Abstract
HER-2 (also called ErbB2 or Neu) tyrosine kinase, one of the four members of ErbB receptor family (ErbBl, i.e., EGFR, ErbB2, ErbB3 and ErbB4), plays a critical role in the control of diverse cellular functions involved in differentiation, proliferation, migration and cell survival via multiple signal transduction pathways. Overexpression of HER-2, observed in HER-2-positive breast cancer patients, is believed to cause the tumor resistance to an array of anti-cancer agents and poor prognosis. Although HER-2 antibodies have shown growth inhibitory effects, more efficient molecular targets against HER-2-mediated tumor resistance need to be developed. The molecular mechanisms underlying HER-2-mediated tumor resistance, especially the connections between HER-2 and therapy-resistant signaling networks, need to be further investigated. NF-κB, a key stress transcription factor that can initiate a pro-survival network, was found to be activated in many cancer cells overexpressing HER-2 and to be responsible for the radiation resistance in HER-2 transfected breast cancer cells. Recent findings in literature and data from this laboratory suggest a possible co-operation between HER-2 and NF-κB in signaling tumor resistance to radiotherapy. This review will discuss the mechanisms of HER-2 mediated NF-κB signaling pathway and potential target for therapeutic intervention.
Keywords: HER-2, NF-κB, herceptin, drug resistance, radiotherapy resistance, review
Introduction
HER-2 is a proto-oncogene which encodes a 185 kDa (1255 amino acids) transmembrane receptor tyrosine kinase (RTK) in various tissues of epithelial, mesenchymal and neuronal origin (1, 2). It maps to human chromosome 17q21, and is closely related in structure to the epidermal growth factor receptor (EGFR) (3, 4). EGFR proteins exist as monomers in the cell membrane. Upon ligand binding (except HER-2), these receptors form homo- or heterodimers to regulate diverse biological processes, such as proliferation, differentiation, cell mobility and apoptosis, via different signal transduction pathways (5, 6). Although there is no specific ligand for HER-2, it appears that it acts as a preferred co-receptor to form heterodimers with other EGFR members for the initiation of signal transduction (7, 8). In cells overexpressing HER-2, including those of the breast, spontaneously activated homodimers can occur in the absence of a ligand and constitutive receptor activation (9). Following dimerization, HER-2 undergoes autophosphorylation on specific tyrosine residues within the regulatory domain (10, 11). HER-2 is localized to the cell membrane with two cysteine-rich extracellular dimerization domains, a transmembrane domain and an intracellular tyrosine kinase domain (3, 12, 13). Although HER-2 is a membrane-bound protein, it was found to enter the nucleus by endocytosis (14) and function as a transcriptional regulator (15).
HER-2 overexpression or amplification, found frequently in many types of human cancers, including breast, ovarian, lung, gastric and oral cancers (2, 16–22), increases cell proliferation and survival (23), and induces tumor resistance to anticancer therapies (16, 24). Breast cancer is the most common cancer and the second leading cause of cancer related death in women in the United States (25). Although a normal level of HER-2 is required for the regulation of normal breast growth and development (26), amplification and overexpression of HER-2 causes the disruption of normal cellular control and the formation of aggressive breast tumor cells (18, 27). HER-Z level is considered as the predictive marker for the diagnosis of metastatic breast cancer and it is an important factor for treatment plan design (28, 29). Many breast cancer patients benefit from radiotherapy combined with chemotherapeutic agents. These combined modalities improve the local control of tumor growth and increase survival rates. However, accumulating reports suggest that chemoresistance can be induced following radiation (radio-chemoresistance), which challenges the overall effectiveness of the combined modality therapy. Most importantly, therapy-resistance is strikingly increased when tumor cells are HER-2 positive. For instance, overexpression of HER-2 has been related to an increased risk of local relapse in breast cancer patients who received conservative surgery and radiation therapy (29). These results suggest that HER-2-mediated therapy-resistance involves the anti-radiation signaling network.
HER-2 and Breast Cancer
Breast cancer cells expressing high levels of EGF receptors are associated with an aggressive clinical behavior (30). Approximately 30% of breast cancer patients showed genetic alterations in the HER-2 gene causing an increased amount of the growth factor receptor protein on the tumor cell surface. Patients with HER-2 postive cancers show a more aggressive disease, greater likelihood of recurrence, poorer prognosis, and decreased survival compared to patients with HER-2-negative breast cancer. A causal link between HER-2 overexpression and tumor progression was further evidenced by experimental results that HER-2 transfected cells showed increased metastasis in vivo, and if HER-2 is inhibited by monoclonal antibody, antisense constructs or adenovirus 5 ELA gene products, the malignant phenotype was reversed (31). Therefore, targeting HER-2 has the potential to be an efficient approach in anticancer treatments. However, HER-2 monoclonal antibody "trastuzumab" (marketed as "herceptin"; a recombinant humanized monoclonal antibody against the extracellular domain of HER-2) produces remission in 11-15% of patients with metastatic breast cancer. This result indicates that the majority of the patients become resistant to herceptin, although they initially respond to this monotherapy. Another monoclonal antibody pertuzumab, which blocks dimerization of HER-2, alone or together with herceptin could not significantly reduce the viability of herceptin-resistant breast cancer cells (32, 33). Also, a combined therapy of herceptin and chemotherapeutic agents (e.g., paclitaxel and docetaxel) resulted in little increase in patient survival (34). These clinical data strongly suggest that targeting HER-2 alone is insufficient for breast cancer patients and the signaling network causing HER-2-mediated tumor resistance needs to be elucidated.
Radiotherapy is an important therapeutic intervention following breast conservative surgery (35, 36). Richard el al. reported that HER-2 monoclonal antibody (rhuMAbs) modulates repair of IR-induced DNA damage and enhances radiosensitivity of HER-2 positive breast cancer cells (37). The potential role of HER-2 in the modulation of sensitivity to radiation is assumed to be due to the signal generated by antibody binding to HER-2 receptor that blocks DNA repair in HER-2-overexpressing cells (38). This concept is supported by clinical studies that demonstrate earlier local relapse in HER-2-positive breast cancer patients following conservative surgery accompanied by radiotherapy. Although it is well documented that genetic factors contribute to the outcome of radiotherapy (39), redox imbalance, repair of DNA damage, cell cycle adjustment, by-stander effects, genomic instability, as well as adaptive tumor resistance may contribute to a complex network that determines the efficacy of anticancer radiotherapy. The exact molecular mechanisms regulating tumor response to radiation are largely unknown, although a group of stress responsive effector genes were found to be responsible for increased resistance to radiotherapy (40, 41). Recently, data reported by our group and others strongly suggest a pro-survival signaling network involving NF-κB and HER-2 (40, 42, 43). HER-2 was found to regulate the constitutive activation of NF-κB in HER-2-overexpressing breast cancer cells (40, 44). Therefore, targeting both HER-2 and NF-κB could be an efficient strategy in the management of HER-2 positive breast cancer patients.
