Abstract
The unfolded protein response (UPR) is a conserved, intracellular signaling pathway activated by endoplasmic reticulum (ER) stress. In mammalian cells, the UPR is controlled by three ER-resident transmembrane proteins: inositol-requiring enyzme-1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor-6 (ATF6), by which cytoprotective mechanisms are initiated to restore ER functions. However, if cellular homeostasis is not restored by the UPR’s initial events, UPR signaling triggers apoptotic cell death, which correlates with the pathogenesis of a wide range of human diseases. The intrinsic function of the UPR in regulating cell survival and death suggests its importance as a mechanistic link between ER stress and disease pathogenesis. Understanding UPR regulatory molecules or signaling pathways involved in disease pathogenesis is critical to establishing therapeutic strategies. For this purpose, several experimental tools have been developed to evaluate individual UPR components. In this chapter, we present methods to monitor and quantify activation of individual UPR signaling pathways in mammalian cells and tissues, and we review strategies to artificially and selectively activate individual UPR signaling pathways using chemical–genetic approaches.
1. Introduction
The endoplasmic reticulum (ER) is the entrance site for newly synthesized polypeptides of membrane and secreted proteins. In the ER, residential chaperones and enzymes fold and assemble polypeptides before export to the rest of the secretory pathway. The ER’s role in protein quality control is pivotal to maintaining cellular homeostasis. Pathologic and physiologic processes can disrupt ER protein folding and assembly, and under these circumstances, misfolded proteins accumulate in the ER lumen, leading to ER stress. ER stress is correlated with and causally linked to the pathogenesis of a wide range of human diseases, including neurodegenerative disease, metabolic and inflammatory disease, infection, and subtypes of cancer (He, 2006; Hotamisligil, 2010; Lin et al., 2008; Moenner et al., 2007). Why and how ER stress causes diseases and what molecular and cellular changes lead to diseases are questions that remain largely unanswered and require further investigation.
The unfolded protein response (UPR) is a conserved, intracellular signaling process activated by ER stress (Ron and Walter, 2007). In mammalian cells, the UPR comprises at least three parallel intracellular signaling pathways controlled by ER-resident transmembrane proteins: inositol-requiring enzyme-1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor-6 (ATF6). IRE1, PERK, and ATF6 detect disrupted ER homeostasis and then transmit this information to the rest of the cell by activating downstream signal transduction effectors. In experimentally induced conditions of acute ER stress, all three UPR branches are simultaneously activated and initiate cytoprotective events that suppress global protein translation to reduce the protein folding burden of the ER, upregulate the transcription of molecular chaperones and protein folding enzymes to increase folding capacity of the ER, and enhance the degradation of unfolded proteins via proteasomal machinery. However, if cellular homeostasis is not restored despite these actions, the UPR then triggers apoptosis.
The intrinsic function of the UPR in regulating cell survival and death in response to ER stress suggests its importance as a mechanistic link between ER stress and disease pathogenesis. However, the concomitant activation of multiple UPR signaling pathways by ER stress in mammalian cells has hindered precise assessments of the contribution of each specific UPR signaling pathway to disease pathogenesis. Identifying specific UPR regulatory molecules or signaling pathways involved in disease pathogenesis is critical to developing targeted therapies that prevent the development or progression of disease. Here, we review methods to monitor and quantify activation of individual UPR signaling pathways in mammalian cells and tissues, and we review strategies to artificially and selectively activate individual UPR signaling pathways using chemical–genetic approaches.
2. Monitoring Mammalian UPR
Here we present conventional protocols to detect and quantify the activity of IRE1, PERK, or ATF6 signaling in mammalian cell lines and tissues. Additionally, we review reporter systems developed for monitoring mammalian UPR activity.
