variables. For the former, established cut-off scores for ASD and ASD-like traits were as follows: ASD ≥ 4.5, language impairment ≥ 1, social impairment ≥ 2, ritualistic/repetitive ≥ 1. Based on the high sensitivity and specificity demonstrated for the A-TAC in identifying ASD (using the cut-off score above), A-TAC scores meeting cut-off for the ASD module were treated as a research proxy for clinical diagnosis of ASD. Such cases are hereafter referred to as ASD.
Birth weight
Birth weight information was collected through the Swedish Medical Birth Register obtained at the time of birth. Register information was compared to parental report of birth order and birth weight to confirm correct assignment of birth weight to each twin. If twin data did not match exactly (2117 exact matches), the following criteria were used for determining agreement between the parental report and register data, which had to be available for at least one twin: (1) both register data and parental report must have agreed on who was the heavier child; and (2) the parental report must have been within 300g of the medical registry birth weight. This resulted in 99 discrepant pairs, who were excluded from analyses. Following prior work (O'Brien et al., 1986; Blickstein & Lancet, 1988; Blickstein, 1991; Talbot et al., 1997; Branum & Schoendorf, 2003), we classified twin-pairs as birth weight discordant when there was ≥ 400 gram difference in birth weight between the twins in a pair, or if one twin was at least 15% lighter than the other in the pair.
Statistical Analyses
The primary analyses were conducted with both MZ and DZ twins among same-sex pairs. As noted previously, this enabled us to control for (unmeasured) confounding of genetic and environmental factors that can impact other study designs. Analyses were performed stratified by zygosity (MZ, DZ) and by sex, given that sex differences are observed both in ASD and birth weight. Three complementary analyses were conducted to address the hypothesis that low birth weight confers a risk for ASD: (1) McNemar’s test was used to calculate odds ratios (ORs) for pairs discordant for both birth weight and ASD, in order to examine the odds of ASD given lighter or heavier status within discordant pairs; (2) conditional logistic regression was used to estimate ORs, viewing the data as a matched case-control study among ASD discordant twins (where the twin with ASD was considered the case and the co-twin without ASD was considered the control), with birth weight entered into the model as a continuous exposure; and (3) generalized estimating equation (GEE) with a Poisson link function was used to examine the relative effect of birth weight on ASD symptoms in the entire sample, controlling for zygosity, sex, and concordance. The first and second analyses were also applied to each ASD-like trait: restricted/repetitive behaviors, and language and social impairment. Because no formal correction for multiple comparisons was made, for secondary analyses of ASD-like traits, statistical significance was established conservatively at .01. All analyses were conducted using SAS, version 9.2 (Littell et al., 1996).
RESULTS
Descriptive Data
Table 1 provides the prevalence of ASD and the ASD-like traits for the total sample (N=5,561), independent of twin status and categorized by standard birth weight categories (<1,500g; 1,500–2,499g; 2,500–3,499g; ≥3,500g (note, however, that twins tend to have lower birth weights than singletons), sex, and zygosity. The proportion of cases meeting cut-off for ASD and the ASD-like traits (defined using A-TAC cut-off definitions, previously described) is also provided for each sub-grouping. With few exceptions, the prevalence of ASD and the ASD-like traits was highest for the lowest birth weight group (decreasing as weight increased). Males had a higher prevalence of ASD than females in all birth weight categories. The higher prevalence of ASD and ASD-like traits among males compared to females was also apparent among the unlike-sexed DZ twins (which control for unmeasured familial confounding); the odds of ASD were 2.1 (95% CI: 1.2, 3.7; p<.01) times higher among males compared to females. Subsequent analyses were performed on same-sexed twins only. There did not appear to be any distinct patterns of prevalence across zygosity classification.
Table 1.
Prevalence of ASD and ASD-like traits measured by the A-TAC by sex, zygosity, and birth weight category (grams), independent of twin status for the full sample (N=5,561).
