Abstract
Purpose
This study evaluates the proapoptotic function of integrin β3 in human hepatocellular carcinoma (HCC).
Experimental Design
The expression of integrin β3 in 67 HCC specimens paired with corresponding neighboring nontumorous tissue was studied by quantitative real-time PCR and Western blot. The proapoptotic function of integrin β3 in SMMC-7721 human hepatoma cells overexpressing ITGB3 (gene coding integrin β3) was determined through colony formation, serum starvation, and anoikis assay.
Results
Compared with neighboring pathologically normal liver tissue, ~60% of the HCC specimens showed a significantdown-regulated level of integrin β3 expression. Transient expression of integrin β3 in SMMC-7721resulted in an enhanced level of apoptosis and suppression of colony formation. Cell growth inhibition on serum/ligand deprivation and incidences of anoikis were remarkably increased in SMMC-7721with stable expression of integrin β3 in comparison with vector control transfectants. In addition, expression of fibrinogen and vitronectin, two native ligands for integrin αvβ3 in liver, was inhibited, which was correlated with the decreased integrin β3 expression. Replenishing these ligands to the starved SMMC-7721 stable transfectants effectively restored the proapoptotic function of integrin β3.
Conclusions
Down-regulation of integrin β3 and its ligands in liver is related to the aggressive growth of HCC. Thus, reconstitution of integrin β3 in HCC may be a potential therapeutic approach to inhibit aggressive growth of liver cancer.
Integrins comprise a family of cell surface receptors mediating cell-matrix and cell-cell adhesion. Due to various α/β subunit combinations and alternative splicing, more than 20 integrin heterodimers have been described and each exhibits ligand-binding specificity and distinct tissue expression pattern (1). Most integrin ligands are shown to be extracellular matrix (ECM) components, as well as their degraded segments such as fibronectin, laminin, collagen, and endostatin (2). Integrins usually recognize their ligands through the consensus RGD (Arg-Gly-Asp) motif (3). Multiple ligands can bind the same integrin and vice versa, making a multifaceted function of integrin in regulating cellular functions. Whereas ligands such as collagen or laminin are shown to promote endothelial cell adhesion and survival, native antiangiogenesis factors such as endostatin and tumostatin inhibit cell proliferation and migration through binding to integrins (4–6). The expression pattern and exact function of integrins in regulation of tumor growth and invasion remain to be elucidated.
It is well documented that integrins play an essential role in cell adhesion and movement. Once an integrin is activated by ligand occupancy, it recruits downstream signaling proteins and cytoskeleton components such as focal adhesion kinase and talin to organize focal adhesion complex and stress fibers in the cell cortex (7, 8). Integrins on membrane cluster through homotypic or heterotypic aggregation at the site of cell-matrix contact to accelerate integrin-mediated cell movement (9). However, evidence is emerging to reveal that integrins can regulate more aspects of cell behavior. For example, integrins are shown to interact with epidermal growth factor receptor and platelet-derived growth factor receptor to modulate their intracellular signals for cell growth (10, 11). Disruption of cell-ECM interaction with mediated by specific integrin results in a specific apoptotic cell death, termed as anoikis (Greek word for homelessness; refs. 12, 13). In addition, overexpression of integrins protects cells from apoptosis induced by chemotherapeutic agents and serum withdrawal through up-regulation of antiapoptotic proteins such as bcl-2 (14–16). Although considerable discrepancies exist about integrin functions, the exact biological function of integrins seems to be integrin type and tissue/cell type specific, ensuring strict control of cell events and physiologic processes, which is thus far unclear in tumor cells.
Many studies of integrins are carried out on normal cells including endothelial cells and fibroblast cells where integrins are universally expressed. However, recent studies show varied expression levels of integrins in different tumors. However, mechanisms of tumor-associated integrins in regulating tumor growth and progression still remain unclear. In nude mice, decreased integrin α5β1 in several tumor types was reportedly correlated with its inhibitory function in tumorigenesis (17 – 20), whereas some melanoma and prostatic cells transfected with integrin αvβ3 showed a higher motility and an elevated tumor metastatic property (21–23). It is also noteworthy that microinjection of an integrin αvβ3–blocking antibody was able to suppress breast cancer progression (24). Moreover, the function of integrin αvβ3 in increasing tumor invasion and metastasis is consistent with its high expression level in melanoma and breast carcinoma (25, 26). However, current knowledge of expression and function of integrin β3 in hepatocellular carcinoma (HCC) is very limited. Studies in the view of tumor heterogeneity and integrin specificity are required to unravel the exact function of integrins in signaling the malignant phenotype of tumor cells.
Using paired tumor specimens versus normal tissues from HCC patients and stable integrin β3-expressing HCC cell lines, here we present the first evidence that down-regulated integrin β3 in HCC contributes to tumor aggressive growth. Compared with the counterpart normal tissues, integrin β3 is remarkably down-regulated in ~60% of HCC specimens in both mRNA and protein expression. Overexpression of integrin β3 induces apoptosis and inhibits clonogenic survival in three hepatoma cell lines. Although stable transfectants show low integrin expression level and similar growth ability as control cells under normal culture condition, these transfectants exhibit significant growth inhibition when the serum/ligands are withdrawn and elevated anoikis. In addition, the major native ligands for integrin β3 in liver (fibrinogen and vitronectin) are down-regulated in the same HCC specimens, and addition of such ligands in the medium rescues integrin-induced apoptotic cell death. These results show a novel proapoptotic function of integrin β3 in human hepatoma cells. Thus, integrin β3 may be a potential therapeutic target for the treatment of human liver cancer.
Materials and Methods
Tumor specimens
Fresh surgical specimens of HCC, including tumor tissues and the neighboring pathologically nontumorous liver tissues, were obtained from liver cancer patients at Zhongshan Hospital, Shanghai, China. All the samples were immediately frozen in liquid nitrogen after surgery and then later stored at −80°C before further analysis.