2.1. Monitoring IRE1 activity
Upon induction of ER stress, IRE1α endonuclease (RNase) is activated and subsequently cleaves its substrate, X-box binding protein-1 (Xbp-1) messenger RNA (mRNA) (Calfon et al., 2002; Yoshida et al., 2001). Xbp-1 mRNA has two conserved, overlapping open reading frames (ORFs). Activated IRE1α removes a 26-nucleotide (nt) intron from unspliced Xbp-1 mRNA, leading to a translational frame shift to produce a protein encoded from the two ORFs. The product from spliced Xbp-1 mRNA, XBP-1s, is an active transcription factor that upregulates the expression of ER associated degradation (ERAD) components and ER chaperones. Specificity of Xbp-1 splicing by activated IRE1α is evidenced by mutation or knockout of IRE1α (Lee et al., 2002). Therefore, monitoring Xbp-1 splicing status is a specific molecular readout for IRE1α activity.
2.1.1. Xbp-1 splicing assay for mammalian cells and tissues
Determination of Xbp-1 splicing is performed by reverse transcriptase PCR (RT-PCR). Total RNA is extracted from cells or tissue lysate, and cDNA is synthesized by RT reaction with oligo-dT primer and Superscript III (Invitrogen, Carlsbad, CA). Sample cDNA is used as a template for PCR amplification with specific primers for Xbp-1 (Table 11.1). PCR conditions are as follows: Step 1: 95 °C for 5 min; Step 2: 95 °C for 1 min; Step 3: 58 °C for 30 s; Step 4: 72 °C for 30 s Repeat steps 2–4 for 35 cycles Step 5: 72 °C for 5 min.
Table 11.1.
PCR primer sets
| Sequence (5′–3′) |
||||
|---|---|---|---|---|
| Gene | Species | Forward | Reverse | Product (bp) |
| RPL19 | Human | ATGTATCACAGCCTGTACCTG | TTCTTGGTCTCTTCCTCCTTG | 233 |
| Mouse | ATGCCAACTCCCGTCAGCAG | TCATCCTTCTCATCCAGGTCACC | 198 | |
| Rat | TGGACCCCAATGAAACCAAC | TACCCTTCCTCTTCCCTATGCC | 180 | |
| Hamster | ATGCCAACTCCCGTCAGCAG | TCATCCTTCTCATCCAGGTCACC | 198 | |
| BiP/Grp78 | Human | CGGGCAAAGATGTCAGGAAAG | TTCTGGACGGGCTTCATAGTAGAC | 211 |
| Mouse | CCTGCGTCGGTGTGTTCAAG | AAGGGTCATTCCAAGTGCG | 201 | |
| Rat | CCTGCGTCGGTGTATTCAAG | AAGGGTCATTCCAAGTGCG | 201 | |
| Hamster | TGGACCTGTTCCGATCTACC | GACAGCAGCACCGTATGCTA | 206 | |
| Chop | Human | ACCAAGGGAGAACCAGGAAACG | TCACCATTCGGTCAATCAGAGC | 201 |
| Mouse | ACGGAAACAGAGTGGTCAGTGC | CAGGAGGTGATGCCCACTGTTC | 219 | |
| Rat | ACGGACCACGAGTGGTCAGTGC | CAGGAGGTGATGCCAACAGTTC | 219 | |
| Hamster | AACGAGAGGAAAGTGGCTCA | TGTTCTCCCTACCCAGCATC | 222 | |
| Xbp-1a | Human | TTACGAGAGAAAACTCATGGC | GGGTCCAAGTTGTCCAGAATGC | s-257, u-283 |
| Mouse | GAACCAGGAGTTAAGAACACG | AGGCAACAGTGTCAGAGTCC | s-179, u-205 | |
| Rat | GAGTGGAGTAAGGCTGGTGG | TGGGTAGACCTCTGGGAGTTCC | s-223, u-249 | |
| Hamster | TTGAGAGAGAAAACTCATGGC | GGGTCCAACTTGTCCAGAATGC | s-263, u-289 | |
Spliced form of xbp-1 (s); unspliced form of xbp-1 (u)
Differentiation of unspliced and spliced Xbp-1 can be resolved on a 2.0–3.0% agarose/1×TAE gel. A minor hybrid amplicon species consisting of unspliced Xbp-1 annealed to spliced Xbp-1 is also typically observed and appears above the unspliced amplicon (Back et al., 2005; Lin et al., 2007).