HER-2-mediated Chemoresistance
Tumor resistance to multiple chemotherapeutic agents, a major cause of failure of anti-cancer therapy, is believed to be related to intrinsic, as well as acquired during treatment (45, 46). The most commonly used anti-neoplastic agents in the treatment of disseminated breast cancer are adriamycin, methotrexate and cyclophosphamide. Cell lines selected for resistance to adriamycin often develop cross-resistance to structurally dissimilar anti-neoplastic drugs with different mechanisms of cytotoxic action, a phenomenon called pleiotropic or multidrug resistance (MDR) (45). One of the mechanisms that may contribute to chemoresistance is the activation of oncogenes, including HER-2, Bcl-2, Bcl-XL, Ras, c-Jun, c-Fos, or mutant p53 (46). HER-2 transfected human lung cancer cells showed enhanced resistance to chemotherapies (47). The level of HER-2 is the only independent predictor for chemoresistance to doxorubicin, etoposide, and probably cisplatin. Although intrinsic chemoresistance almost certainly is a multifactorial process, overexpression of HER-2 may be an important factor for the chemoresistance of non-small cancer lung cells (48). A clinical study conducted by Gusterson et al. demonstrates that HER-2-overexpressing breast cancer cells are less responsive to adjuvant chemotherapy regimens consisting of cyclophosphamide, Methotrexate and 5-fluorouracil (CMF) than tumors that have normal expression of HER-2 (49). In another clinical study, patients with metastatic breast cancers showed that elevated HER-2 serum protein levels are associated with a lower rate of response to chemotherapy compared to those with normal HER-2 levels (29% versus 59%) (50).
Herceptin has been shown to induce therapeutic responses in patients with primary operable breast cancer through antibody-dependent cellular cytotoxicity (ADCC) (51). It activates the PTEN phophatase, which results in rapid dephosphorylation of Akt and inhibits cell proliferation (52). Although clinical studies established that herceptin is active against HER-2-overexpressing breast cancer cells (16, 17), the time to disease progression is short (median duration is 9 months) (17). Therefore, herceptin monotherapy, approved in 1998 by the US Food and Drug Administration, is not an efficient treatment for many cancer patients. Recent studies using herceptin combined with chemotherapeutic drugs, including paclitaxel and docetaxel, increases the time to disease progression and the survival of breast cancer patients. However, in most patients disease progression begins again within a year (34). These results indicate that targeting other key signaling elements as well as HER-2 is critical to improve the survival of HER-2-overexpressing breast cancer patients.
HER-2-mediated Radioresistance
Radiotherapy is widely used in the treatment of breast cancer and reduces the risk of loco-regional recurrence (53). Patients treated with breast conservation surgery routinely receive radiotherapy as an additional treatment. However, breast cancer cells have been shown to be relatively refractory to IR-induced DNA damage and apoptosis (54), although the molecular mechanism underlying the radioresistant phenotype is largely unknown. Several clinical studies showed that the recurrence risk after treatment with surgery and radiation is higher among HER-2-positive breast cancer patients. Stimulated with epidermal growth factors, MCF-7 human breast cancer cells showed more radioresistance than the untreated cells (55). Compared to parental cell line, HER-2 transfected MCF-7 cells showed enhanced resistance to IR-induced apoptosis and increased post-radiation clonogenic survival (37, 56). Previous studies indicated that ionizing radiation itself can mimic the function of growth factors to activate EGFR, which then activates the downstream mitogen-activated protein kinase (MAPK) pathway (57, 58). Other radiation-induced factors, such as p53 status of the cell, induction of Bax and Bcl-2 families of proteins, NF-κB activation, relative levels of insulin like growth factor and insulin-like growth factor binding proteins, and PI3K/Akt pathway activation, may modulate the apoptotic response to DNA-damaging agents (54).
EGFRs, especially HER-2, have become the target to treat cancers that are resistant to radiotherapy (59). Anti-HER-2 monoclonal antibody, rhumAbHER2 (trasluzamab/herceptin), enhances radiation-induced growth inhibition in human head and neck carcinomas (60). Rao et al. found that a potent EGFR tyrosine kinase inhibitor CI-1033 that inhibits the proliferation of HER-2-overexpressing breast cancer cells, reduced clonogenic survival by 65-fold with radiation compared to cells that received irradiation alone (59). The study conducted by Pietras et al. indicated that herceptin is able to radiosensitize HER-2-overexpressing MCF-7 cells. They found that the combination of herceptin and radiation can synergistically reduce tumor formation in nude mice (37). Another study showed that breast cancer cells treated with herceptin significantly sensitized the apoptosis induced by radiation in cell lines with high levels of HER-2 (such as BT474, SKBR3, and MDA453), but not in cell lines with low levels of HER-2 (such as MCF7, ZR75B and MDA468) (61). Herceptin is hypothesized to sensitize HER-2-positive tumors to irradiation by blocking the growth-promoting signals required to induce cell proliferation and survival through HER-2, although the signaling pathways have not been clearly identified. Further research indicated that MAPK and PI3K/Akt pathways are involved in HER-2-mediated resistance to radiation-induced apoptosis in breast cancer cells (53, 56). Wortmannin, a PI3K inhibitor, induces radiosensitization in MCF-7 and other cell lines (62), and inhibition of MAPK decreases radioresistance in A431 human squamous carcinoma cells (63).
Mechanisms of NF-κB Activation
NF-κB is a dimeric transcription factor, which acts as a master regulator of stress and immune responses in many cell types, since it constitutes a primary means of relaying an extracellular, immunologically relevant signal into nucleus to initiate a genetic program. NF-κB was originally identified as a protein bound to a sequence in the immunoglobulin kappa light chain enhancer in B cells (64). It tightly controls genes for stress responses, inflammation and apoptosis. NF-κB family consists of five members of the Rel family: RelA (also called p65), RelB, c-Rel, p50/p105 (also called NF-κB1) and p52/p100 (also called NF-κB2). Although the heterodimer of p50 and p65 is shown to be the most abundant form of NF-κB (65), different combinations of homo- or hetero-dimers can be formed to regulate the intrinsic NF-κB specificity (66–69). NF-κB DNA binding sites that are present in the promoter region of many genes are capable of binding to p50 homodimers, p50/p65 or p50/c-Rel heterodimers, suggesting that NF-κB can regulate the expression of different effector genes (70).