| Sex | ASD | Restricted/ Repetitive |
Language | Social | ||
|---|---|---|---|---|---|---|
| BW (g) | N | n (%) | n (%) | n (%) | n (%) | |
| Males | ≤1,499 | 249 | 8 (3.2) | 38 (15.3) | 37 (14.9) | 5 (2.0) |
| 1,500–2,499 | 1751 | 40 (2.3) | 219 (12.5) | 220 (12.6) | 32 (1.8) | |
| 2,500–3,499 | 3178 | 69 (2.2) | 319 (10.0) | 309 (9.7) | 52 (1.6) | |
| ≥3,500 | 426 | 5 (1.2) | 37 (8.6) | 43 (10.0) | 6 (1.4) | |
| Missing BW | 88 | |||||
| Females | ≤1,499 | 256 | 6 (2.3) | 22 (8.5) | 27 (10.5) | 5 (2.0) |
| 1,500–2,499 | 1916 | 19 (1.0) | 106 (5.5) | 103 (5.4) | 21 (1.1) | |
| 2,500–3,499 | 2920 | 16 (0.6) | 146 (5.0) | 116 (4.0) | 18 (0.6) | |
| ≥3,500 | 227 | 0 (0.0) | 6 (2.6) | 10 (4.4) | 0 (0.0) | |
| Missing BW | 111 | |||||
| Zygosity MZ | ≤1,499 | 160 | 3 (1.9) | 20 (12.5) | 21 (13.1) | 3 (1.3) |
| 1,500–2,499 | 1175 | 16 (1.4) | 76 (6.5) | 114 (9.7) | 21 (1.8) | |
| 2,500–3,499 | 1716 | 19 (1.1) | 106 (6.2) | 130 (7.6) | 16 (0.9) | |
| ≥3,500 | 139 | 1 (0.7) | 5 (3.6) | 14 (10.0) | 0 (0.0) | |
| Missing BW | 76 | |||||
| DZ1 | ≤1,499 | 279 | 8 (2.9) | 32 (11.4) | 33 (11.7) | 5 (1.8) |
| 1,500–2,499 | 2167 | 38 (1.8) | 221 (10.2) | 186 (8.6) | 28 (1.3) | |
| 2,500–3,499 | 3831 | 57 (1.5) | 311 (8.1) | 248 (6.5) | 49 (1.3) | |
| ≥3,500 | 457 | 3 (0.7) | 34 (7.4) | 33 (7.2) | 5 (1.1) | |
| Missing BW | 106 | |||||
| Unknown | ≤1,499 | 66 | 3 (4.6) | 8 (12.1) | 10 (15.2) | 3 (4.6) |
| 1,500–2,499 | 325 | 5 (1.5) | 28 (8.6) | 23 (7.0) | 4 (1.2) | |
| 2,500–3,499 | 551 | 9 (1.6) | 48 (8.7) | 47 (8.5) | 5 (0.9) | |
| ≥3,500 | 57 | 1 (1.8) | 4 (7.0) | 6 (10.5) | 1 (1.8) | |
| Missing BW | 17 |
BW=Birth weight; N= total number of individuals; n= number of individuals meeting cut-off criteria for ASD or ASD-like traits; (%) = percentage meeting cut-offs on the A-TAC;
DZ includes both same-sex and opposite-sex twin-pairs
Effect of Birth Weight Discordance (>400g or 15%)
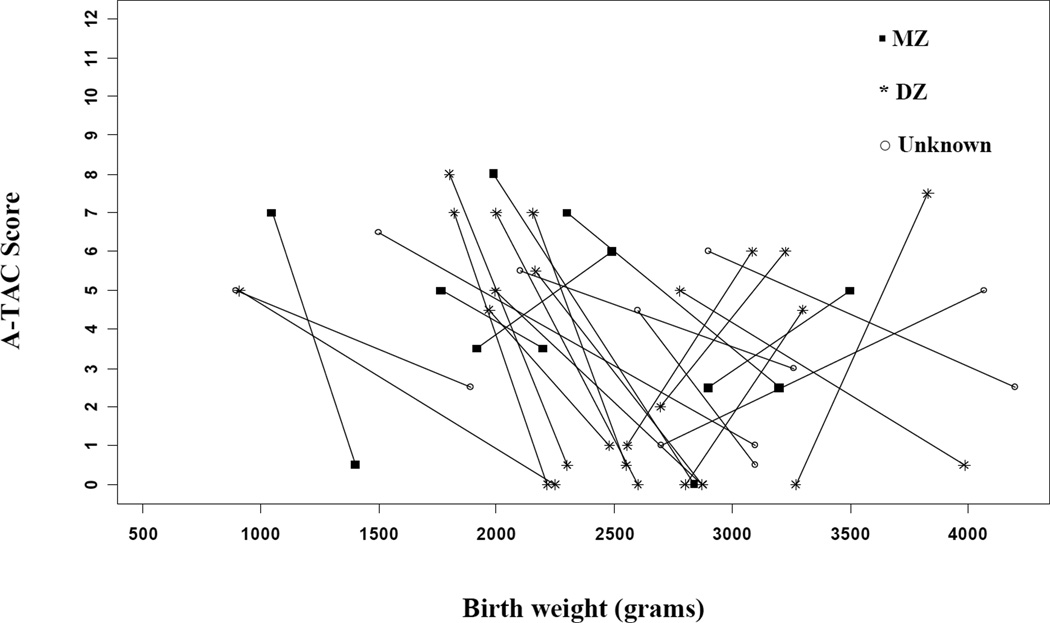
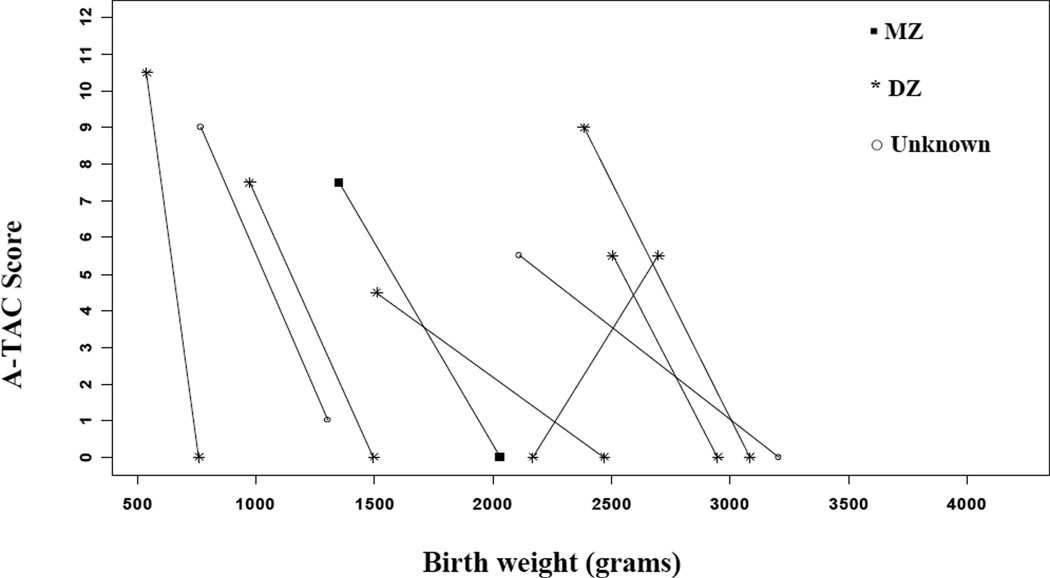
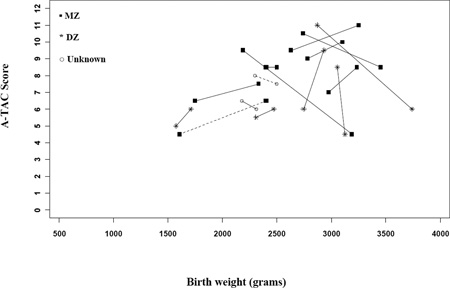
Figures 2a (males) and 2b (females) demonstrate that for the majority (26/34) of same-sex pairs discordant for ASD and birth weight, the lighter twin exhibited higher A-TAC scores (i.e., more severe symptomatology reported). The odds of meeting cut-off for ASD were 3.25 times higher for the lighter twin (95% CI: 1.47–7.18; p≤0.01). The odds ratios (ORs) were similar for MZ and DZ twin-pairs, as well as for