2.2. Monitoring PERK activity
In response to ER stress, PERK forms dimers and undergoes autophosphorylation (Harding et al., 1999). Consequently, PERK’s kinase activity phosphorylates the alpha subunit of eukaryotic translation initiation factor-2 (eIF2α). Phosphorylation of eIF2α impairs ribosomal assembly on mRNA, thereby attenuating global protein translation (Harding et al., 2000b). However, translation of activating transcription factor 4 (ATF4) is increased when eIF2α is phosphorylated because of the presence of multiple upstream ORFs in its cognate Atf4 mRNA (Harding et al., 2000a; Lu et al., 2004a; Vattem and Wek, 2004). ATF4 subsequently induces expression of its target genes, including transcription factor C/EBP-homologous protein (CHOP). CHOP, ATF4, and phosphorylated eIF2α are therefore useful molecular markers for monitoring activity of PERK.
2.2.1. Quantitative PCR (qPCR) analysis of CHOP
Relative Chop mRNA expression is evaluated by qPCR. Total RNA extraction and RT reaction is performed to generate cDNA. Rpl19, a gene for ribosomal protein L19, whose transcription is not affected by ER stress, can be used as an internal control for normalization (Hollien and Weissman, 2006). Primer information for Chop and Rpl19 is shown in Table 11.1 and qPCR conditions are as follows: Step 1: 95 °C for 5 min; Step 2: 95 °C for 30 s; Step 3: 58 °C for 30 s; Step 4: 72 °C for 30 s Repeat steps 2–4 for 40 cycles.
2.2.2. Western blotting of eIF2α and ATF4
Cells are homogenized by sonication in 1% NP-40 or 6 M urea, 20 mM HEPES pH = 8.0, containing protease and phosphatase inhibitors (Pierce, Rockford, IL). Twenty to thirty micrograms of total cell lysate are loaded onto SDS-PAGE mini gels and analyzed by Western blot. The following antibody dilutions are used: anti-eIF2α at 1:2000 (Cell Signaling, Natick, MA); anti-phospho-eIF2α at 1:500 (Cell Signaling); anti-ATF4 at 1:2000 (Santa Cruz Biotechnologies, Santa Cruz, CA). After overnight incubation with primary antibody, membranes are washed in PBS (or TBS) containing 0.1% Tween-20 and incubated in horseradish peroxidase (HRP)-coupled secondary antibody (Amersham, Piscataway, NJ) diluted 1:5000 in wash buffer. Immunoreactivity is detected using the enhanced chemiluminescence assay (Pierce). These conditions have been optimized for human protein samples and may require modification when applied to other mammalian cell types and tissues.
2.3. Monitoring ATF6 activity
The third stress sensor for ER stress in mammalian cells is ATF6 (Haze et al., 1999). Upon induction of ER stress, ATF6 translocates from the ER to the Golgi apparatus where Site-1 Protease (S1P) and Site-2 Protease (S2P) cleave it (Ye et al., 2000). After cleavage by S1P/S2P, the amino-terminus of ATF6, containing a basic leucine zipper (bZIP) transactivating domain, translocates to the nucleus as an active transcription factor. ATF6 increases ER protein folding capacity and assembly by transcriptional upregulation of molecular chaperones and/or enzymes. In particular, immunoglobulin heavy chain binding protein (BiP/Grp78) is robustly induced in response to ER stress and is a direct transcriptional target of ATF6 (Haze et al., 1999). A simple and effective way to evaluate ATF6 activity is to measure BiP/Grp78 mRNA or protein levels. In addition, Ron Prywes and colleagues established a method to monitor proteolytic cleavage using a FLAG-tagged ATF6 construct (Shen and Prywes, 2005). In this section, we briefly describe these methods of evaluating ATF6 activation.
2.3.1. qPCR analysis of BiP
Conditions for analysis of BiP/Grp78 by qPCR are identical to Chop. Primer sequences for BiP/Grp78 are shown in Table 11.1.
2.3.2. Western blotting of FLAG-ATF6
Cells are transfected with a p3×FLAG-ATF6 plasmid and protein lysate is prepared with lysis buffer as described above (Shen and Prywes, 2005). Detection is performed by Western blotting as described above using a 1:5000 dilution of primary antibody to FLAG (Sigma, St. Louis, MO).