Under resting conditions, the NF-κB complex is formed by a p50 homodimer or a p50/p65 heterodimer bound to a member of the IκB family (70, 71). In the cytosol of unstimulated cells, the nuclear localization signal of NF-κB is effectively hidden through the non-covalent binding with IκB. Members of the mammalian IκB family include IκBα, IκBβ, IκBγ, IκBε, Bcl3, p105 and p100, of which the most studied is IκBα. Following stimulation, DNA-binding subunits p50 and p52 that carry a Rel homology domain (RHD) are proteolytically released from p105 and p100, respectively. The RHD contains a nuclear localization sequence (NLS) and is involved in dimerization, sequence-specific DNA binding and interaction with the inhibitory IκB proteins. Bcl3 functions as a transcriptional activator with p50 or p52 homodimers, rather than an inhibitor of NF-κB. Using various stimuli, including TNF-α, PMA, LPS, interleukins and UV or IR, it has been well established in many cell lines that signal-induced activation of NF-κB typically occurs through phosphorylation of IκB proteins at Ser-32 and Ser-36 in IκBα, and Ser-19 and Ser-23 in IκBβ via ubiquitin-dependent protein kinase, followed by ubiquitination at nearby lysine residues and degradation by the 26S proteasome (72–74). Upon degradation of IκB protein, NF-κB translocates to the nucleus where it either binds to a specific 10-base-pair consensus site GGGPuNNPyPyCC (Pu = purine, Py = pyrimidine and N = any base) or interacts with other transcription factors thereby regulating gene transcription. Although it has been suggested that the degraded IκB may still be associated with NF-κB in mammalian cells, activated NF-κB typically exists as a dimeric protein, and this transcriptionally active form possesses both DNA-binding and transactivation domains. NF-κB activates transcription from a wide variety of promoters, including that of its own inhibitor IκBα. The newly synthesized IκBα, enters the nucleus and removes NF-κB from its DNA-binding sites and transports it back to the cytoplasm, thereby terminating NF-κB-dependent transcription (75, 76).
Acetylation and deacetylation events are post-translational control mechanisms that play critical roles in the activation of NF-κB. Evidence suggests that NF-κB-dependent transcription requires multiple co-activators i.e., p300/CBP, P/CAF and SRC-1/NcoA-1 that possess histone acetyltransferase (HAT) activity (77–79). The interactions between NF-κB and these HATs suggest a link between acetylation events and NF-κB-mediated transactivation. A role for acetylation in the regulation of NF-κB-mediated transactivation has emerged with the finding that histone deacetylase inhibitors (HDACi) (such as trichostatin A or sodium butyrate) enhance NF-κB-dependent gene expression in the presence of TNF-α (80–82). In addition, NF-κB heterodimer p50/p65 can be acetylated at multiple lysine residues (83), which is believed to regulate the function of transcriptional activation, DNA-binding affinity and IκBα affinity. Interestingly, the p50 subunit, that does not possess a transactivation domain, is acetylated in vitro by p300/CBP (84). The enhanced p50 acetylation in vivo is found to be correlated with increased p50 binding to the cyclooxygenase-2 (COX-2), an important NF-κB-regulated effector (85). Acetylation of p65 is also detected in vivo (86) with stimulation of TNF-α and PMA (87). In both cases, p65 is acetylated following overexpression of p300/CBP and p65 is deacetylated through a specific interaction with histone deacetylase-3 (HDAC-3) (82, 87, 88). These results strongly suggest that acetylation of p65 increases its ability to bind to DNA. The exact role of the association of NF-κB with HATs and HDACs remains to be elucidated.
NF-κB Activation in Human Breast Cancers
Aberrant activation of NF-κB is associated with various human cancers (89–91), which indicates that this transcription factor may play crucial roles in neoplastic cell growth. The constitutive NF-κB activation in some cancers is believed to be caused by genetic alterations in genes encoding NF-κB components, IκBS, or upstream regulators of NF-κB, e.g., deregulated expression or activation of upstream kinases. Activated NF-κB was detected in cultured cells from ER-negative breast cancer cells (92). Also, Debajit et al. found activated NF-κB predominantly in the ER-negative and ER-negative/HER-2 positive compared to ER-positive breast cancers (93), suggesting the importance of NF-κB signaling in specific classes of breast cancers. Selective inhibition of NF-κB in an ER-negative and HER-2 positive human breast tumor cell line (SKBr3) blocked heregulin-induced proliferation and resulted in apoptosis (93). Lack of NF-κB activation in ER-positive breast cancer cells is consistent with the observation that stimulation of the ER-positive pathway blocks NF-κB activation (94). A recent publication by Akane et al. showed that a novel IKKβ inhibitor IMD-0354 prevented the proliferation of ER-positive (MCF-7) and -negative (MDA-MB231 and HMC1-8) breast cancer cells (95). This result of NF-κB activation in ER-positive MCF-7 cells is contrasted with that reported by Debajit et al. Overall, the constitutive activation of NF-κB in breast tumor cells indicates an active role of this transcription factor in signaling the response to anticancer therapy. The relationship between NF-κB and HER-2-mediated breast cancer resistance is an important issue to be investigated.
HER-2 and NF-κB Connection in Signaling Tumor Resistance
It has been well-documented that overexpression of HER-2 increases cell proliferation and survival (23) and causes NF-κB activation (42). The PI3K/Akt pathway is involved in HER-2 mediated NF-κB activation (44). Both IKK-dependent and independent pathways contribute to the deregulation of NF-κB in breast cancers. The IKK-independent pathway involves calpain-mediated IκBα degradation (44). This pathway also requires PI3K and its downstream kinase Akt, which is subject to inhibition by the tumor suppressor phosphatase PTEN. In another study, Akt-mediated NF-κB activation blocked apoptosis in HER-2-expressing cells (96). It is therefore highly possible that PI3K/Akt pathway mediated by HER-2 expression is involved in radio- or chemo-induced NF-κB activation that regulates downstream effector genes required for HER-2-mediated tumor resistance against therapeutic regimens. To support this notion, the following section describes a tight correlation between Akt and HER-2-mediated NF-κB activation in radiation-treated HER-2 overexpressing cells (40, 44).