males, but slightly higher for females. However, small sample sizes resulted in fairly wide confidence intervals around these estimates (poor precision), particularly for females (see Table 2). In addition, the odds meeting criteria for ASD-like traits on the A-TAC were higher for the lighter child in birth weight discordant pairs. The magnitude of these relationships was similar when stratifying based on zygosity and sex. Finally, we also examined whether discordance in birth weight would show an effect on symptom severity within the 16 ASD concordant twins. As illustrated in the Appendix, there does not appear to be an effect related to birth weight among ASD concordant twins (p = 0.67).
Figure 2.
a: ASD symptoms and birth weight differences for discordant male twin-pairs.
b: ASD symptoms and birth weight differences for discordant female twin-pairs.
Table 2.
Odds ratios for pairs discordant for birth weight and ASD (and ASD-like traits) on the A-TAC using McNemar’s test.
| Pairs | ASD | Restricted/ Repetitive |
Language | Social | |
|---|---|---|---|---|---|
| Overall | No. discordant pairs* | 34 | 151 | 120 | 37 |
| No. lighter child meeting cut-off | 26 | 92 | 80 | 26 | |
| OR (95% CI) | 3.25‡ (1.47, 7.18) | 1.56‡ (1.12, 2.16) | 2.00‡ (1.37, 2.92) | 2.36† (1.17, 4.78) | |
| MZ | No. discordant pairs* | 7 | 38 | 41 | 8 |
| No. lighter child meeting cut-off | 5 | 23 | 30 | 6 | |
| OR (95% CI) | 2.50 (0.49, 12.89) | 1.53 (0.80, 2.94) | 2.72‡ (1.37, 5.44) | 3.00 (0.61, 14.86) | |
| DZ | No. discordant pairs* | 19 | 88 | 62 | 22 |
| No. lighter child meeting cut-off | 14 | 54 | 41 | 15 | |
| OR (95% CI) | 2.80† (1.01, 7.77) | 1.59† (1.03, 2.44) | 1.95† (1.15, 3.30) | 2.14 (0.87, 5.26) | |
| Males | No. discordant pairs* | 25 | 98 | 81 | 24 |
| No. lighter child meeting cut-off | 18 | 63 | 52 | 16 | |
| OR (95% CI) | 2.57† (1.07, 6.16) | 1.80‡ (1.19, 2.72) | 1.79† (1.14, 2.82) | 2.00 (0.86, 4.67) | |
| Females | No. discordant pairs* | 9 | 63 | 39 | 13 |
| No. lighter child meeting cut-off | 8 | 29 | 28 | 10 | |
| OR (95% CI) | 8.00† (1.00, 63.96) | 1.21 (0.70, 2.08) | 2.55‡ (1.27, 5.11) | 3.33 (0.92, 12.11) |
OR=Odds Ratio; CI=Confidence Interval;
Discordant for birth weight and ASD (or ASD-like traits) on the A-TAC. Birth weight discordance defined as ≥ 400 gram difference in birth weight between the twins in a pair, or one twin at least 15% lighter than the other in the pair;
p≤0.05;
p≤ 0.01.