2.4. Alternative approaches to UPR evaluation
Several reporter systems have been developed to monitor mammalian UPR activity. ER stress response elements (ERSE) (Yoshida et al., 1998) and UPR elements (UPRE) (Yoshida et al., 2000) have been identified within the promoter regions of UPR target genes, and UPR-dependent transcription factors bind to these regions to start their transcription. Constructs with conventional reporter proteins, such as luciferase and β-galactosidase, fused to UPRE and ERSE sequences can report ER stress, as evidenced by increased luciferase and β-galactosidase activities, in both cell culture and transgenic mice (Mao et al., 2004; Wang et al., 2000; Yoshida et al., 1998). However, in these systems, the contribution of individual UPR pathways (IRE1 vs. PERK vs. ATF6) cannot be resolved.
Additionally, Iwawaki et al. (2004) established in vitro and in vivo green fluorescent protein (GFP) reporter systems to monitor the IRE1 pathway. They constructed a reporter plasmid containing a FLAG-tagged, partial sequence (410–633 nt) of hXBP-1 and a GFP variant (Venus) sequence under the control of the chicken β-actin promoter. This partial sequence of hXBP-1 includes a 26-nt intron that is spliced out by activated IRE1. Under normal condition, expression of Venus-fused hXbp-1 mRNA contains the 26-nt intron, thereby halting translation via the stop codon located between the coding regions of hXBP-1 and Venus. Under ER stress conditions, the 26-nt intron is spliced out, inducing a frame shift and resulting in production of the hXBP-1-Venus fusion protein. Using this construct observation or quantification of Venus fluorescence allows monitoring of IRE1 activation. However, interpreting the on/off kinetics of UPR signaling might be difficult with this GFP-based approaches because the intrinsic stability of GFP may result in a prolonged fluorescence signal that remains elevated even after cessation of ER stress or the activation of a particular UPR pathway being monitored.
3. Chemical–Genetic Manipulation of Mammalian UPR
Common physiological and pharmacological inducers of ER stress (e.g., hypoxia, tunicamycin, and thapsigargin) concomitantly activate all UPR signaling pathways. To precisely identify the downstream effects of each signaling pathway, multiple chemical–genetic tools have been developed to selectively activate one UPR pathway at a time. Here we review chemical–genetic tools that enable selective activation of individual UPR signaling pathways in mammalian cells independent of ER stress.
The techniques described within take advantage of UPR initiation events by creating cell lines that mimic the activation of IRE1, PERK, or ATF6 proteins upon addition of cell-permeable, small molecules. Unlike simple gene deletion or constitutive overexpression, drug-induced activation acts quickly and reversibly. Furthermore, it avoids activating compensatory mechanisms in the cell arising from long-term UPR signaling. The establishment of stable, isogenic cell lines to study each pathway is recommended to (1) minimize ER stress arising from transfection or transduction protocols; (2) minimize epigenetic influences resulting from variable transgenic insertion; (3) permit longer analysis since the transgene is stably expressed; and (4) prevent overexpression bias by selecting stable clones with no elevation in basal ER stress compared to wild-type cells.