Although HER-2 has been shown to initiate signaling responsible for tumor response to chemo- and radiotherapy (97), the key elements causing HER-2-mediated radio- or chemo-resistance have not been well established. We found that NF-κB and its radioresistant effector genes are activated with HER-2 overexpression (40). Overexpression of HER-2 also enhanced NF-κB activation and stable transfection of mutant IκB (MCF-7/Her-2/mIκB) or treatment with herceptin, inhibited NF-κB activation and radiosensitized MCF-7/HER-2 cells (40). Basal and IR-induced Akt was found to be activated in MCF-7/HER-2 cells and inhibited by herceptin. To determine the downstream signaling effectors, cyclin B1, cyclin D1, Bcl-2 and Bcl-XL, but not the pro-apoptotic Bad and Bax, were found to be up-regulated in MCF-7/HER-2 cells with a striking enhancement in expression of Bcl-2 and Bcl-XL. IR further induced the expression of cyclin B1 and cyclin D1 that can be reduced by herceptin treatment. These results thus suggest that overexpression of HER-2 is able to enhance NF-κB in response to IR, and a specific pro-survival network downstream of NF-κB is required for HER-2-mediated radioresistance (40). However, although our data showed an agreement between the decreased apoptotic cells and increased clonogenic survival, we cannot exclude the possibility that apoptosis-independent cell death (e.g., necrosis and mitosis-linked cell death) may also contribute to the radioresistant phenotype observed in MCF-7/Her-2 cells. Clinically, expression of cyclin B1 has been identified as an important factor when evaluating radioresistance of patients with squamous cell carcinoma (98, 99) and those with regional recurrence of head and neck tumors treated by radiotherapy (100). Together with HER-2-induced activation of Bcl-2 and Bcl-XL, our results demonstrate a tight regulation of cyclin B1 with the prosurvival network regulated by NF-κB in HER-2-overexpressing cells. Using IR treated human breast carcinoma MCF-7 cells (41) and human keratinocytes (101), we have reported a temporary radioresistance phenotype associated with activation of both NF-κB and mitochodrial antioxidant MnSOD. Overexpression of MnSOD has been shown to protect cells from mitochondria-initiated apoptosis (102, 103). Since NF-κB is able to induce MnSOD expression by TNF-α and IL-β (104) and NF-κB binding sites have been located in the regulatory regions of the SOD2 gene that encodes MnSOD (104–106), NF-κB-mediated MnSOD activation might function as a major downstream effector of the HER-2 pathway. Alteration of mitochondrial apoptosis in HER-2-overexpressing cells is worth being investigated.
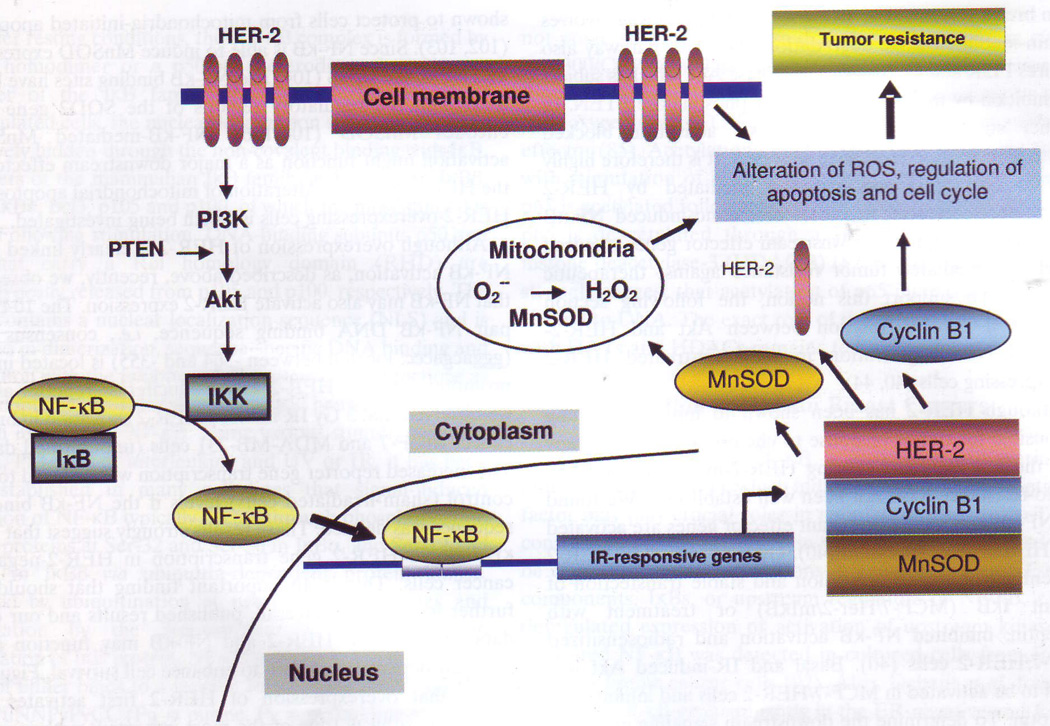
Although overexpression of HER-2 is clearly linked with NF-κB activation, as described above, recently, we observed that NF-κB may also activate HER-2 expression. The 10-base-pair NF-κB DNA binding sequence, i.e., consensus site (gggacgaccc; located between -364 and -355) is located in the promoter region of HER-2. Using a luciferase reporter assay, we observed that 5 Gy IR induced HER-2 activation in breast cancer MCF-7 and MDA-MB-231 cells (unpublished data). The increased reporter gene transcription was reduced to the control (sham-irradiated cells) levels if the NF-κB binding sequence was deleted. These results strongly suggest that NF-κB induces HER-2 gene transcription in HER-2-negative cancer cells. This is an important finding that should be further investigated. Overall, published results and our own data indicate that HER-2 and NF-κB may function in a mutually dependent pattern to enhance cell survival. Figure 1 shows that overexpression of HER-2 first activates the PI3K/Akt pathway (one of the most important pathways for cell growth, proliferation, and survival) for NF-κB activation that, in turn, up-regulates a group of pro-survival genes including mitochondrial antioxidant enzyme MnSOD (involved in mitochondrial function), cyclin B1 (invovled in cell cycle regulation and apoptosis) as well as HER-2 itself, leading to the radio- and/or chemo-resistant phenotype in HER-2 positive cancer cells.
Figure 1.
Signaling network of HER-2-mediated NF-κB activation in radio- andlor chemo-resistant breast cancer cells. overexpression of HER-2 firstly activates pI3K/Akt pathway that, in turn, activates NF-κB. Activated NF-κB then induces a group of pro-survival genes including cyclin B1, mitochondrial antioxidant MnSOD and importantly, the HER-2 gene itself, leading to the radio-and/or chemo-resistant phenotype in HER-2-positive cancer cells.