Birth Weight as a Continuous Risk Factor
Birth weight was analyzed as a continuous exposure variable in a conditional logistic model for pairs discordant for ASD as measured by the A-TAC. For these analyses, the twin with ASD was used as the case and the twin without ASD was used as the control. Increasing birth weight was significantly associated with a decreased risk of ASD. A 100 gram increase in birth weight resulted in a 13% reduction in risk of ASD (OR=0.87; 95% CI: 0.78 –0.96) (see Table 3). In the stratified analyses, the ORs for the MZ and DZ male pairs were similar to the overall OR, whereas the female pairs had a slightly higher, but less precise, risk reduction (28%; OR=0.72; 95% CI: 0.51–1.00). Similarly, increased birth weight was associated with a reduction in the risk of each ASD-like trait, though only language impairment was significant at the 0.01 level. No trends were observed in the stratified analyses.
Table 3.
Odds ratios 100g increase in birth weight for twin-pairs discordant for ASD and ASD-like traits on the A-TAC using conditional logistic regression.
| ASD | Restricted/ Repetitive |
Language | Social | ||
|---|---|---|---|---|---|
| Overall | No. discordant pairs* | 78 | 375 | 309 | 78 |
| OR 100g increase in BW (95% CI) | 0.87‡ (0.78, 0.96) | 0.95† (0.90, 0.99) | 0.90‡ (0.85, 0.95) | 0.91† (0.82, 1.00) | |
| MZ | No. discordant pairs* | 20 | 108 | 106 | 25 |
| OR 100g increase in BW (95% CI) | 0.86† (0.76, 0.97) | 0.93 (0.85, 1.03) | 0.85‡ (0.77, 0.95) | 0.88 (0.73, 1.06) | |
| DZ | No. discordant pairs* | 44 | 214 | 161 | 44 |
| OR 100g increase in BW (95% CI) | 0.86† (0.74, 1.00) | 0.94 (0.88, 1.01) | 0.91† (0.85, 0.98) | 0.91 (0.78, 1.05) | |
| Males | No. discordant pairs | 58 | 239 | 201 | 53 |
| OR 100g increase in BW (95% CI) | 0.89† (0.80, 0.99) | 0.94† (0.89, 0.99) | 0.92‡ (0.87, 0.98) | 0.94 (0.84, 1.05) | |
| Females | No. discordant pairs* | 20 | 136 | 108 | 25 |
| OR 100g increase in BW (95% CI) | 0.72† (0.51, 1.00) | 0.96 (0.89, 1.05) | 0.85‡ (0.76, 0.95) | 0.84 (0.69, 1.02) |
BW=Birth Weight; OR=Odds Ratio; CI=Confidence Interval;
Discordant for ASD (or component feature) measured by A-TAC cut-off scores;
p≤0.05;
p≤ 0.01
The association between birth weight and ASD symptoms was also examined in the full study sample, with birth weight analyzed as a discrete variable and ASD analyzed as a continuous variable, controlling for zygosity, sex, and concordance using generalized estimating equation (GEE) with a Poisson link function. This analysis corrected for skewness due to the large number of ‘0’ scores (64% of sample) on the A-TAC, as most children in the sample showed no ASD symptoms. Although the data were overdispersed, according to Stokes et al. (Stokes et al., 2000) the robust standard error estimates of GEE help to adjust for overdispersion. In addition, GEE accounted for the correlation between twins of the same pair. Because A-TAC scores could be half or whole units, scores were transformed linearly to allow for use of the Poisson model. Birth weight, zygosity (MZ, DZ, Unknown), concordance (ASD concordant, ASD discordant, or no ASD concordant), and sex were modeled. Results inidicated signficant effects of birth weight on ASD symptoms, controlling for zygosity and sex. As illustrated in Table 4, an increase of 100 grams in birth weight predicted a decrease in ASD severity score by a factor of 0.98, holding constant all other variables. That is, for every 100 gram increase in birth weight a 2% decrease in severity of ASD indexed by A-TAC scores would be expected. Analysis of sex indicated that females would be expected to exhibit ASD severity scores 0.68 of males’ scores (i.e., a 32% decrease in severity compared to males), holding all other variables constant. Zygosity was not statistically significant.
Table 4.
Model of the effect of birth weight on ASD symptoms on the A-TAC using Generalized Estimating Equations with Poisson Link Function (same-sex twins).
| Variable | PR (95% CI) | p-value |
|---|---|---|
| Birth Weight* | 0.98 (0.97, 0.98) | <0.0001 |
| Sex | <0.0001 | |
| Female | 0.68 (0.62, 0.75) | |
| Male | 1 [Reference] | |
| Zygosity | 0.1083 | |
| MZ | 0.94 (0.82, 1.08) | |
| DZ | 1.04 (0.92, 1.19) | |
| Unknown | 1 [Reference] | |
| Concordance | <0.0001 | |
| ASD Concordant | 22.93 (19.50, 26.96) | |
| ASD Discordant | 10.65 (9.77, 11.62) | |
| Non-ASD Concordant | 1 [Reference] |
PR=Prevalence Ratio; CI=Confidence Interval
100 gram change
DISCUSSION
This study examined low birth weight as a potential environmental risk factor associated with ASD in a large cohort of Swedish twins. Analyses of discordant twin-pairs using the cotwin-control design, in which the affected twin serves as the case and unaffected twin the control, indicated that lighter twins in birth weight discordant pairs (> 400 gram or 15% difference) were more than twice as likely to meet criteria for ASD (i.e., score ≥ 4.5 on the ASD module of the A-TAC measure) than the heavier twin in the pair (odds ratio for all twins = 3.25, MZ twins = 2.50, DZ twins = 2.80). Analyses of birth weight as a continuous risk factor indicated a 13% reduction in risk of ASD per 100 gram increase in birth weight. Birth weight was also associated with individual ASD-like traits, with stronger effects detected for social and language features than ritualistic/repetitive behaviors. Examining the effect of birth weight on A-TAC scores in the entire sample population (where the majority of twins did not have meet A-TAC criteria for ASD) revealed a modest but consistent association, with a 2% reduction in A-TAC scores per every increase of 100 grams.