3.1. Chemical–genetic manipulation of IRE1
3.1.1. Activation of wild-type IRE1
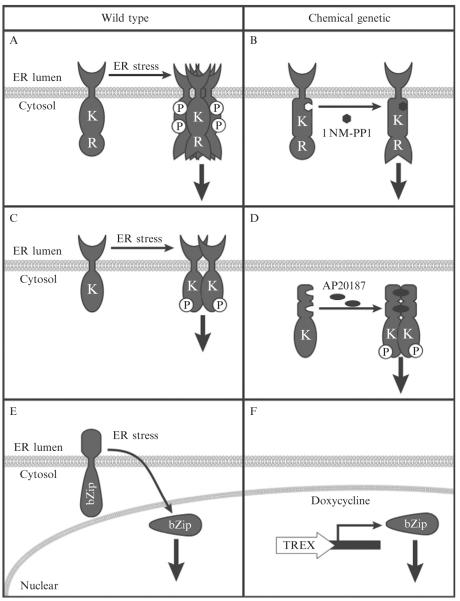
IRE1α is a type I membrane protein consisting of an ER stress-sensor domain protruding into the ER lumen coupled to cytosolic kinase and RNase domains (Cox et al., 1993). In response to ER stress, IRE1α oligomerizes, which induces trans-autophosphorylation of its cytosolic domains and activation of its RNase activity (Cox and Walter, 1996; Shamu and Walter, 1996; Sidrauski and Walter, 1997) (Fig. 11.1A). The activated RNase cleaves Xbp-1 mRNA in the cytosol to generate spliced Xbp-1 mRNA which encodes a potent bZIP-containing transcription factor that upregulates ERAD components and ER chaperones (Lee et al., 2003b). In addition to catalyzing Xbp-1 mRNA splicing, mammalian IRE1α also binds to a growing number of ER membrane and cytosolic proteins including TRAF2 (tumor necrosis factor receptor-association factor 2), BAX (Bcl-2-associated X protein), BAK (Bcl-2 homologous antagonist killer), BI-1 (BAX inhibitor-1), HSP90 (heat shock protein 90), and RACK1 (receptor for activated C-kinase 1) (Hetz et al., 2006; Hetz and Glimcher, 2009; Lisbona et al., 2009; Marcu et al., 2002; Urano et al., 2000). The IRE1–TRAF2 interaction leads to the activation of the c-Jun N-terminal kinase (JNK) signaling pathway (Urano et al., 2000). The physiologic functions of other IRE1 protein–protein interactions are less well understood.
Figure 11.1.
Comparison of UPR activation by ER stress or chemical–genetics. (A) In response to ER stress, wild-type IRE1 oligomerizes, trans-autophosphorylates (P) its kinase domain (K), and activates its endoribonuclease (RNase) activity (R). (B) In response to 1NM-PP1, IRE1[I642G] activates its RNase activity. (C) In response to ER stress, wild-type PERK dimerizes, autophosphorylates, and activates its kinase. (D) In response to AP20187, Fv2E–PERK conditionally dimerizes, autophosphorylates, and activates its kinase function. (E) In response to ER stress, full-length of ATF6 is cleaved and its N-terminus containing a bZip DNA binding domain translocates to the nucleus to activate transcription of target genes. (F) In response to doxycycline, the cleaved form of ATF6 under the control of T-REx promoter is expressed and activates transcription of its target genes.
3.1.2. Activation of IRE1[I642G]
Several chemical–genetic approaches can selectively recapitulate IRE1’s RNase activity in mammalian cells. ATP binding to IRE1’s kinase domain is believed to activate IRE1’s RNase function (Korennykh et al., 2009). A generalized chemical–genetic strategy for sensitizing protein kinases to cell-permeable molecules, while not affecting wild-type kinases, was first applied in yeast (Bishop et al., 2000; Papa et al., 2003) and has since been adapted and demonstrated to be effective in regulating mammalian IRE1α (Han et al., 2008; Hollien et al., 2009; Lin et al., 2007). A human IRE1α mutant bearing an isoleucine-to-glycine missense mutation at the gatekeeper position in the kinase domain, IRE1[I642G], enables ATP-competitive ligands to selectively bind its kinase domain thereby activating the RNase activity of this IRE1α allele (Fig. 11.1B).
1NM-PP1 (1-(tert-butyl)-3-(naphthalen-1-ylmethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine) is the most common ligand used to regulate IRE1 [I642G] and is commercially available (EMD Chemicals, Gibbstown, NJ). Addition of 1NM-PP1 to cells expressing IRE1[I642G] results in rapid Xbp-1 mRNA splicing in multiple mammalian cell types including human embryonic kidney (HEK293), mouse embryonic fibroblast (MEF), and human prostate cancer (PC-3) (Han et al., 2008; Hollien et al., 2009; Lin et al., 2007; Thorpe and Schwarze, 2010). This strategy robustly reproduces IRE1α signaling through Xbp-1 mRNA splicing without perturbing other UPR pathways.