Quantitative real-time PCR
Total RNA was extracted from tissues or cultured cells using Trizol reagent (Invitrogen) and 1 to 2 μg of RNA were applied for reverse transcription using an oligo(dT) (Invitrogen) primer and reverse transcriptase (Invitrogen). Real-time PCR analysis was carried out using SYBR Green Supermix kit (Takara) with the iCycler detection system (Bio-Rad). Properly diluted cDNA was used in a 20-μL real-time PCR reaction in triplicate for each gene. For ITGB3, cycle parameters were 95°C for 5-min hot start and 45 cycles of 95°C for 5 s and 60°C for 30 s. For fibrinogen γ-chain (FGG) and vitronectin (VTN), cycle parameters were 95°C for 5-min hot start and 45 cycles of 95°C for 5 s, 57°C for 10 s, and 72°C for 20 s. Blank controls with no cDNA templates were done to rule out contamination. The specificity of PCR product was confirmed by melting curve analysis and gel electrophoresis. All the gene expression levels were normalized to that of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Primers for ITGB3 were forward, 5′-GGACACAGCCAACAACCCAC-3′, and reverse, 5′-AGGAGGCATTCTGGGACAAAG-3′. Primers for GAPDH were forward, 5′-GAAGGTGAAGGTCGGAGTC-3′, and reverse, 5′-GAAGATGGTGATGGGATTTC-3′. Vitronectin and fibrinogen γ-chain mRNA expression levels were examined using primers previously described (27, 28).
Western blot
Protein samples were separated by SDS-PAGE and then transferred onto nitrocellulose membranes. After blocking, the membranes were incubated with specific antibodies against different proteins at 4°C overnight, followed by incubation with horseradish peroxidase – conjugated secondary antibody. Immunoreactivity was visualized by enhanced chemiluminescence (GE Healthcare). The related antibodies we used included anti – integrin β3 (Chemicon), anti-myc (Sigma), anti – β-actin (Sigma), anti – caspase-8 (Cell Signaling), and rabbit anti-mouse IgG (Calbiochem).
Transient transfection and selection of stable transfectants
ITGB3 cDNA was kindly gifted by Prof. Peter J. Newman (Blood Center of Wisconsin, Milwaukee, WI). This ITGB3 cDNA was subcloned into mammalian expression vector pcDNA3.1a(−) (Invitrogen) containing Myc tag and neomycin resistance gene for establishment of stable transfectants or pCMV-Myc (Clontech) containing only Myc tag, separately. Cells with 80% confluency were transfected using either Lipo2000 (QGY-7703 and SK-Hep1) or Plus and Lipofectamine reagent (SMMC-7721) in parallel. For transient transfection, cells were transfected with pCMV-Myc or pCMV-Myc-ITGB3 together with plasmid pBB14 expressing integral membrane green fluorescent protein as a transfection marker. Cells with green fluorescence were considered to be positive transfectants and were selected in the following analysis. For stable cell line screen, 24 h posttransfection with pcDNA3.1a(−) or pcDNA3.1a(−)-ITGB3, cells were portioned into new dishes and subject to selection with 800 μg/mL G418 (Invitrogen) for ~2 wk until single clone was visible. Independent colonies were isolated and subjected to Western blot with an anti-Myc antibody to select ITGB3-expressing colonies. Control colonies stably transfected with pcDNA3.1a(−) were also generated in parallel.
Flow cytometry analysis
For cell apoptosis analysis, cells were harvested and resuspended in 70% ice-cold alcohol for fixation overnight. DNA was stained with propidium iodide (50 μg/mL) together with RNase (100 μg/mL) before being analyzed by FACSCa-libur (BD Biosciences). At least 10,000 cells were acquired for each sample. Apoptotic cells were calculated as cells in the area corresponding to sub-G1 relative to total cells. For propidium iodide exclusion in cell viability analysis, cells were harvested and washed, followed by propidium iodide staining (10 μg/mL) without permeation treatment. Propidium iodide – negative cells corresponded to the survival population. For Annexin V staining, cell pellets were resuspended in 100 μL of binding buffer and then reacted with Annexin V at room temperature before analysis. Apoptosis data are the percentage of Annexin V – positive cells. Results are representatives of three independent experiments with triplicate samples for each condition.
Cell surface integrin staining
Cells in culture were harvested and resuspended in PBS, then blocked with 5% goat serum and 1% bovine serum albumin for 30 min at room temperature. After incubation with anti – integrin αvβ3 antibody (LM609, Upstate) on ice for 90 min, cells were washed and fixed with 4% formaldehyde, followed by staining with a FITC-conjugated secondary antibody (Upstate) for 30 min on ice. Mouse IgG (Calbiochem) was used as control.
Colony formation assay
Hepatoma cells with 80% confluency were transfected with pcDNA3.1a(−) and pcDNA3.1a(−)-ITGB3 separately. Twenty-four hours later, cells were portioned into new 60-mm dishes at a density of 0.5 × 105 per dish (SMMC-7721 and QGY-7703) or 1 × 105 per dish (SK-Hep1) in duplicate. G418 was added into the medium 24 h later. Colonies were identified by crystal violet staining after about 10 to 14 days of culture. Results are the representative of three independent experiments.
Cell proliferation assay
All SMMC-7721 derivative lines were plated at a density of 1,500 cells per well in 96-well plates with complete medium supplemented with G418. During a 7-d culture period, cells were subjected to 3-(4,5-dimethyl-thiazol-2yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega) everyday. Cell growth curve was expressed as absorbance at 490 nm (n = 6) read by a microtiter reader (Bio-Rad). In the serum deprivation assay, culture medium was replaced by DMEM supplemented with 0.1% bovine serum albumin and G418 (starvation medium) 24 h after cell plating till the end of culture. For fibrinogen rescue experiment, culture medium was replaced by starvation medium with or without additional fibrinogen (Merck). Cells were then incubated in different media separately for 4 d before being subjected to apoptotic analysis.
Anoikis assay
Stable cell lines in normal culture were harvested by trypsinization and suspended at a high density (1 × 106/mL). Suspended cells were either loaded on a revolving turntable to avoid adhesion or plated on poly-HEMA (10 mg/mL; Sigma)– coated dishes for the indicated time before analysis by FACSCalibur as described (29).
Statistical analysis
In real-time PCR, relative gene expression levels normalized by GAPDH were calculated by the formula 2−ΔCt, where ΔCt (critical threshold) = Ct of genes of interest - Ct of GAPDH. For analysis of HCC specimens, fold changes of gene expression levels in tumor specimens relative to corresponding nontumorous specimens (T/N) were calculated by the 2−ΔΔCt method as previously described (30) and transformed to log 2, where ΔΔCt = ΔCttumor - ΔCtnontumorous. A 2-fold change threshold was set for identifying significant changes in gene expression. A two-tailed Student's t test was used to evaluate group-level differences. We considered P < 0.05 (*) and P < 0.01 (**) to be statistically significant. The relationship between ITGB3 expression and ligand expression or ITGB3 expression and clinical characteristics was analyzed by χ2 significance test.