As a whole, these findings add to prior reports of an association between birth weight and ASD (Bryson et al., 1988; Burd et al., 1999; Maimburg & Vaeth, 2006; Kolevzon et al., 2007). They further indicate that birth weight is associated with ASD-like traits, and the social and language impairments associated with ASD in particular, supporting a prior report that differences in MZ twins’ social ASD-like traits were associated with differences in birth weight, whereas ritualistic/repetitive behaviors were not (Ronald et al., 2010). The large population based twin sample employed minimized ascertainment bias. And, by virtue of the cotwin-control design, the present results preclude many additional environmental or genetic (including maternal genetic) factors that could influence birth weight, and which are not fully accounted for in studies of singletons. Similar effects observed in MZ and DZ pairs suggest that the association between birth weight and ASD does not appear to be attributable to confounding environmental and/or genetic factors affecting both features.
In the case of MZ twins, who share gestational age, differences in birth weight can provide an index of differences in fetal growth that may be relevant to clinical outcome in twin-pairs discordant for disease. Findings could therefore support a role of restricted fetal growth in adverse neurodevelopment leading to ASD, although it should be noted that this study examined only birth weight and not any direct measures of fetal growth. It is unclear how fetal growth restriction might influence brain development in ways specific to autism. Additional studies are clearly needed to clarify the pathophysiological mechanisms underlying the association between birth weight and ASD, and the potential role of fetal growth restriction in particular.
Further work is also needed to help clarify the clinical significance of several of the findings reported here. Whereas associations between birth weight and ASD appeared robust in twin-pairs discordant for both ASD and birth weight, this subgroup of twins was relatively small, and confidence intervals were large. Additionally, analysis of the full sample (where the majority of twins were reported to exhibit no symptoms of ASD and thus received a zero on the ASD A-TAC module) indicated only a slight increase in ASD symptoms with incremental decreases in birth weight. Small effect sizes in mean A-TAC scores in the entire sample may nevertheless contain significant effects in individual twin-pairs. We also found no association between birth weight and ASD severity (indexed by total A-TAC scores on the ASD module) in the limited sample of 16 ASD concordant twin-pairs, suggesting that the effects of birth weight on ASD risk criteria in more severely affected concordant pairs are not as apparent as those observed in discordant pairs.
The lack of direct clinical assessment of ASD and reliance on the A-TAC for assigning ASD status is a potential limitation of the study. As with other large-scale twin studies (Constantino & Todd, 2003; Hoekstra et al., 2009; Ronald et al., 2010), direct assessment of the large population based sample assessed in CATSS was not feasible, and assessment of ASD therefore involved informant based proxies for clinical diagnosis of ASD and ASD-like traits using the A-TAC. Two validation studies (Hansson et al., 2005; Larson et al., 2010) have now demostrated that the A-TAC has high sensitivity and specificity to provide research proxies for clinical diagnosis of autism and other targeted disroders. This instrument has also been successfully used as a dimensional measure of ASD-like traits in recent work (Lundstrom et al., 2011), supporting its use in the present investigation. Nevertheless, direct clinical assessment, including evaluation of intellectual disability, would provide important verification of findings, and allow for more thorough investigation of their clinical significance and potential differential relationships with intellectual disability. Additionally, assessment of family members who may exhibit ASD or subclinical ASD-like traits would enrich these data by affording analysis of familial loading related to the relationship between birth weight and ASD.
Finally, it is important to note that findings from twin samples may not be generalizable to singleton samples, further warranting cautious interpretation of results. Twins on average have lower birth weights than singletons (Spellacy et al., 1990), and there has been some suggestion of increased rates of ASD in twin samples (Bailey et al., 1995). The associations detected in this study may therefore not extend to singleton births. With these caveats in mind, results from this study appear to support existing literature implicating low birth weight as a risk factor for ASD. The twin design employed allowed for control of several confounding factors not fully addressed in prior work. Results also highlight several areas for further study, which, together with continued large-scale efforts to identify causal genetic variants, may help to illuminate the complex etiology of ASD.
ACKNOWLEDGMENTS
We gratefully acknowledge the support of the Swedish Council for Working Life and Social Research and the Swedish Research Council. ML and DE also acknowledge support from grant # 1KL2RR025746-01.