When using the IRE1[I642G] mutant, however, it is important to note its differences from WT IRE1α and what consequences it might have in evaluating the IRE1α signaling pathway as a whole. It is unclear whether the mutant IRE1α forms dimers and/or oligomers or whether this step is necessary for activation in this system whereas trans-autophosphorylation is absent and unnecessary for RNase activity in the mutant. Differences in activation mechanism may be critical, especially in light of any cross talk between UPR pathways. While typically thought of as cytoprotective, recently, Hollien et al. (2009) and Han et al. (2009) discovered that mammalian IRE1α promotes the cleavage of numerous additional mRNAs and that IRE1 may also promote apoptosis depending on the specific mRNAs degraded. These authors demonstrated that IRE1α with active phosphotransfer was able to reduce levels of ER-localized mRNA which preceded apoptosis—this activity was absent in kinase catalysis-compromised mutants such as IRE1[I642G]. This suggests that WT IRE1α can send proapoptotic signals independent of Xbp-1 splicing and that IRE1[I642G] uncouples these endonucleolytic outputs. This uncoupling may be therapeutically advantageous but may confound understanding of the endogenous role of IRE1 and the UPR.
Back et al. (2006) employed a different strategy to activate IRE1α via induced dimerization. By fusing the kinase and RNase domains of IRE1α to a modified FK506-binding domain (Fv1E), addition of a small organic molecule, AP20187, can serve as a dimerizer and recapitulate IRE1α’s RNase activity. This strategy has also been adapted for inducible PERK activation and is described in more detail below. Additionally, others have successfully developed inducible spliced XBP-1 (XBP-1s) using the Tet-Off system (Lee et al., 2003a); however, solely evaluating XBP-1s expression ignores additional targets of IRE1α RNase activity that may have physiological significance (Han et al., 2009; Hollien et al., 2009). Notably, neither system is suitable to study the physiologic functions of other IRE1 protein–protein interactions, as described above (Han et al., 2009; Hollien et al., 2009).
3.2. Chemical–genetic manipulation of PERK
3.2.1. Activation of wild-type PERK
PERK is an ER stress sensor protein that has an ER-luminal domain, bearing sequence and functional similarities to IRE1’s luminal domain, and a cytosolic domain encoding a kinase. In response to ER stress, PERK oligomerizes, activating its kinase function (Fig. 11.1C). PERK kinase activity phosphorylates eIF2α which in turn attenuates protein translation by inhibiting ribosome assembly on mRNAs.
3.2.2. Activation of Fv2E–PERK
Chemical–genetic approaches have been developed that selectively recapitulate PERK’s kinase activity in mammalian cells. Dimerization of PERK is a proximal step in its activation following ER stress. Fv2E–PERK comprises PERK’s kinase domain fused to tandem-modified FK506-binding domains, Fv2E. Fv2E-PERK dimerization can be artificially induced by application of AP20187 (Fig. 11.1D). AP20187 is a cell permeable synthetic mimic of FK1012 (dimer of FK506) that can simultaneously bind two Fv2E domains and physically allow dimerization of Fv2E-fused proteins (Pollock and Rivera, 1999). Fv2E–PERK dimerization by AP20187 causes autophosphorylation of Fv2E–PERK, trans-phosphorylation of eIF2α, and downstream PERK signaling (Lu et al., 2004a,b). This strategy robustly reproduces PERK signaling without necessitating ER stress or activating other UPR signaling pathways. AP20187-based activation of the PERK pathway has been demonstrated in Chinese hamster ovary (CHO), HEK293, MEF, and human mammary epithelial (MCF10A) cells, as well as in mice (Lin et al., 2009; Lu et al., 2004a,b; Oyadomari et al., 2008; Sequeira et al., 2007).
Fv2E–PERK fusion proteins can be created using standard cloning techniques and the ARGENT Regulated Homodimerization Kit (ARIAD Pharmaceuticals, Cambridge, MA). Fv2E is a modified FKBP domain with high affinity to AP20187, therefore commercially available anti-FKBP12 antibodies (Affinity Bioreagents, Rockford, IL) can be used to detect expression of the chimeric PERK protein by Western blot. AP20187 is commercially available from ARIAD Pharmaceuticals, can be diluted in DMF (diamethylformamide), and can be added directly to cell culture preparations.