Results
Integrin β3 was down-regulated in HCC
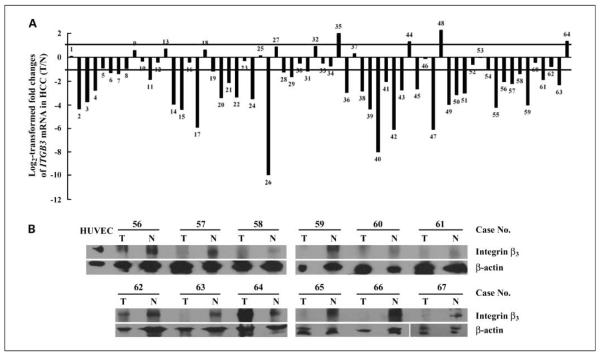
We first determined the levels of mRNA and protein expression of integrin β3 in 64 HCC specimens paired with their corresponding neighboring nontumorous specimens. Quantitative real-time PCR analysis was carried out using specific primers targeting a 349-bp product of ITGB3 gene and a 226-bp product of GAPDH gene. Figure 1A presents the log 2 transformed fold changes of ITGB3 mRNA expression ratio of T/N (tumor/nontumorous). Thirty-eight of 64 (59.38%) cases showed significant reduction of integrin β3 in HCC (log 2 transformed fold change < −1); 22 of 64 (34.38%) cases showed no alteration (−1 < log 2 transformed fold change < 1); and only 4 of 64 (6.25%) cases showed up-regulation (log 2 transformed fold change > 1). The average expression fold change of ITGB3 mRNA in HCC was 32.16% of nontumorous control. To confirm real-time PCR results, total protein of 12 paired HCC specimens was further analyzed by Western blot. As shown in Fig. 1B, under non-reducing condition, integrin β3 was detected at an ~90-kDa band. The positive control of expression of endogenous integrin β3 in human umbilical vascular endothelial cells was included at the left panel and the loading control was shown by reprobing with anti – β-actin antibody. These results show an agreement between lowered levels of integrin β3 protein in tumor compared with their corresponding normal controls and mRNA levels. In particular, consistent expression patterns were detected in nine cases with both mRNA and protein data (case no. 56–64). Further analysis of the pathologic data with integrin expression from 54 HCC cases showed that 83.33% of the specimens with poorly differentiated (grade 3) characteristics showed inhibited integrin β3 expression, although no significant difference is shown (Supplementary Table S1).
Fig. 1.
Down-regulated integrin β3 expression in HCC specimens. A, ITGB3 mRNA expression levels were analyzed in 64 paired HCC specimens with their corresponding neighboring nontumorous specimens (no. 1–64) by quantitative real-time PCR and the 2−ΔΔCt method as mentioned in Materials and Methods. Values were expressed as log 2 transformed relative fold decrease or fold increase in the mRNA expression with respect to the adjacent nontumorous tissues after normalization to the housekeeping gene GAPDH. A positive log 2 transformed fold change indicates higher expression levels in tumorous specimens compared with the neighboring nontumorous specimens, whereas a negative value means relatively decreased levels. A 2-fold change threshold was set for identifying significant changes in gene expression. B, protein samples extracted from 12 paired specimens (no. 56–67) were subjected to Western blot under nonreducing conditions with anti – integrin β3 monoclonal antibody. Primary human umbilical vascular endothelial cell (HUVEC) lysate was used as positive control to recognize endogenous integrin protein. β-Actin was used as the loading control.
Overexpression of ITGB3-induced apoptosis in serial human hepatoma cells
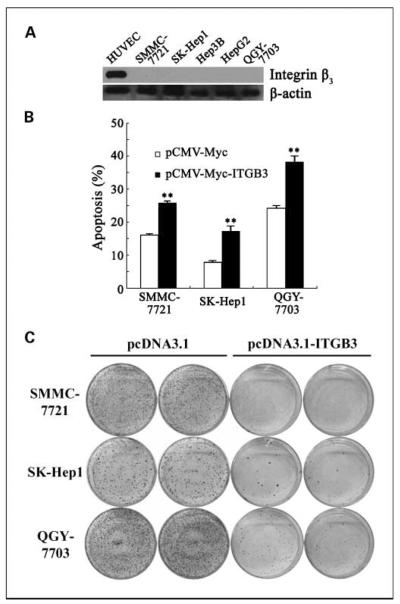
Based on the strikingly down-regulated levels of integrin β3 expression in HCC specimens, we further investigated the ability of integrin β3 overexpression in hepatoma cells on inhibiting tumor cell malignance. Consistent with the observation in HCC, five hepatoma cell lines showed little or no integrin β3 protein expression (Fig. 2A). pCMV-Myc-ITGB3 construct was transiently transfected into SMMC-7721 hepatoma cells; after 36 hours, flow cytometry and apoptosis assay were done to measure the effect on cell cycle and cell death. No obvious difference was observed in major peak distribution during the cell cycle but a progressive aggregation in sub-G1 phase appeared in cells expressing integrin β3, indicating the influence of integrin β3 on cell apoptosis, but not cell cycle (data not shown).
Fig. 2.
Overexpression of ITGB3 induced apoptosis in hepatoma cells. A, expression of ITGB3 was determined in five indicated hepatoma cells through Western blot. Lysate from ECV304 (a transformed endothelial cell line) was used as positive control. B, cell apoptosis was analyzed in SMMC-7721, SK-Hep1, and QGY-7703 through Annexin V staining. Cells were transiently transfected with pCMV-Myc or pCMV-Myc-ITGB3 together with pBB14 separately, harvested 36 h posttransfection, and stained with Annexin V before being subjected to flow cytometric analysis. Cell apoptosis was calculated as the percentage of Annexin V – positive population compared with total cells. Columns, mean (n = 3); bars, SD. **, P < 0.01. C, representative images of colony formation assay in SMMC-7721, SK-Hep1, and QGY-7703. Cells were transiently transfected with pcDNA3.1 or pcDNA3.1-ITGB3 separately, split into new dishes 24 h posttransfection, and cultured in the presence of G418 48 h posttransfection. Colonies were identified by crystal violet staining after about 10 to 14 d of culture.