Abbreviations
- MZ
Monozygotic
- DZ
Dizygotic
- ASD
Autism Spectrum Disorder
- Odds Ratio
OR
- A-TAC
Autism - Tics ADHD and other Comorbidities Inventory
Appendix
Figure A1. ASD symptoms and birth weight differences for concordant twin-pairs, among all same-sex pairs. (Only two female pairs fell into this group, and are depicted with dashed lines.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- Anckarsäter H, Larson T, Hansson SL, Carlström E, Ståhlberg O, Gillberg C, Råstam R, Gillberg C, Lichtenstein P. Child neurodevelopmental and behavioral problems at intercorrelated and dimensionally distributed in the general population. The Open Psychiatry Journal. 2008;2:5–11. [Google Scholar]
- Anckarsäter H, Lichtenstein P, Carlstedt E, Ståhlberg O, Gillberg C. Psychometric development of The Autism - Tics, AD/HD and other comorbidities (A-TAC) inventory. Full version with gate structure; based on clinic and general population data. 2007 [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychological Medicine. 1995;25(1):63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, Nagaraja HN, Cooley WC, Gaelic SE, Bauman ML. Timing of prenatal stressors and autism. Journal of Autism and Developmental Disorders. 2005;35(4):471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- Blickstein I. The definition, diagnosis, and management of growth-discordant twins: An international census survey. Acta Geneticae Medicae et Gemellologiae. 1991;40(3–4):345–351. doi: 10.1017/s0001566000003536. [DOI] [PubMed] [Google Scholar]
- Blickstein I, Lancet M. The growth discordant twin. Obstetrical and Gynecological Survey. 1988;43(9):509–515. doi: 10.1097/00006254-198809000-00002. [DOI] [PubMed] [Google Scholar]
- Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, Bailey A, Rutter M. A Case-Control Family History Study of Autism. Journal of Child Psychology and Psychiatry. 1994;35(5):877–900. doi: 10.1111/j.1469-7610.1994.tb02300.x. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Griffiths PD. Association of tuberous sclerosis of temporal lobes with autism and atypical autism. Lancet. 1997;349(9049):392–395. doi: 10.1016/S0140-6736(97)80012-8. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Murphy M, Macdonald H, Whitlock B, Pickles A, Rutter M. Obstetric complications in autism: consequences or causes of the condition? Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(2):272–281. doi: 10.1097/00004583-199702000-00018. [DOI] [PubMed] [Google Scholar]
- Branum AM, Schoendorf KC. The effect of birth weight discordance on twin neonatal mortality. Obstetrics & Gynecology. 2003;101(3):570–574. doi: 10.1016/s0029-7844(02)03119-8. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Smith IM, Eastwood D. Obstetrical suboptimality in autistic children. Journal of the American Academy of Child & Adolescent Psychiatry. 1988;27(4):418–422. doi: 10.1097/00004583-198807000-00006. [DOI] [PubMed] [Google Scholar]
- Burd L, Severud R, Kerbeshian J, Klug MG. Prenatal and perinatal risk factors for autism. Journal of Perinatal Medicine. 1999;27(6):441–450. doi: 10.1515/JPM.1999.059. [DOI] [PubMed] [Google Scholar]
- Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Diseases in Canada. 2010;30(4):125–134. [PubMed] [Google Scholar]
- CDC. Prevalence of autism spectrum disorders --- Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR CDC Surveillance Summaries. 2009;58(SS10):1–20. [PubMed] [Google Scholar]
- Chess S. Autism in children with congenital rubella. Journal of Autism and Childhood Schizophrenia. 1971;1(1):33–47. doi: 10.1007/BF01537741. [DOI] [PubMed] [Google Scholar]
- Chess S. Follw-up report on autism in congenital rubella. Journal of Autism and Childhood Schizophrenia. 1977;7(1):69–81. doi: 10.1007/BF01531116. [DOI] [PubMed] [Google Scholar]
- Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. British Journal of Obstetrics and Gynaecology. 2000;107(3):375–381. doi: 10.1111/j.1471-0528.2000.tb13234.x. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Archives of General Psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrance and the genetic epidemiology of autism. American Journal of Psychiatry. 2010;167(11):1349–1356. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan E, Byrne M, O'donovan A, O'callaghan E. A case-control study of obstetric complications and later autistic disorder. Journal of Autism and Developmental Disorders. 1996;26(4):453–460. doi: 10.1007/BF02172829. [DOI] [PubMed] [Google Scholar]
- Deb S, Prasad KB, Seth H, Eagles JM. A comparison of obstetric and neonatal complications between children with autistic disorder and their siblings. Journal of Intellectual Disability Research. 1997;41(Pt 1):81–86. doi: 10.1111/j.1365-2788.1997.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Falconer DS. Introduction to Quantitative Genetics. London/New York: Longmans Green; 1981. [Google Scholar]
- Folstein S, Rutter M. Infantile autism: A genetic study of 21 twin pairs. Journal of Child Psychology and Psychiatry. 1977;18(4):297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Gielen M, Lindsey PJ, Derom C, Loos RJF, Souren NY, Paulussen ADC, Zeegers MP, Derom R, Vlietinck R, Nijhuis JG. Twin-specific intrauterine ‘growth’ charts based on cross-sectional birthweight data. Twin Research and Human Genetics. 2008;11(2):224–235. doi: 10.1375/twin.11.2.224. [DOI] [PubMed] [Google Scholar]