3.3. Chemical–genetic activation of ATF6
3.3.1. Activation of wild-type ATF6
In contrast to IRE1 and PERK, ATF6 is a type II transmembrane protein with a carboxyl-terminus sensor domain facing the ER lumen coupled to a bZIP-containing transcription factor domain in its cytosolic amino terminus. In response to ER stress, ATF6 exits the ER and translocates to the Golgi (Shen et al., 2002). ATF6 is sequentially cleaved into its active form by two proteases, S1P and S2P (Ye et al., 2000) in the Golgi. Cleavage of ATF6 liberates the cytosolic fragment of ATF6 containing the bZIP transcription factor. The cleaved ATF6 fragment then translocates to the nucleus and upregulates transcription of target genes including ER protein-folding enzymes and ERAD proteins (Fig. 11.1E) (Haze et al., 1999; Wu et al., 2007; Yamamoto et al., 2007).
3.3.2. Activation of TET-ATF6
Chemical–genetic approaches have been developed that selectively recapitulate ATF6’s transcriptional factor activity in mammalian cells. Tetracycline-inducible induction of the cleaved ATF6 fragment encoding the cytosolic bZIP transactivational domain reproduces native ATF6 signaling in human cervical cancer cells (HeLa) and HEK293 cells (Okada et al., 2002). In this system, the ATF6 fragment is placed under the control of a strong promoter containing multiple regulatory tet operator elements. In the absence of tetracycline or its derivative, doxycycline, tet repressors bind to the tet operators and inhibit transcription of ATF6. However, in the presence of doxycycline, the tet repressors bind the drug and undergo a conformational change that dissociates it from the tet operator. As a result, transcription of ATF6 is strongly induced and downstream transcriptional targets of ATF6 are upregulated (Fig. 11.1F). Other UPR signaling pathways are unaffected under these conditions.
There are several ATF6 isoforms; however, amino acid residues 1–373 of human ATF6α correspond to the active product of S1P/S2P-mediated proteolysis. This truncated ATF6α constitutively exists in the nucleus (Haze et al., 1999). We recommend establishing stable Tet-On cell lines expressing this truncated form of ATF6α. For the establishment of a Tet-On system, there are several commercially available vectors and cell lines such as the T-REx system (Invitrogen, Carlsbad, CA) that makes creating of a stable ATF6α(373) cell line convenient and efficient.
Conditional regulation of ATF6 activity can also be achieved with the ERT2 (mutated estrogen receptor) system (Thuerauf et al., 2007). Using this system, the functional domain of cleaved ATF6 is fused to ERT2. In the absence of ERT2 ligands (e.g., tamoxifen), ERT2 forms a complex with various cellular proteins, such as Hsp90, which mask ATF6. The addition of tamoxifen, however, blocks Hsp90 binding to ERT2, allowing ERT2–ATF6 to induce transcriptional activity of ATF6.
4. Concluding Remarks
UPR signaling pathways are likely to contribute to the pathogenesis and progression of numerous human diseases arising from protein misfolding or ER stress. Strategies to monitor and manipulate individual UPR signaling pathways can offer valuable tools to investigate the precise role of the UPR in disease pathogenesis and may offer new ways to combat diseases arising from ER stress.
ACKNOWLEDGMENTS
We thank C. Hetz, H. Li, C. Zhang, and members of the Lin laboratory for comments and suggestions.