To confirm this result, three hepatoma cells, including SMMC-7721, SK-Hep1, and QGY-7703, were subjected to standard Annexin V staining analysis to measure cell apoptosis. Cells were stained with Annexin V 36 hours posttransfection of ITGB3. As shown in Fig. 2B, ITGB3-expressing cells exhibited an elevated cell apoptotic level (P < 0.01, Student's t test) compared with parental controls transfected with vector only in all three cell lines. This result gave us the first hint that overexpression of integrin β3 might be a positive mediator of cell apoptosis in hepatoma cells.
This finding was further confirmed by colony formation assay (Fig. 2C). Expression of integrin expression dramatically reduced the number of survival colonies in all three hepatoma cells under G418 selection compared with control groups with transfection of pcDNA3.1 vector only. Taken together, our results suggested that overexpression of integrin β3 induces apoptosis in human hepatoma cells.
Stable expression of ITGB3 in SMMC-7721 cells promoted apoptosis in the absence of its ligands
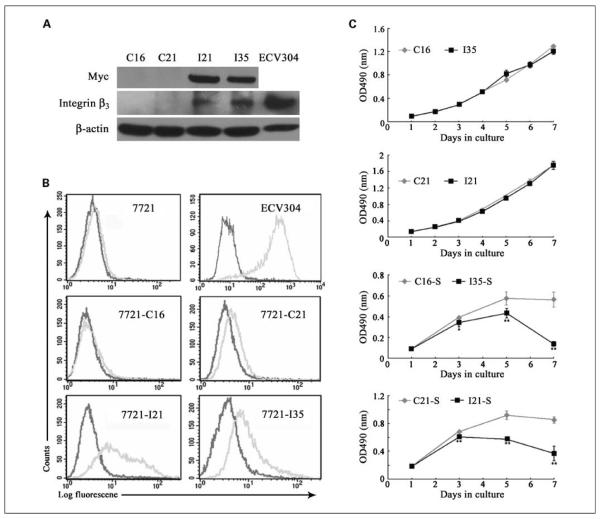
To get insights on integrin β3 function, we then established stable transfectants in hepatoma cells. Although several cell lines have been tested, due to extensive apoptosis in transient transfection of integrin β3, we only obtained two ITGB3-positive colonies (termed as I21 and I35) with very low integrin expression level in comparison with endogenous integrin in ECV304 (Fig. 3A). C16 and C21 were two control colonies selected from stable transfectants with empty vector. Analysis of cell surface molecules by flow cytometry was done to confirm cell surface expression of integrin using an antibody specifically against integrin αvβ3, the sole heterodimer formed by integrin β3 in most cell types except platelets (Fig. 3B). Fluorescence signals of integrin antibody (gray) in I21 and I35 showed obvious, but not large, rightward shifts compared with control IgG (black), further showing the functional expression of integrin β3 at a low level on SMMC-7721 cell surface in comparison with ECV304. The slight shift in two control cells could be explained by the endogenous expression of integrin as shown in parental SMMC-7721 cells.
Fig. 3.
Stable transfection of ITGB3 into SMMC-7721 led to cell death under serum starvation. A, two positive clones (I21 and I35) of SMMC-7721 stable cell line expressing integrin β3 and two control clones (C16 and C21) were identified and subjected to Western blot. Antibodies were indicated on the left and β-actin was used as the loading control. Lysate from ECV304 was designed as a positive control. B, representative flow cytometric profile of integrin expression in stable cell lines as well as parental SMMC-7721, ECV304. Gray and dark lines, anti – integrin αvβ3 monoclonal antibody and mouse IgG, respectively. C, cell proliferation assay was carried out under normal culture conditions (top) or in the absence of serum (bottom) with MTS kit in a 7-d culture period among four cell lines randomly grouped. Cell growth curve was expressed as the absorbance at 490 nm with a microtiter reader. Points, mean (n = 6); bars, SD. *, P < 0.05; **, P < 0.01.
Based on the proapoptotic effect of integrin β3, we first studied cell apoptosis and viability. Data in Supplementary Fig. S1 show that under normal culture condition, both I21 and I35 exhibited a slight tendency of apoptosis with a consistent decrease of cell viability. However, this difference in apoptosis level seemed to have little effect on cell growth (Fig. 3C, top), which may be due to a low ectopic integrin expression in the transfectants. In a 7-day period of culture, all four SMMC-7721 stable lines displayed similar growth. However, when cells were cultured in the absence of serum, significant cell death was induced in the transfectants I21 and I35 (indicated as I21-S and I35-S). In Fig. 3C (bottom), as the culture time was prolonged, cell numbers of both I21-S and I35-S decreased dramatically from day 3, resulting in much fewer cells than C16-S and C21-S by the end of culture.
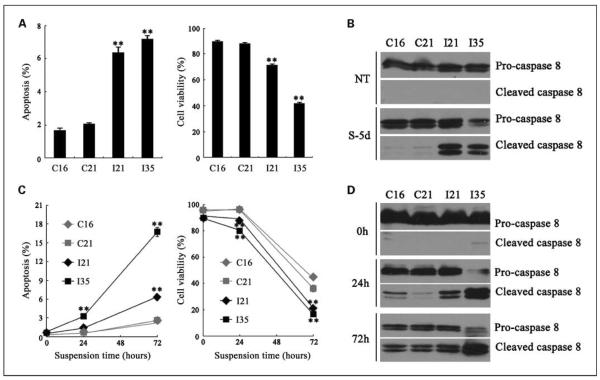
We then further investigated cell apoptosis and viability 5 days after serum deprivation. Both I21 and I35 cell lines were prone to apoptotic death in contrast to control cells, which was correlated to the reduced cell viability (Fig. 4A). Cell lysates after starvation were further subjected to Western blot with anti–caspase-8 monoclonal antibody to identify integrin-mediated apoptosis pathways (31). As shown in Fig. 4B, nontreated cells showed no detectable cleaved caspase-8 in spite of low level of apoptosis shown in flow cytometric analysis, probably due to lower sensitivity of Western blot. However, caspase-8 from ITGB3-expressing cells starved for 5 days (S-5d) were obviously cleaved in contrast to control cells.