- Hack M, Taylor HG, Schluchter M, Andreias L, Drotar D, Klein N. Behavioral outcomes of extremely low birth weight children at age 8 years. Journal of Developmental & Behavioral Pediatrics. 2009;30(2):112–130. doi: 10.1097/DBP.0b013e31819e6a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Archives of General Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.76. archgenpsychiatry.2011.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson SL, Rojvall AS, Rastam M, Gillberg C, Anckarsater H. Psychiatric telephone interview with parents for screening of childhood autism - tics, attention-deficit hyperactivity disorder and other comorbidities (A-TAC): preliminary reliability and validity. The British Journal of Psychiatry. 2005;187(3):262–267. doi: 10.1192/bjp.187.3.262. [DOI] [PubMed] [Google Scholar]
- Hoekstra RA, Happe F, Baron-Cohen S, Ronald A. Association between extreme autistic traits and intellectual disability: Insights from a general population twin study. British Journal of Psychiatry. 2009;195:531–536. doi: 10.1192/bjp.bp.108.060889. [DOI] [PubMed] [Google Scholar]
- Hrubec Z, Robinette CD. The study of human twins in medical research. New England Journal of Medicine. 1984;310(7):435–441. doi: 10.1056/NEJM198402163100706. [DOI] [PubMed] [Google Scholar]
- Indredavik MS, Vik T, Heyerdahl S, Kulseng S, Fayers P, Brubakk AM. Psychiatric symptoms and disorders in adolescents with low birth weight. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2004;89(5):F445–F450. doi: 10.1136/adc.2003.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzchak EB, Lahat E, Zachor DA. Advanced parental ages and low birth weight in autism spectrum disorders-Rates and effect on functioning. Research in Developmental Disabilities. 2011 doi: 10.1016/j.ridd.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Juul-Dam N, Townsend J, Courchesne E. Prenatal, perinatal, and neonatal factors in autism, pervasive dvelopmental disorder-not otherwise specified, and the general population. Pediatrics. 2001;107(4):E63. doi: 10.1542/peds.107.4.e63. [DOI] [PubMed] [Google Scholar]
- Kolevzon A, Gross R, Reichenberg A. Prenatal and Perinatal Risk Factors for Autism. Archives of Pediatrics and Adolescent Medicine. 2007;161(4):326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- Kuban KC, O'shea TM, Allred EN, Tager-Flusberg H, Goldstein DJ, Leviton A. Positive screening on the Modified Checklist for Autism in Toddlers (M-CHAT) in extremely low gestational age newborns. Journal of Pediatrics. 2009;154(4):535–540. doi: 10.1016/j.jpeds.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson T, Anckarsater H, Gillberg C, Stahlberg O, Carlstrom E, Kadesjo B, Rastam M, Lichtenstein P. The autism--tics, AD/HD and other comorbidities inventory (A-TAC): further validation of a telephone interview for epidemiological research. BMC Psychiatry. 2010;10:1. doi: 10.1186/1471-244X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HJ, Eaton WW, Madsen KM, Vestergaard M, Olesen AV, Agerbo E, Schendel D, Thorsen P, Mortensen PB. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. American Journal of Epidemiology. 2005;161(10):916–925. doi: 10.1093/aje/kwi123. [DOI] [PubMed] [Google Scholar]
- Lauritsen MB, Pedersen CB, Mortensen PB. Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. Journal of Child Psychology and Psychiatry. 2005;46(9):963–971. doi: 10.1111/j.1469-7610.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- Levy S, Zoltak B, Saelens T. A comparison of obstetrical records of autistic and nonautistic referrals for psychoeducational evaluations. Journal of Autism and Developmental Disorders. 1988;18(4):573–581. doi: 10.1007/BF02211875. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Carlström C, Rastam M, Gillberg C, Anckarsater H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. The American Journal of Psychiatry. 2010;167:1357–1363. doi: 10.1176/appi.ajp.2010.10020223. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlström C, Björk C, Svartengren M, Wolk A, Klareskog L, De Faire U, Schalling M, Palmgren J, Pedersen NL. The Swedish Twin Registry in the third millenium - an update. Twin Research and Human Genetics. 2006;9(6):875–882. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- Limperpoulos C. Autism spectrum disorders in survivors of extreme prematurity. Clinical Perinatology. 2009;36(4):791–805. doi: 10.1016/j.clp.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute, Inc.; 1996. [Google Scholar]
- Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B(4):424–433. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom S, Chang Z, Kerekes N, Gumpert CH, Rastam M, Gillberg C, Lichtenstein P, Anckarsater H. Autistic-like traits and their association with mental health problems in two nationwide twin cohorts of children and adults. Psychological Medicine. 2011;00(00):1–11. doi: 10.1017/S0033291711000377. [DOI] [PubMed] [Google Scholar]