Conclusion
Clinically, humanized HER-2 antibody herceptin has been used over the past 7 years alone or together with chemotherapeutic drugs as an important anti-cancer agent for the treatment of HER-2-positive breast cancer patients. However, the majority of patients who initially respond to herceptin generally acquire resistance within 1 year. Therefore, further elucidation of HER-2-mediated therapy-resistance will allow for the design of more efficient targets to enhance herceptin anticancer efficiency. Data presented in this review strongly suggest that HER-2-activated NF-κB plays a critical role in HER-2-mediated tumor resistance. Importantly, NF-κB may, in turn, activates HER-2 gene expression causing radio-and/or chemo-resistance in HER-2-negative cancer cells. Therefore, NF-κB and HER-2 appear to be mutually dependent in signaling pro-survival in breast cancer cells. Thus, combination of NF-κB inhibitor and herceptin may promise a novel therapeutic strategy for breast cancer pateints.
Acknowledgements
We thank our collaborators, postdoctoral fellows and graduate students at the School of Health Sciences, Purdue University for their perceptive discussion and support. This work was partially supported by the National Institutes of Health (RO1 CA101990) and by the Office of Science (BER), DOE low-dose radiation research program (Grant No. DE-FG02-05ER63945) to JJ Li.
Abbreviations
- ER
estrogen receptor
- EGFR
epidermal growth factor receptor
- HAT
histone acetyltransferase
- HDAC
histone deacetylase
- HER-2
human epidermal growth factor receptor-2
- IκB
inhibitor kappa B
- IKK
inhibitor kappa B kinase
- IR
ionizing radiation
- MAPK
mitogen-activated protein kinase
- MnSOD
manganese-containing superoxide dismutase
- NF-κB
nuclear factor-kappaB
- PI3K
phosphatidyl inositol 3 kinase
- PTEN
phosphatase and tensin homolog deleted on chromosome ten
References
- 1.Soomro S, Shousha S, Taylor P, Shepard HM, Feldmann M. c-erbB-2 expression in different histological types of invasive breast carcinoma. J Clin Pathol. 1991;44:211–214. doi: 10.1136/jcp.44.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olayioye MA. Update on HER-2 as a target for cancer therapy: intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res. 2001;3:385–389. doi: 10.1186/bcr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, Weinberg RA. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- 5.Way TD, Lin JK. Role of HER2/HER3 co-receptor in breast carcinogenesis. Fut Oncol. 2005;1:841–849. doi: 10.2217/14796694.1.6.841. [DOI] [PubMed] [Google Scholar]
- 6.Hellstrom I, Goodman G, Pullman J, Yang Y, Hellstrom KE. Overexpression of HER-2 in ovarian carcinomas. Cancer Res. 2001;61:2420–2423. [PubMed] [Google Scholar]
- 7.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. Embo J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klapper LN, Glathe S, Vaisman N, Hlmes NE, Andrews GC, Sela M, Yarden Y. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci USA. 1999;96:4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hynes NE, Stern DF. The biolory of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 10.Hazan R, Margolis B, Dombalagian M, Ullrich A, Zilberstein A, Schlessinger J. Identification of autophosphorylation sites of HER2/neu. Cell Growth Differ. 7990;1:3–7. [PubMed] [Google Scholar]
- 11.Reese DM, Slamon DJ. HER-2/neu signal transduction in human breast and ovarian cancer. Stem Cells. 1997;15:1–8. doi: 10.1002/stem.150001. [DOI] [PubMed] [Google Scholar]
- 12.Rubin I, Yarden Y. The basic biology of HER2. Ann Oncol. 2001;12(Suppl 1):S3–S8. doi: 10.1093/annonc/12.suppl_1.s3. [DOI] [PubMed] [Google Scholar]
- 13.Brand FX, Ravanel N, Gauchez AS, Pasquier D, Payan R, Fagret D, Mousseau M. Prospect for anti-her2 receptor therapv in breast cancer. Anticancer Res. 2006;26:715–722. [PubMed] [Google Scholar]
- 14.Giri DK, Ali-Seyed M, Li LY, Lee DF, Ling P, Bartholomeusz G, Wang SC, Hung MC. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol. 2005;25:11005–11018. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, Ali-Seyed M, Lee DF, Bartholomeusz G, Ou-Yang F, Giri DK, Hung MC. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6:251–261. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:777–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 17.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 18.Yu D, Hung MC. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene. 2000;19:6715–6721. doi: 10.1038/sj.onc.1203972. [DOI] [PubMed] [Google Scholar]
- 19.Hou L, Shi D, Tu SM, Zhang HZ, Hung MC, Ling D. Oral cancer progression and c-erbB-2/neu proto-oncogene expresslon. Cancer Lett. 1992;65:215–220. doi: 10.1016/0304-3835(92)90234-m. [DOI] [PubMed] [Google Scholar]
- 20.Schneider PM, Hung MC, Chiocca SM, Manning J, Zhao XY, Fang K, Roth JA. Differential expression of the c-erbB-2 gene in human small cell and non-small cell lung cancer. Cancer Res. 1989;49:4968–4971. [PubMed] [Google Scholar]
- 21.Weiner DB, Nordberg J, Robinson R, Nowell PC, Gazdar A, Greene MI, Williams WV, Cohen JA, Kern JA. Expression of the neu gene-encoded protein (P185neu) in human non-small cell carcinomas of the lung. Cancer Res. 1990;50:421–425. [PubMed] [Google Scholar]
- 22.Yokota J, Yamamoto T, Miyajima N, Toyoshima K, Nomura N, Sakamoto H, Yoshida T, Terada M, Sugimura T. Genetic alterations of the c-erbB-2 oncogene occur frequently in tubular adenocarcinoma of the stomach and are often accompanied by amplification of the v-erbA homologue. Oncogene. 1988;2:283–287. [PubMed] [Google Scholar]
- 23.Kurokawa H, Arteaga CL. Inhibition of erbB receptor (HER) tyrosine kinases as a strategy to abrogate antiestrogen resistance in human breast cancer. Clin Cancer Res. 2001;7:4436s–4442s. discussion 4411s-4412s. [PubMed] [Google Scholar]