Fig. 4.
Serum deprivation and cell-ECM disruption induced elevated apoptosis in ITGB3-expressing cell lines. After serum starvation for 5 d, cells were harvested and subjected to apoptosis (A, left) and viability (A, right) analyses by flow cytometry. Whole-cell lysates from starved cells (S-5d) as well as nontreated cells (without starvation; NT) were subjected to Western blot with anti – caspase-8 monoclonal antibody (B). The antibody simultaneously recognized procaspase-8 (57 kDa; top) and cleaved-caspase-8 as indicated (43/41kDa; bottom). Stable cell lines were kept in suspension to induce anoikis in a 72-h period. After the indicated suspension time periods, cells were collected and subjected to apoptosis (C, left) and viability (C, right) analyses by flow cytometry as described. Cells were also lysed and subjected to anti – caspase-8 blot at the same indicated time (D). Columns and points, mean (n = 3); bars, SD. *, P < 0.05; **, P < 0.01.
Normal endothelial cells often die from anoikis in which integrin-ligand ligation was completely interrupted, whereas anchorage-independent tumor cells usually escape from anoikis through gene expression alternation (32, 33). We then investigated whether ITGB3 expression affected anoikis level in SMMC-7721 cells. SMMC-7721 stable cell lines were kept in suspension for 0 to 72 hours to induce anoikis. Both cell apoptosis and cell viability were examined by flow cytometric analysis at variable time periods. An extensive cell apoptotic death was observed from four cell lines as suspension time was prolonged. However, ectopic ITGB3 expression could significantly enhance cell apoptosis level to 2- to 6-fold of control cells and decrease cell viability to 50% to 70% of control cells after 72-hour suspension (Fig. 4C). Caspase-8 cleavage was subsequently examined (Fig. 4D). Although caspase-8 activation was detected in control cells with a long suspension time, I21 and I35 clones showed significant stronger activation of caspase-8. Notably, the stronger proapoptotic effect of integrin in anoikis than in serum deprivation may be due to complete disruption of integrin-ligand ligation in anoikis. Another experiment to study cell anoikis by using poly-HEMA–coated dishes was also carried out, and similar results were obtained (data not shown). Therefore, we concluded that stable transfectants with relative low expression of integrin β3 enhanced apoptosis in hepatoma cells under unligated conditions.
To expand the magnitude of our conclusion, another hepatoma cell line, SK-Hep1, was subjected to integrin β3 stable line screen, as summarized in Supplementary Fig. S2. Two positive stable clones (S40 and S41) as well as two control clones (SC2 and SC3) were selected and subjected to assays as described above. Consistent with SMMC-7721 cells, although integrin expression did not induce alteration in cell growth in SK-Hep1 cells under normal condition, both S40 and S41 cell lines showed an enhanced apoptosis under serum deprivation in comparison with control cells.
Fibrinogen partially rescued the proapoptotic function of ectopic integrin β3
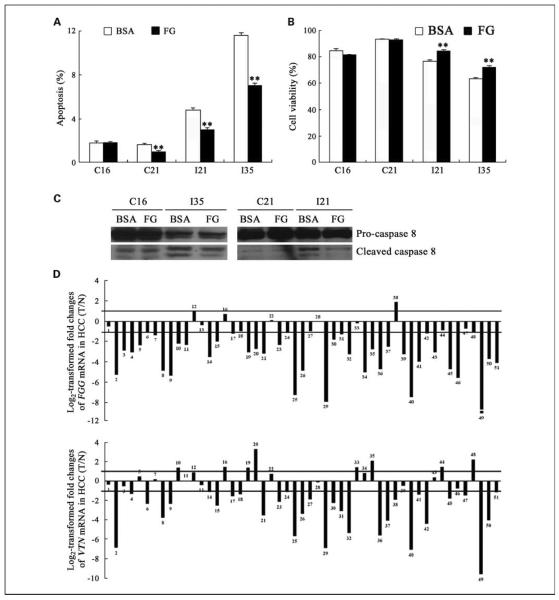
To confirm that ITGB3-expressing cells are prone to apoptosis due to lack of appropriate ligands, we investigated the effect of integrin β3 ligands on SMMC-7721 cells with stable expression of integrin β3. Fibrinogen is a well-defined ligand for integrin β3 and one of the most abundant metabolic proteins in liver (34). SMMC-7721 stable cell lines were cultured in the absence of serum with or without fibrinogen for 4 days before being subjected to analysis. When fibrinogen was present, the apoptotic death of both I21 and I35 was significantly inhibited (Fig. 5A), resulting in consistent higher cell viability (Fig. 5B) and less caspase-8 cleavage (Fig. 5C). In contrast, C16 showed no obvious changes on fibrinogen stimulus. There is only a slight but statistically significant difference in the apoptosis level of C21 under fibrinogen incubation, but no consistent difference was shown in cell viability data. Interestingly, another ligand for integrin β3, vitronectin, could also partially rescue the apoptotic effect of integrin β3 (data not shown). Moreover, integrin-stable transfectants of SK-Hep1 under serum starvation also exhibited inhibition of cell apoptosis on fibrinogen incubation as shown in Supplementary Fig. S2E and F. Thus, the reversal function of integrin β3 ligands supports that insufficient ligand supplement in the extracellular microenvironment is associated with the proapoptotic effect of integrin β3.
Fig. 5.
Fibrinogen partially rescued the proapoptotic function of ITGB3 expression in SMMC-7721 cells and was down-regulated in HCC specimens. SMMC-7721 stable cell lines were cultured in starvation medium with or without fibrinogen (FG) for 4 d before being analyzed by flow cytometry. Fibrinogen partially prevented cell apoptosis (A), as indicated by sub-G1 analysis, and enhanced cell viability (B), as indicated by propidium iodide exclusion. Cells after treatment were also lysed and subjected to caspase-8 blot (C). Less cleaved caspase-8 were shown in the cell lysate after fibrinogen incubation by comparison with that from bovine serum albumin (BSA) treatment only. Columns, mean (n = 3); bars, SD. *, P < 0.05; **, P < 0.01. D, 51 pairs of HCC specimens and their corresponding neighboring nontumorous specimens were examined at mRNA level by quantitative real-time PCR and statistically analyzed by the 2−ΔΔCt method as detailed in Materials and Methods. Values are the log 2 transformed relative fold changes of FGG (top) and VTN (bottom) mRNA expression ratios of T/N. A 2-fold change threshold was set for identifying significant changes at mRNA level.