REFERENCES
- Back SH, Schroder M, Lee K, Zhang K, Kaufman RJ. ER stress signaling by regulated splicing: IRE1/HAC1/XBP1. Methods. 2005;35:395–416. doi: 10.1016/j.ymeth.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Back SH, Lee K, Vink E, Kaufman RJ. Cytoplasmic IRE1alpha-mediated XBP1 mRNA splicing in the absence of nuclear processing and endoplasmic reticulum stress. J. Biol. Chem. 2006;281:18691–18706. doi: 10.1074/jbc.M602030200. [DOI] [PubMed] [Google Scholar]
- Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, Wood JL, Morgan DO, et al. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- Han D, Upton JP, Hagen A, Callahan J, Oakes SA, Papa FR. A kinase inhibitor activates the IRE1alpha RNase to confer cytoprotection against ER stress. Biochem. Biophys. Res. Commun. 2008;365:777–783. doi: 10.1016/j.bbrc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000a;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000b;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13:393–403. doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- Hetz C, Glimcher LH. Fine-tuning of the unfolded protein response: Assembling the IRE1alpha interactome. Mol. Cell. 2009;35:551–561. doi: 10.1016/j.molcel.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwawaki T, Akai R, Kohno K, Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat. Med. 2004;10:98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457:687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl. Acad. Sci. USA. 2003a;100:9946–9951. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003b;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Walter P, Yen TS. Endoplasmic reticulum stress in disease pathogenesis. Annu. Rev. Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Li H, Zhang Y, Ron D, Walter P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS ONE. 2009;4:e4170. doi: 10.1371/journal.pone.0004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, Glavic A, Kress C, Lin JH, Walter P, Reed JC, Glimcher LH, et al. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol. Cell. 2009;33:679–691. doi: 10.1016/j.molcel.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 2004a;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004b;23:169–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Dong D, Little E, Luo S, Lee AS. Transgenic mouse model for monitoring endoplasmic reticulum stress in vivo. Nat. Med. 2004;10:1013–1014. doi: 10.1038/nm1004-1013. author reply 1014. [DOI] [PubMed] [Google Scholar]
- Marcu MG, Doyle M, Bertolotti A, Ron D, Hendershot L, Neckers L. Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha. Mol. Cell. Biol. 2002;22:8506–8513. doi: 10.1128/MCB.22.24.8506-8513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moenner M, Pluquet O, Bouchecareilh M, Chevet E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007;67:10631–10634. doi: 10.1158/0008-5472.CAN-07-1705. [DOI] [PubMed] [Google Scholar]
- Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem. J. 2002;366:585–594. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephos-phorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa FR, Zhang C, Shokat K, Walter P. Bypassing a kinase activity with an ATP-competitive drug. Science. 2003;302:1533–1537. doi: 10.1126/science.1090031. [DOI] [PubMed] [Google Scholar]
- Pollock R, Rivera VM. Regulation of gene expression with synthetic dimerizers. Meth. Enzymol. 1999;306:263–281. doi: 10.1016/s0076-6879(99)06017-6. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Sequeira SJ, Ranganathan AC, Adam AP, Iglesias BV, Farias EF, Aguirre-Ghiso JA. Inhibition of proliferation by PERK regulates mammary acinar morphogenesis and tumor formation. PLoS ONE. 2007;2:e615. doi: 10.1371/journal.pone.0000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamu CE, Walter P. Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 1996;15:3028–3039. [PMC free article] [PubMed] [Google Scholar]
- Shen J, Prywes R. ER stress signaling by regulated proteolysis of ATF6. Methods. 2005;35:382–389. doi: 10.1016/j.ymeth.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Thorpe JA, Schwarze SR. IRE1alpha controls cyclin A1 expression and promotes cell proliferation through XBP-1. Cell Stress Chaperones. 2010;15:497–508. doi: 10.1007/s12192-009-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuerauf DJ, Marcinko M, Belmont PJ, Glembotski CC. Effects of the isoform-specific characteristics of ATF6 alpha and ATF6 beta on endoplasmic reticulum stress response gene expression and cell viability. J. Biol. Chem. 2007;282:22865–22878. doi: 10.1074/jbc.M701213200. [DOI] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman RJ, Prywes R. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J. Biol. Chem. 2000;275:27013–27020. doi: 10.1074/jbc.M003322200. [DOI] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev. Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Ye J, Rawson RB, Komuro R, Chen X, Dave UP, Prywes R, Brown MS, Goldstein JL. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement basic leucine zipper transcription factors. J. Biol. Chem. 1998;273:33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Okada T, Haze K, Yanagi H, Yura T, Negishi M, Mori K. ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cisacting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 2000;20:6755–6767. doi: 10.1128/mcb.20.18.6755-6767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]