- 24.Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS., Jr Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene. 2000;19:1123–1131. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- 25.Flagg EW, Coates RJ, Calle EE, Potischman N, Thun MJ. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort Food Frequency Questionnaire. Epidemiology. 2000;11:462–468. doi: 10.1097/00001648-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Carraway KL, 3rd, Weber JL, Unger MJ, Ledesma J, Yu N, Gassmann M, Lai C. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature. 1997;387:512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- 27.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 28.Hicks DG, Tubbs RR. Assessment of the HER2 status in breast cancer by fluorescence in situ hybridization: a technical review with interpretive guidelines. Hum Pathol. 2005;36:250–261. doi: 10.1016/j.humpath.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Haffty BG, Brown F, Carter D, Flynn S. Evaluation of HER-2 neu oncoprotein expression as a prognostic indicator of local recurrence in conservatively treated breast cancer: a case-control study. Int J Radiat Oncol Biol Phys. 1996;35:751–757. doi: 10.1016/0360-3016(96)00150-2. [DOI] [PubMed] [Google Scholar]
- 30.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 31.Yu D, Hamada J, Zhang H, Nicolson GL, Hung MC. Mechanisms of c-erbB2/neu oncogene-induced metastasis and repression of metastatic properties by adenovirus 5 ElA gene products. Oncogene. 1992;7:2263–2270. [PubMed] [Google Scholar]
- 32.Tanner M, Kapanen AI, Junttila T, Raheem O, Grenman S, Elo J, Elenius K, Isola J. Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther. 2004;3:1585–1592. [PubMed] [Google Scholar]
- 33.Nahta R, Yuan LX, Zhang B, Kobayashi R, Esteva FJP. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 34.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 35.Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med. 1995;333:1444–1455. doi: 10.1056/NEJM199511303332202. [DOI] [PubMed] [Google Scholar]
- 36.Lichter AS, Lawrence TS. Recent advances in radiation oncology. N Engl J Med. 1995;332:371–379. doi: 10.1056/NEJM199502093320607. [DOI] [PubMed] [Google Scholar]
- 37.Pietras RJ, Poen JC, Gallardo D, Wongvipat PN, Lee HJ, Slamon DJ. Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res. 1999;59:1347–1355. [PubMed] [Google Scholar]
- 38.Hancock MC, Langton BC, Chan T, Toy P, Monahan JJ, Mischak RP, Shawver LK. A monoclonal antibody against the c-erbB-2 protein enhances the cytotoxicity of cis-diamminedichloroplatinum against human breast and ovarian tumor cell lines. Cancer Res. 1991;51:4575–4580. [PubMed] [Google Scholar]
- 39.Fernet M, Hall J. Genetic biomarkers of therapeutic radiation sensitivity. DNA Repair (Amst) 2004;3:1237–1243. doi: 10.1016/j.dnarep.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 40.Guo G, Wang T, Gao Q, Tamae D, Wong P, Chen T, Chen WC, Shively JE, Wong JY, Li JJ. Expression of ErbB2 enhances radiation-induced NF-kappaB activation. Oncogene. 2004;23:535–545. doi: 10.1038/sj.onc.1207149. [DOI] [PubMed] [Google Scholar]
- 41.Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Longmate JA, Huang TT, Spitz DR, Oberley LW, Li JJ. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol. 2003;23:2362–2378. doi: 10.1128/MCB.23.7.2362-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galang CK, Garcia-Ramirez J, Solski PA, Westwick JK, Der CJ, Neznanov NN, Oshima RG, Hauser CA. Oncogenic Neu/ErbB-2 increases ets, AP-1, and NF-kappaB-dependent gene expression, and inhibiting ets activation blocks Neu-mediated cellular transformation. J Biol Chem. 1996;271:7992–7998. doi: 10.1074/jbc.271.14.7992. [DOI] [PubMed] [Google Scholar]
- 43.Raziuddin A, Court D, Sarkar FH, Liu YL, Kung H, Raziuddin RA. c-erbB-2 promoter-specific nuclear matrix protein from human breast tumor tissues mediates NF-kappaB DNA binding activity. J Biol Chem. 1997;272:15715–15720. doi: 10.1074/jbc.272.25.15715. [DOI] [PubMed] [Google Scholar]
- 44.Pianetti S, Arsura M, Romieu-Mourez R, Coffey RJ, Sonenshein GE. Her-2/neu overexpression induces NF-kappaB via a PI3-kinase/Akt pathway involving calpain-mediated degradation of IkappaB-alpha that can be inhibited by the tumor suppressor PTEN. Oncogene. 2001;20:1287–1299. doi: 10.1038/sj.onc.1204257. [DOI] [PubMed] [Google Scholar]
- 45.Giai M, Biglia N, Biglia P. Chemoresistance in breast tumors. Eur J Gynaecol Oncol. 1991;12:359–373. [PubMed] [Google Scholar]
- 46.el-Deiry WS. Role of oncogenes in resistance and killing by cancer therapeutic agents. Curr Opin Oncol. 1997;9:79–87. doi: 10.1097/00001622-199701000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Tsai CM, Yu D, Chang KT, Wu LH, Perng RP, Ibrahim NK, Hung MC. Enhanced chemoresistance by elevation of p185neu levels in HER-2/neu-transfected human lung cancer cells. J Natl Cancer Inst. 1995;87:682–684. doi: 10.1093/jnci/87.9.682. [DOI] [PubMed] [Google Scholar]
- 48.Tsai CM, Levitzki A, Wu LH, Chang KT, Cheng CC, Gazit A, Perng RP. Enhancement of chemosensitivity by tyrphostin AG825 in high-p185(neu) expressing non-small cell lung cancer cells. Cancer Res. 1996;56:1068–1074. [PubMed] [Google Scholar]
- 49.Gusterson BA, Gelber RD, Goldhirsch A, Price KN, Save-Soderborgh J, Anbazhagan R, Styles J, Rudenstam CM, Golouh R, Reed R, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992;10:1049–1056. doi: 10.1200/JCO.1992.10.7.1049. [DOI] [PubMed] [Google Scholar]
- 50.Fehm T, Maimonis P, Katalinic A, Jager WH. The prognostic significance of c-erbB-2 serum protein in metastatic breast cancer. Oncology. 1998;55:33–38. doi: 10.1159/000011832. [DOI] [PubMed] [Google Scholar]
- 51.Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, Castiglioni F, Villani L, Magalotti C, Gibelli N, Oliviero B, Ballardini B, Da Prada G, Zambelli A, Costa A. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 52.Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006;232:123–138. doi: 10.1016/j.canlet.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 53.Soderlund K, Perez-Tenorio G, Stal O. Activation of the phosphatidylinositol 3-kinase/Akt pathway prevents radiation-induced apoptosis in breast cancer cells. Int J Oncol. 2005;26:25–32. [PubMed] [Google Scholar]