Both fibrinogen and vitronectin were remarkably down-regulated in HCC and correlated with integrin expression
The above reversal function of appropriate ligands to overexpressing integrins suggests a potential relationship between integrin β3 and its ligands in vivo. We then address the question of whether integrin β3 and its ligands function in a cooperative way to promote tumor cell survival via inhibition of apoptosis in HCC. Quantitative real-time PCR was done to analyze the expression pattern of two ligands for integrin αvβ3 abundant in liver, fibrinogen and vitronectin, in the majority of HCC specimens subjected to integrin analysis. An amplified 101-bp product of human fibrinogen γ-chain fragment (FGG) was used to measure the expression level of fibrinogen heterotriplet in vivo according to a previous report (35). Primers specific to human vitronectin (VTN) were also used to amplify a 240-bp product as previously described (27). Both fibrinogen and vitronectin exhibited significant down-regulated expression pattern in most HCC specimens, as analyzed by Student's t test (FGG: P = 6.83E-11; VTN: P = 2.03E-05), consistent with the data from other laboratories by large-scale analysis (34). As shown in Fig. 5D (top), of 51 cases, 39 (76.47%) cases showed significantly reduced fibrinogen expression, 4 (7.84%) cases showed no significant changes, and 8 (15.69%) cases showed expression up-regulation. Similarly, vitronectin expression was significantly reduced in 30 (58.82%) cases; 8 (15.69%) cases had no significant changes; and 13 (25.49%) cases showed up-regulated expression (Fig. 5D, bottom). The average decreases in mRNA expression of FGG and VTN were 84.83% and 71.33%, respectively, for HCC tissues relative to nontumorous control. Inhibited protein levels of fibrinogen and vitronectin were also confirmed by Western blot and immunohistochemical staining with anti-vitronectin and anti-fibrinogen antibodies in HCC specimens (Supplementary Fig. S3).
To further study the correlation between endogenous integrin expression and its ligands, mRNA expression levels of integrin and its ligands were analyzed by χ2 significance test. Table 1 shows statistical correlation results between integrin β3 and its ligands in HCC specimens (χ2 = 4.449, P = 0.035). The number of specimens with down-regulated integrin β3 as well as FGG and VTN was significantly higher compared with that of specimens with down-regulation of either integrin or ligands. Integrin expression in the specimens with either down-regulated FGG or VTN seemed to be reduced, although no statistical difference was detected (FGG: Supplementary Table S2, χ2 = 2.946, P = 0.086; VTN: Supplementary Table S3, χ2 = 3.757, P = 0.052). The expression levels of FGG and VTN also seemed be correlated with each other (Supplementary Table S4, χ2 = 2.946, P = 0.086). These results suggest that down-regulated integrin β3 expression correlated with inhibition of fibrinogen and vitronectin is related to antiapoptotic response and aggressive growth of HCC.
Table 1.
Relationship between FGG, VTN, and ITGB3 expressions in HCC
| HCC specimens |
FGG, VTN down-regulation |
Positive ratio (%) | P | ||
|---|---|---|---|---|---|
| + | − | ||||
| ITGB3 | + | 19 | 11 | 63.33 | 0.035 |
| Down-regulation | − | 7 | 14 | 33.33 | |
Discussion
Our study shows the down-regulation of integrin β3 in HCC compared with paired counterpart normal liver tissues from clinical liver cancer patients. Overexpression of ectopic integrin β3 significantly induced apoptosis in hepatoma cells in a ligand-dependent manner. These results show that inhibited integrin β3 and its ligands are responsible for the aggressive growth of HCC.
In contrast with some reports showing enhanced expression of integrin β3 in poorly differentiated HCC (36) and up-regulation of vitronectin and fibrinogen in HCC (37, 38), the present study reveals the evidence that the expression of integrin β3 is down-regulated in HCC at both mRNA and protein levels (Fig. 1). Compared with normal tissues, tumors often show alterations in integrin expression. Although it still remains to be experimentally shown, the disregulated expression of integrin may significantly contribute to the malignant phenotype of tumor cells. Integrin β3 (or its only heterodimer in most cell types, integrin αvβ3) is widely studied on its potential function in tumor invasion and metastasis in melanoma and breast cancer, which correlated with its elevated expression level as we mentioned above. However, studies of integrin β3 in HCC are limited. These discrepancies could be explained by data collection from different cancer specimens. For instance, the present study used samples from nonmetastatic HCC, and data from other groups mentioned above were reportedly from invasive HCC cells. Our in vitro data further suggest that inhibited integrin β3 expression is correlated with its proapoptotic function in hepatoma cells in an integrinligation–dependent manner. Two native ligands of integrin β3 rich in the ECM of liver were found to be down-regulated and statistically associated with integrin expression in HCC specimens (Table 1), further indicating that integrin β3 functions cooperatively with its ligands in initiating a proapoptotic pathway in liver cancer cells.
Due to the potential function of integrin β3 in tumor progression, the mechanism responsible to integrin down-regulation could be an interesting topic. Regulatory effects on integrin β3 expression have been reported on different pathways, including stimulus of transforming growth factor or estrogen and sustained activation of Ras-induced Raf/mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase pathway, supporting the role of diverse and complicated factors in integrin β3 transcriptional regulation (39–41). Moreover, components of ECM where cells reside not only cooperate with integrins but also show an ability to regulate integrin β3 expression. Evidence from different groups has further shown that osteopontin and angiotensin II up-regulates, but endostatin inhibits, integrin expression (42–44). As such, our current data suggest that inhibition of integrin β3 is, at least partially, under the influence of fibrinogen and/or vitronectin expression alternation. However, this still remains to be experimentally shown. On the transcriptional regulation, several transcriptional factors have been reported in integrin β3 expression (45, 46). Elucidation of detailed transcriptional and translational regulation of integrin will lead to a better understanding of integrin and its function in tumor development and invasive growth.