- Lundstrom S, Haworth CM, Carlström E, Gillberg C, Mill J, Rastam M, Hultman CM, Ronald A, Anckarsater H, Plomin R, Lichtenstein P, Reichenberg A. Trajectories leading to autism spectrum disorders are affected by paternal age: findings from two nationally representative twin studies. Journal of Child Psychology and Psychiatry. 2010;51(7):850–856. doi: 10.1111/j.1469-7610.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- Magnus P, Gjessing HK, Skrondal A, Skjaerven R. Paternal contribution to birth weight. Journal of Epidemiology and Community Health. 2001;55(12):873–877. doi: 10.1136/jech.55.12.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimburg RD, Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatrica Scandinavica. 2006;114(4):257–264. doi: 10.1111/j.1600-0447.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- Markowitz PI. Autism in a child with congenital cytomegalovirus infection. Journal of Autism and Developmental Disorders. 1983;13(3):249–253. doi: 10.1007/BF01531564. [DOI] [PubMed] [Google Scholar]
- Mason-Brothers A, Ritvo ER, Pingree C, Petersen PB, Jenson WR, Mcmahon WM, Freeman BJ, Jorde LB, Spencer MJ, Mo A, et al. The UCLA-University of Utah epidemiologic survey of autism: prenatal, perinatal, and postnatal factors. Pediatrics. 1990;86(4):514–519. [PubMed] [Google Scholar]
- O'Brien WF, Knuppel RA, Scerbo JC, Rattan PK. Birth weight in twins: An analysis of discordancy and growth retardation. Obstetrics and Gynecology. 1986;67(4):483–486. [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson S, Carver LJ, Constantino JN, Dobkins K, Hutman T, Iverson JM, Landa R, Rogers SJ, Sigman M, Stone WL. Recurrence Risk for Autism Spectrum Disorders: A Baby Siblings Research Consortium Study. Official Journal of the American Academy of Pediatrics. 2011 doi: 10.1542/peds.2010-2825. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Palmer P, Landa R, Santangelo S, Jacobi D, Childress D. Personality and language characteristics in parents from multiple-incidence autism families. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 1997;74(4):398–411. [PubMed] [Google Scholar]
- Piven J, Simon J, Chase GA, Wzorek M, Landa R, Gayle J, Folstein S. The Etiology of Autism: Pre-, Peri- and Neonatal Factors. Journal of American Academy of Child & Adolescent Psychiatry. 1993;32(6):1256–1263. doi: 10.1097/00004583-199311000-00021. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Gross R, Weiser M, Bresnahan M, Silverman J, Harlap S, Rabinowitz J, Shulman C, Malaspina D, Lubin G, Knobler H, Davidson M, Susser E. Advancing paternal age and autism. Archives of General Psychiatry. 2006;63(9):1026–1032. doi: 10.1001/archpsyc.63.9.1026. [DOI] [PubMed] [Google Scholar]
- Ronald A, Happe F, Dworzynski K, Bolton P, Plomin R. Exploring the relation between prenatal and neonatal complications and later autistic-like features in a representative community sample of twins. Child Development. 2010;81(1):166–182. doi: 10.1111/j.1467-8624.2009.01387.x. [DOI] [PubMed] [Google Scholar]
- Schendel D, Bhasin TK. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics. 2008;121(6):1155–1164. doi: 10.1542/peds.2007-1049. [DOI] [PubMed] [Google Scholar]
- Skranes J, Vangberg TR, Kulseng S, Indredavik MS, Evensen KA, Martinussen M, Dale AM, Haraldseth O, Brubakk AM. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130(Pt 3):654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- Spellacy WN, Handler A, Ferre CD. A case-control study of 1253 twin pregnancies from a 1982–1987 perinatal data base. Obstetrics & Gynecology. 1990;75(2):168–171. [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. Journal of Child Psychology and Psychiatry. 1989;30(3):405–416. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using the SAS System. Cary, NC: SAS Institute Inc.; 2000. [Google Scholar]
- Szatmari P. Is Autism, at Least in Part, a Disorder of Fetal Programming? Archives of General Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.99. archgenpsychiatry.2011.2099. [DOI] [PubMed] [Google Scholar]
- Talbot GT, Goldstein RF, Nesbitt T, Johnson JL, Kay HH. Is size discordancy an indication for delivery of preterm twins? American Journal of Obstetrics and Gynecology. 1997;177(5):1050–1054. doi: 10.1016/s0002-9378(97)70013-9. [DOI] [PubMed] [Google Scholar]
- Ward AJ. A comparison and analysis of the presence of family problems during pregnancy of mothers of "autistic" children and mothers of normal children. Child Psychiatry & Human Development. 1990;20(4):279–288. doi: 10.1007/BF00706020. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Arking DE, Consortium GDPOJHA, Daly MJ, Chakravarti A. A genome-wide linkage and association scan reveals novel loci for autism. Nature. 2009;461(7265):802–808. doi: 10.1038/nature08490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira MA, Green T, Platt OS, Ruderfer DM, Walsh CA, Altshuler D, Chakravarti A, Tanzi RE, Stefansson K, Santangelo SL, Gusella JF, Sklar P, Wu BL, Daly MJ. Association between microdeletion and microduplication at 16p11.2 and autism. New England Journal of Medicine. 2008;358(7):667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Szatmari P, Jones MB, Bryson SE, Maclean JE, Mahoney WJ, Bartolucci G, Tuff L. Pregnancy and birth complications in autism and liability to the broader autism phenotype. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(5):572–579. doi: 10.1097/00004583-200205000-00015. [DOI] [PubMed] [Google Scholar]