- 54.Gewirtz DA. Growth arrest and cell death in the breast tumor cell in response to ionizing radiation and chemotherapeutic agents which induce DNA damage. Breast Cancer Res Treat. 2000;62:223–235. doi: 10.1023/a:1006414422919. [DOI] [PubMed] [Google Scholar]
- 55.Wollman R, Yahalom J, Maxy R, Pinto J, Fuks Z. Effect of epidermal growth factor on the growth and radiation sensitivity of human breast cancer cells in vitro. Int J Radiat Oncol Biol Phys. 1994;30:91–98. doi: 10.1016/0360-3016(94)90523-1. [DOI] [PubMed] [Google Scholar]
- 56.Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther. 2003;2:1113–1120. [PubMed] [Google Scholar]
- 57.Schmidt-Ullrich RK, Mikkelsen RB, Dent P, Todd DG, Valerie K, Kavanagh BD, Contessa JN, Rorrer WK, Chen PB. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15:1191–1197. doi: 10.1038/sj.onc.1201275. [DOI] [PubMed] [Google Scholar]
- 58.Kavanagh BD, Dent P, Schmidt-Ullrich RK, Chen P, Mikkelsen RB. Calcium-dependent stimulation of mitogen-activated protein kinase activity in A431 cells by low doses of ionizing radiation. Radiat Res. 1998;149:579–587. [PubMed] [Google Scholar]
- 59.Rao GS, Murray S, Ethier SP. Radiosensitization of human breast cancer cells by a novel ErbB family receptor tyrosine kinase inhibitor. I. nt J Radiat Oncol Biol Phys. 2000;48:1519–1528. doi: 10.1016/s0360-3016(00)01358-4. [DOI] [PubMed] [Google Scholar]
- 60.Uno M, Otsuki T, Kurebayashi J, Sakaguchi H, Isozaki Y, Ueki A, Yata K, Fujii T, Hiratsuka J, Akisada T, Harada T, Imajo Y. Anti-HER2-antibody enhances irradiation-induced growth inhi-bition in head and neck carcinoma. Int J Cancer. 2001;94:474–479. doi: 10.1002/ijc.1493. [DOI] [PubMed] [Google Scholar]
- 61.Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther. 2003;2:7113–1120. [PubMed] [Google Scholar]
- 62.Price BD, Youmell MB. The phosphatidylinositol 3-kinase inhibitor wortmannin sensitizes murine fibroblasts and human tumor cells to radiation and blocks induction of p53 following DNA damage. Cancer Res. 1996;56:246–250. [PubMed] [Google Scholar]
- 63.Carter S, Auer KL, Reardon DB, Birrer M, Fisher PB, Valerie K, Schmidt-Ullrich R, Mikkelsen R, Dent P. Inhibition of the mitogen activated protein (MAP) kinase cascade potentiates cell killing by low dose ionizing radiation in A431 human squamous carcinoma cells. Oncogene. 1998;16:2787–2796. doi: 10.1038/sj.onc.1201802. [DOI] [PubMed] [Google Scholar]
- 64.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein NF-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 65.Nabel GJ, Verma IM. Proposed NF-kappa B/I kappa B family nomenclature. Genes Dev. 1993;7:2063. doi: 10.1101/gad.7.11.2063. [DOI] [PubMed] [Google Scholar]
- 66.Kuriyan J, Thanos D. Structure of the NF-kappa B transcription factor: a holistic interaction with DNA. Structure. 1995;3:135–141. doi: 10.1016/s0969-2126(01)00143-5. [DOI] [PubMed] [Google Scholar]
- 67.Ghosh G, van Duyne G, Ghosh S, Sigler PB. Structure of NF-kappa B p50 homodimer bound to a kappa B site. Nature. 1995;373:303–310. doi: 10.1038/373303a0. [DOI] [PubMed] [Google Scholar]
- 68.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracycline in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 69.Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 70.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 71.Piette J, Piret B, Bonizzi G, Schoonbroodt S, Merville MP, Legrand-Poels S, Bours V. Multiple redox regulation in NF-kappaB transcription factor activation. Biol Chem. 1997;378:1237–1245. [PubMed] [Google Scholar]
- 72.Chen JY, Penco S, Ostrowski J, Balaguer P, Pons M, Stanett JE, Reczek P, Chambon P, Gronemeyer H. RAR-specific agonist/antagonists which dissociate transactivation and AP1 transrepression inhibit anchorage-independent cell proliferation. EMBO J. 1995;14:1187–1197. doi: 10.1002/j.1460-2075.1995.tb07102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Traenckner EB, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human I kappa B-alpha on serines 32 and 36 controls I kappa B-alpha proteolysis and NF-kappa B activation in response to diverse stimuli. Embo J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baeuerle PA, Rupec RA, Pahl HL. Reactive oxygen intermediates as second messengers of a general pathogen response. Pathol Biol (Paris) 1996;44:29–35. [PubMed] [Google Scholar]
- 75.Ben-Neriah Y. Regulatory functions of ubiquitination in the immune system. Nat Immunol. 2002;3:20–26. doi: 10.1038/ni0102-20. [DOI] [PubMed] [Google Scholar]
- 76.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 77.Gerritsen ME, Williams AJ, Neish AS, Moore S, Shi Y, Collins T. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Na SY, Lee SK, Han SJ, Choi HS, Im SY, Lee JW. Steroid receptor coactivator-1 interacts with the p50 subunit and coactivates nuclear factor kappaB-mediated transactivations. J Biol Chem. 1998;273:10831–10834. doi: 10.1074/jbc.273.18.10831. [DOI] [PubMed] [Google Scholar]