Integrins are cell surface receptors sensing dynamic environment cues especially during tissue remodeling and tumor progression. Our results provide additional evidence indicating that integrin β3 can function as an important apoptotic regulator on changes in extracellular microenvironment. According to previous reports, integrin-associated apoptosis includes at least three forms. First, integrin αvβ3 and α5β1 expression usually preserve cell viability in response to serum starvation or chemotherapeutic agent treatment in a ligation-dependent manner through intrinsic apoptosis pathway (14–16). However, once the ligation is absent, overexpression of integrin α5β1 could lead to cell growth arrest and decreased tumorigenicity (18). In our experiment, expression of integrin β3 promoted cell apoptosis on serum deprivation, and addition of ligands effectively restored this phenomenon (Figs. 4A–B and 5A–C), similarly due to unligated condition of ectopic integrin β3, further confirming that appropriate and sufficient ligands in ECM are critical to switch the biosensory roles of integrins. However, caspase-8 cleavage is shown in our assay, indicating an extrinsic apoptosis pathway initiated by unligated integrin β3, but detailed mechanism still remains to be unraveled in future work. Second, anoikis is an anchorage-dependent cell apoptosis wherein integrin-mediated cell-ECM connection is completely disrupted, first described by Frisch in endothelial cells (13). In the work of Berman et al., activation of integrin αvβ3 strongly stimulates anoikis in human intestinal carcinoma cells (29). Similarly, integrin α5β1 expression induced by p16INK4a is also a positive anoikis inducer in different tumor cell lines, including melanoma cells and hepatoma cells (47). Using in vitro anoikis assay, the present study provides the evidence that integrin β3 expression induces anoikis in HCC cells (Fig. 4C), indicating a more general role of integrin β3 expression in tumor cell anoikis. In particular, caspase-8 is also cleaved in this apoptosis pathway (Fig. 4D). Another form of apoptosis that integrins may take part in is called integrin-mediated death, reported by Stupack et al. (31), which may be tested in the future. Taken together, our results from different assays support the notion that lack of appropriate ligands is the key factor to switch on the proapoptotic function of integrin β3 in hepatoma cells in an undefined extrinsic pathway.
In summary, our studies with paired HCC and normal liver specimens and cultured hepatoma cell lines suggest that inhibition of integrin β3 and its ligation is responsible for tumor progression in human liver cancer. Clarification of the mechanism accounting for expression inhibition and the apoptosis pathway would provide us a new therapeutic approach for liver cancer.
Translational Relevance.
The observation of expression alternation of integrins in tumor indicated a promising future of studying integrins in clinical tumor therapy. The long-term goal of this study is to explore the potential of integrin β3 as a supplementary marker in hepatocellular carcinoma (HCC) diagnosis as well as an effective therapeutic target in HCC treatment. The significant down-regulated expression pattern of integrin β3 and correlated ligand expression detected at both mRNA and protein levels in HCC show the feasibility of this goal. Moreover, by understanding the underlying mechanism, drugs selectively activating integrin β3–associated apoptotic pathways may be discovered and applied to effectively target hepatoma cells in clinical study.
Acknowledgments
We thank Dr. Peter J. Newman (Blood Center of Wisconsin, Milwaukee, WI) for providing pcDNA3-ITGB3 plasmid; Dr. Hanfei Ding (Biochemistry and Cancer Biology, The University of Toledo, Toledo, OH); Dr. Haojie Huang (Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN); and Dr. Xianmei Yang (State Key Laboratory of Genetic Engineering, Fudan University, Shanghai, P.R. China) for their helpful discussion.
Grant support: National 973 Programs of China grant 2004CB518605 (L. Yu), National 863 Projects of China grants 2006AA020501 and 2002BA711A02-4 (L. Yu), The Projectof Shanghai Municipal Science and Technology Commission grant 03dz14086 (L. Yu), National Natural Science Foundation of China grant 30024001 (L. Yu), and National Cancer Institute, NIH grant RO1101990 (J.J. Li).
Footnotes
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 2.Sottile J. Regulation of angiogenesis by extracellular matrix. Biochim Biophys Acta. 2004;1654:13–22. doi: 10.1016/j.bbcan.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–8. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 4.Herbst TJ, McCarthy JB, Tsilibary EC, Furcht LT. Differential effects of laminin, intact type IV collagen, and specific domains of type IV collagen on endothelial cell adhesion and migration. J Cell Biol. 1988;106:1365–73. doi: 10.1083/jcb.106.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc Natl Acad Sci U S A. 2003;100:4766–71. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 6.DeHahn KC, Gonzales M, Gonzalez AM, et al. The α4 laminin subunit regulates endothelial cell survival. Exp Cell Res. 2004;294:281–9. doi: 10.1016/j.yexcr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–91. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 8.Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yauch RL, Felsenfeld DP, Kraeft SK, et al. Mutational evidence for control of cell adhesion through integrin diffusion/clustering, independent of ligand binding. J Exp Med. 1997;186:1347–55. doi: 10.1084/jem.186.8.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moro L, Venturino M, Bozzo C, et al. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 1998;17:6622–32. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneller M, Vuori K, Ruoslahti E. αvβ3 integrin associates with activated insulin and PDGFβ receptors and potentiates the biological activity of PDGF. EMBO J. 1997;16:5600–7. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meredith JE, Jr., Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–61. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethi T, Rintoul RC, Moore SM, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5:662–8. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Vuori K, Reed JC, Ruoslahti E. The α5β1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci U S A. 1995;92:6161–5. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matter ML, Ruoslahti E. A signaling pathway from the α5β1 and αvβ3 integrins that elevates bcl-2 transcription. J Biol Chem. 2001;276:27757–63. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- 17.Giancotti FG, Ruoslahti E. Elevated levels of the α5β1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990;60:849–59. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 18.Varner JA, Emerson DA, Juliano RL. Integrin α5β1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol Biol Cell. 1995;6:725–40. doi: 10.1091/mbc.6.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stallmach A, von Lampe B, Matthes H, Bornhoft G, Riecken EO. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumour transformation. Gut. 1992;33:342–6. doi: 10.1136/gut.33.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinel RJ, Rosendahl A, Neumann K, et al. Expression and function ofVLA-α2, -α3, -α5 and -α6-integrin receptors in pancreatic carcinoma. Int J Cancer. 1992;52:827–33. doi: 10.1002/ijc.2910520526. [DOI] [PubMed] [Google Scholar]
- 21.Gehlsen KR, Davis GE, Sriramarao P. Integrin expression in human melanoma cells with differing invasive and metastatic properties. Clin Exp Metastasis. 1992;10:111–20. doi: 10.1007/BF00114587. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Regezi J, Ross FP, et al. Integrin αvβ3 mediates K1735 murine melanoma cell motility in vivo and in vitro. J Cell Sci. 2001;114:2665–72. doi: 10.1242/jcs.114.14.2665. [DOI] [PubMed] [Google Scholar]
- 23.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via αvβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–64. [PubMed] [Google Scholar]
- 24.Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–22. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albelda SM, Mette SA, Elder DE, et al. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 1990;50:6757–64. [PubMed] [Google Scholar]
- 26.Gasparini G, Brooks PC, Biganzoli E, et al. Vascular integrin αvβ3: a new prognostic indicator in breast cancer. Clin Cancer Res. 1998;4:2625–34. [PubMed] [Google Scholar]
- 27.Ozaki S, Johnson LV, Mullins RF, Hageman GS, Anderson DH. The human retina and retinal pigment epithelium are abundant sources of vitronectin mRNA. Biochem Biophys Res Commun. 1999;258:524–9. doi: 10.1006/bbrc.1999.0672. [DOI] [PubMed] [Google Scholar]
- 28.Rox JM, Muller J, Potzsch B. Absence of fibrinogen α-, β- and γ-chain mRNA in human platelets. Br J Haematol. 2005;130:647–8. doi: 10.1111/j.1365-2141.2005.05654.x. [DOI] [PubMed] [Google Scholar]
- 29.Kozlova NI, Morozevich GE, Chubukina AN, Berman AE. Integrin αvβ3 promotes anchorage-dependent apoptosis in human intestinal carcinoma cells. Oncogene. 2001;20:4710–7. doi: 10.1038/sj.onc.1204619. [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155:459–70. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yawata A, Adachi M, Okuda H, et al. Prolonged cell survival enhances peritoneal dissemination of gastric cancer cells. Oncogene. 1998;16:2681–6. doi: 10.1038/sj.onc.1201792. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Lu H, Dazin P, Kapila Y. Squamous cell carcinoma cell aggregates escape suspension-induced, p53-mediated anoikis: fibronectin and integrin αv mediate survival signals through focal adhesion kinase. J Biol Chem. 2004;279:48342–9. doi: 10.1074/jbc.M407953200. [DOI] [PubMed] [Google Scholar]
- 34.Xu XR, Huang J, Xu ZG, et al. Insight into hepatocellular carcinogenesis at transcriptome level by comparing gene expression profiles of hepatocellular carcinoma with those of corresponding noncancerous liver. Proc Natl Acad Sci U S A. 2001;98:15089–94. doi: 10.1073/pnas.241522398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirota-Kawadobora M, Tozuka M, Yamauchi K, et al. Quantitative RT-PCR analysis demonstrates that synthesis of the recombinant fibrinogen is dependent on the transcription and synthesis of γ-chain. Clin Chim Acta. 2002;319:67–73. doi: 10.1016/s0009-8981(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 36.Volpes R, van den Oord JJ, Desmet VJ. Integrins as differential cell lineage markers of primary liver tumors. Am J Pathol. 1993;142:1483–92. [PMC free article] [PubMed] [Google Scholar]
- 37.Uematsu F, Takahashi M, Yoshida M, et al. Distinct patterns of gene expression in hepatocellular carcinomas and adjacent non-cancerous, cirrhotic liver tissues in rats fed a choline-deficient, l-amino acid-defined diet. Cancer Sci. 2005;96:414–24. doi: 10.1111/j.1349-7006.2005.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kondoh N, Wakatsuki T, Ryo A, et al. Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res. 1999;59:4990–6. [PubMed] [Google Scholar]
- 39.Pechkovsky DV, Scaffidi AK, Hackett TL, et al. Transforming growth factor β1 induces αvβ3 integrin expression in human lung fibroblasts via a β3 inte grin-, c-Src-, and p3 8 MAPK-dependent pathway. J Biol Chem. 2008;283:12898–908. doi: 10.1074/jbc.M708226200. [DOI] [PubMed] [Google Scholar]
- 40.Saintier D, Burde MA, Rey JM, et al. 17β-Estradiol down-regulates β3-integrin expression in differentiating and mature human osteoclasts. J Cell Physiol. 2004;198:269–76. doi: 10.1002/jcp.10406. [DOI] [PubMed] [Google Scholar]
- 41.Woods D, Cherwinski H, Venetsanakos E, et al. Induction of β3-integrin gene expression by sustained activation of the Ras-regulated Raf-MEK-extracellular signal-regulated kinase signaling pathway. Mol Cell Biol. 2001;21:3192–205. doi: 10.1128/MCB.21.9.3192-3205.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao J, Dong L, Lu B, et al. Down-regulation of osteopontin suppresses growth and metastasis of hepatocellular carcinoma via induction of apoptosis. Gastroenterology. 2008;135:956–68. doi: 10.1053/j.gastro.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 43.Li SM, Wang XM, Qiu J, et al. Inhibitory effects of α-zearalenol on angiotensin II-induced integrin β3 mRNA via suppression of nuclear factor-κB. Biomed Environ Sci. 2005;18:314–20. [PubMed] [Google Scholar]
- 44.Zhang M, Yang Y, Yan M, Zhang J. Down-regulation of vascular endothelial growth factor and integrin β3 by endostatin in a mouse model of retinal neovascularization. Exp Eye Res. 2006;82:74–80. doi: 10.1016/j.exer.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Crotti TN, Flannery M, Walsh NC, et al. NFATc1 regulation of the human β3 integrin promoter in osteoclast differentiation. Gene. 2006;372:92–102. doi: 10.1016/j.gene.2005.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crotti TN, Sharma SM, Fleming JD, et al. PU.1 and NFATc1 mediate osteoclastic induction of the mouse β3 integrin promoter. J Cell Physiol. 2008;215:636–44. doi: 10.1002/jcp.21344. [DOI] [PubMed] [Google Scholar]
- 47.Plath T, Detjen K, Welzel M, et al. A novel function for the tumor suppressor p16(INK4a): induction of anoikis via up-regulation of the α(5)β(1) fibronectin receptor. J Cell Biol. 2000;150:1467–78. doi: 10.1083/jcb.150.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]