- 79.Sheppard KA, Rose DW, Haque ZK, Kurokawa R, McInerney E, Westin S, Thanos D, Rosenfeld MG, Glass CK, Collins T. Transcriptional activation by NF-kappaB requires multiple coactivators. Mol Cell Biol. 1999;19:6367–6378. doi: 10.1128/mcb.19.9.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quivy V, Adam E, Collette Y, Demonte D, Chariot A, Vanhulle C, Berkhout B, Castellano R, de Launoit Y, Burny A, Piette J, Bours V, Van Lint C. Synergistic activation of human immunodeficiency virus type 1 promoter activity by NF-kappaB and inhibitors of deacetylases: potential perspectives for the development of therapeutic strategies. J Virol. 2002;76:11091–11103. doi: 10.1128/JVI.76.21.11091-11103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adam E, Quivy V, Bex F, Chariot A, Collette Y, Vanhulle C, Schoonbroodt S, Goffin V, Nguyen TL, Gloire G, Carrard G, Friguet B, De Launoit Y, Burny A, Bours V, Piette J, Van Lint C. Potentiation of tumor necrosis factor-induced NF-kappa B activation by deacetylase inhibitors is associated with a delayed cytoplasmic reappearance of I kappa B alpha. Mol Cell Biol. 2003;23:6200–6209. doi: 10.1128/MCB.23.17.6200-6209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen BC, Lin WW. PKC- and ERK-dependent activation of I kappa B kinase by lipopolysaccharide in macrophages: enhancement by PZY receptor-mediated CaMK activation. Br J Pharmacol. 2007;134:1055–1065. doi: 10.1038/sj.bjp.0704334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henrie MS, Kurimasa A, Burma S, Menissier-de Murcia J, de Murcia G, Li GC, Chen DJ. Lethality in PARP-1/Ku80 double mutant mice reveals physiological synergy during early embryogenesis. DNA Repair (Amst) 2003;2:151–158. doi: 10.1016/s1568-7864(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 84.Furia B, Deng L, Wu K, Baylor S, Kehn K, Li H, Donnelly R, Coleman T, Kashanchi F. Enhancement of nuclear factor-kappa B acetylation by coactivator p300 and HIV-1 Tat proteins. J Biol Chem. 2002;277:4973–4980. doi: 10.1074/jbc.M107848200. [DOI] [PubMed] [Google Scholar]
- 85.Deng L, Yang J, Zhao XR, Deng XY, Zeng L, Gu HH, Tang M, Cao Y. Cells in G2/M phase increased in human nasopharyngeal carcinoma cell line by EBV-LMP1 through activation of NF-kappaB and AP-1. Cell Res. 2003;13:187–194. doi: 10.1038/sj.cr.7290163. [DOI] [PubMed] [Google Scholar]
- 86.Chen F, Ding M, Castranova V, Shi X. Carcinogenic metals and NF-kappaB activation. Mol Cell Biochem. 2001;222:159–171. [PubMed] [Google Scholar]
- 87.Kiernan R, Bres V, Ng RW, Coudart MP, E1 Messaoudi S, Sardet C, Jin DY, Emiliani S, Benkirane M. Post-activation turn-off of NF-kappa B-dependent transcription is regulated by acetylation of p65. J Biol Chem. 2003;278:2758–2766. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- 88.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. Embo J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ, Jr, Sledge GW., Jr Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol. 1997;17:3629–3639. doi: 10.1128/mcb.17.7.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 91.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 92.Nakshatri H, Goulet RJ., Jr NF-kappaB and breast cancer. Curr Probl Cancer. 2002;26:282–309. doi: 10.1067/mcn.2002.129977. [DOI] [PubMed] [Google Scholar]
- 93.Biswas DK, Shi Q, Baily S, Strickland I, Ghosh S, Pardee AB, Iglehart JD. NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA. 2004;101:10137–10142. doi: 10.1073/pnas.0403621101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu H, Lee ES, Gajdos C, Pearce ST, Chen B, Osipo C, Loweth J, McKian K, De Los Reyes A, Wing L, Jordan VC. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95:1586–1597. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- 95.Tanaka A, Muto S, Konno M, Itai A, Matsuda H. A new IkappaB kinase beta inhibitor prevents human breast cancer progression through negative regulation of cell cycle transition. Cancer Res. 2006;66:419–426. doi: 10.1158/0008-5472.CAN-05-0741. [DOI] [PubMed] [Google Scholar]
- 96.Yang HY, Zhou BP, Hung MC, Lee MH. Oncogenic signals of HER-2/neu in regulating the stability of the cyclin-dependent kinase inhibitor p27. J Biol Chem. 2000;275:24735–24739. doi: 10.1074/jbc.C000147200. [DOI] [PubMed] [Google Scholar]
- 97.Summers RW, Maves BV, Reeves RD, Arjes LJ, Oberley LW. Irradiation increases superoxide dismutase in rat intestinal smooth muscle. Free Radic Biol Med. 1989;6:261–270. doi: 10.1016/0891-5849(89)90053-1. [DOI] [PubMed] [Google Scholar]
- 98.Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, Mao L. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000;60:4000–4004. [PubMed] [Google Scholar]
- 99.Hassan KA, El-Naggar AK, Soria JC, Liu D, Hong WK, Mao L. Clinical significance of cyclin B1 protein expression in squamous cell carcinoma of the tongue. Clin. Cancer Res. 2001;7:2458–2462. [PubMed] [Google Scholar]
- 100.Hassan KA, Ang KK, El-Naggar AK, Story MD, Lee JI, Liu D, Hong WK, Mao L. Cyclin B1 overexpression and resistance to radiotherapy in head and neck squamous cell carcinoma. Cancer Res. 2002;62:6414–6477. [PubMed] [Google Scholar]
- 101.Chen X, Shen B, Xia L, Khaletzkiy A, Chu D, Wong JY, Li JJ. Activation of nuclear factor kappaB in radioresistance of TP53-inactive human keratinocvtes. Cancer Res. 2002;62:l213–1221. [PubMed] [Google Scholar]
- 102.Wong GH, Elwell JH, Oberley LW, Goeddel DV. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989;58:923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- 103.Siemankowski LM, Morreale J, Briehl MM. Antioxidant defenses in the TNF-treated MCF-7 cells: selective increase in MnSOD. Free Radic Biol Med. 1999;26:979–924. doi: 10.1016/s0891-5849(98)00273-1. [DOI] [PubMed] [Google Scholar]
- 104.Xu Y, Kiningham KK, Devalaraja MN, Yeh CC, Majima H, Kasarskis EJ, St Clair DK. An intronic NF-kappaB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-alpha and interleukin-1beta. DNA Cell Biol. 1999;18:709–722. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]
- 105.Wan XS, Devalaraja MN, St Clair DK. Molecular structure and organization of the human manganese superoxide dismutase gene. D. NA Cell Biol. 7994;13:1127–1136. doi: 10.1089/dna.1994.13.1127. [DOI] [PubMed] [Google Scholar]
- 106.Duttaroy A, Parkes T, Emtage P, Kirby K, Boulianne GL, Wang X, Hilliker AJ, Phillips JP. The manganese superoxide dismutase gene of Drosophila: structure, expression, and evidence for regulation by MAP kinase. DNA Cell Biol. 1997;16:391–399. doi: 10.1089/dna.1997.16.391. [DOI] [PubMed] [Google Scholar]